Significance
Oxytocin promotes positive social behaviors in several species and therefore may be a therapeutic tool for neurodevelopmental disorders. It remains untested, however, whether oxytocin may affect infants, and whether effects may vary depending on infants’ social skills or interest. To test these predictions, we administered nebulized oxytocin to rhesus macaque newborns. Macaques, like humans, engage in complex face-to-face mother–infant interactions. Oxytocin increased infants’ affiliative communicative gestures and decreased salivary cortisol, and higher oxytocin levels were associated with greater social interest. Infants with stronger imitative skills were most positively influenced by oxytocin, suggesting that oxytocin sensitivity may underlie early social motivation. These results suggest that oxytocin may be a promising early intervention for infants at risk for abnormal social functions.
Keywords: imitation recognition, neonatal imitation, individual differences
Abstract
Early caregiver–infant interactions are critical for infants’ socioemotional and cognitive development. Several hormones and neuromodulators, including oxytocin, affect these interactions. Exogenous oxytocin promotes social behaviors in several species, including human and nonhuman primates. Although exogenous oxytocin increases social function in adults—including expression recognition and affiliation—it is unknown whether oxytocin can increase social interactions in infants. We hypothesized that nebulized oxytocin would increase affiliative social behaviors and such effects would be modulated by infants’ social skills, measured earlier in development. We also hypothesized that oxytocin’s effects on social behaviors may be due to its anxiolytic effects. We tested these hypotheses in a blind study by nebulizing 7- to 14-d-old macaques (n = 28) with oxytocin or saline. Following oxytocin administration, infants’ facial gesturing at a human caregiver increased, and infants’ salivary oxytocin was positively correlated with the time spent in close proximity to a caregiver. Infants’ imitative skill (measured earlier in development: 1–7 d of age) predicted oxytocin-associated increases in affiliative behaviors—lip smacking, visual attention to a caregiver, and time in close proximity to a caregiver—suggesting that infants with higher propensities for positive social interactions are more sensitive to exogenous oxytocin. Oxytocin also decreased salivary cortisol, but not stress-related behaviors (e.g., scratching), suggesting the possibility of some anxiolytic effects. To our knowledge, this study provides the first evidence that oxytocin increases positive social behaviors in newborns. This information is of critical importance for potential interventions aimed at ameliorating inadequate social behaviors in infants with higher likelihood of developing neurodevelopmental disorder.
Oxytocin is a neuropeptide that has wide-ranging effects on social behaviors and social perception, including increased emotion recognition and prosocial behavior (1, 2). Animal studies present convergent evidence of oxytocin’s positive effects on social behavior (2–6), including humans (1, 7). In recent years, an increasing literature on human and nonhuman primates suggests an association between oxytocin levels—either endogenous or exogenously administered—and prosocial behaviors (8–10). In both humans and macaques, exogenous oxytocin appears to enhance social attention, prosocial behaviors, sensitivity to gaze, and sensitivity to facial expressions (for reviews, see refs. 1 and 2).
Oxytocin, therefore, may be a tool for promoting social behaviors, especially in clinical populations in which social faculties are compromised (8, 11–13). In the last few years, in fact, oxytocin has been tested in autistic individuals, and it appears to increase social attention and improve emotion recognition (e.g., refs. 14–19; although see ref. 20; for a recent review, see ref. 21). Given the importance of early assessments in the diagnosis of autism (22), studies clarifying the role of oxytocin in early development are critically important. For example, human infants actively participate in face-to-face caregiver–infant interactions; failure to engage with caregivers in this way can disrupt the development of healthy emotion regulation and socioemotional skills (23–25). In both caregivers and neonates, complex cortical and limbic brain networks are prepared to sustain such exchanges (26–28), and several hormones and neuromodulators regulate the affective components of face-to-face caregiver–infant interactions (29–32). However, to our knowledge, studies investigating the role of infants’ oxytocin levels in these early intersubjective exchanges have not been carried out. Only one study to date measured endogenous oxytocin levels in newborns and reported that higher levels of oxytocin in newborns’ cerebrospinal fluid (CSF) were associated with higher levels of social engagement, including actively seeking parental social interaction for soothing and a greater interest in social interaction (33). No studies to date, however, have administered oxytocin to infants to determine its effects on social behavior, despite the fact that a more thorough understanding of oxytocin and its behavioral consequences may provide a potential tool for interventions aimed at promoting social affiliation in individuals with social impairments (11–15, 34). The necessity to fill this gap motivated the present study.
Our first goal was to determine whether oxytocin influences newborn macaques’ behaviors during an interaction with a human caregiver. We predicted that oxytocin, compared with saline, would increase positive social behavior, including facial gestures [i.e., lip smacking (LPS) and tongue protrusion (TP)], visual attention to a human caregiver, and time spent in close proximity to a human caregiver (Table 1). We chose macaques because they have an extended period of parental care and exhibit complex mother–infant face-to-face interactions, e.g., mutual gaze, “motherese,” and facial imitation (35). As adults, macaques display positive behavioral changes in response to exogenous oxytocin (2–6), as in humans (1).
Table 1.
Ethogram for 12 behaviors scored during imitation recognition
| Behavior | Operational definition | |
| Events | LPS | Lip smacking. Rapid opening and closing of the mouth |
| TP | Protrusion and retraction of the tongue | |
| States | Vis attn | Visual attention. Looking at the face of the human caregiver model |
| Prox | Proximity. Infant torso is within 5 cm (infant arm’s reach) from cage front | |
| Events | Scratch | Common use |
| Yawn | Common use | |
| States | Self-suck | Insertion into mouth of fingers/hands, toes/feet |
| Self-clasp | Hand or foot closed on fur or some body part | |
| Surrogate | Any touching of surrogate mother | |
| Loco | Locomotion. Directed movement of torso (>15 cm within 5 s) | |
| Explore | Exploration. Manipulating toys or bedding | |
| Sleep | Infant lying down with head on floor of cage |
An additional motivation for the present study was to examine individual differences in sensitivity to oxytocin. We predicted that individual differences in infants’ social skills might moderate the effects of oxytocin. In particular, in the first week of life, macaques, like humans, imitate facial gestures (36); this response reflects the emergence of infants’ early social skills in tuning their own behavior with that of their mothers (36). Despite large individual differences in imitative ability (37), the neurochemical mechanism mediating these responses remains unknown. Early imitative abilities are associated with some aspects of later social cognitive development (37–39) and may reflect general social interest (for a review, see ref. 40). For example, macaque infants who consistently imitate in the first week of life, compared with those who do not, are better at recognizing human caregivers (38) and visually attend more to caregivers (39). Together, these lines of evidence suggest that the capacity to imitate at birth is associated with a range of social-cognitive skills, and that the interindividual differences in such skills may rely on neurobiological substrates mediated by oxytocin. Given that infants may vary in their social interest, and that oxytocin may enhance intrinsic social motivation (2, 4), we predicted infants’ imitative skill—a measure of social interest—may predict their sensitivity to exogenous oxytocin.
A final motivation was to assess infants’ salivary oxytocin and cortisol levels, to determine the influence of inhaled oxytocin. Other studies report that administering oxytocin results in a dose-dependent decrease in plasma cortisol (41) and reduces anxiety, which increases affiliative motivation (42). We predicted that inhaled oxytocin would increase infants’ salivary oxytocin and decrease salivary cortisol. We also measured anxiolytic effects behaviorally by examining self-directed behaviors that have been associated with stress (43), including scratching, yawning, self-sucking, self-clinging, and interactions with the surrogate (Table 1).
To test these hypotheses, we assessed infant macaques’ neonatal imitation of facial gestures in the first week of life (for rearing and testing details, see SI Methods). In the second week of life, we carried out a procedure on 2 consecutive days, in which infants were nebulized with oxytocin or saline (one per day). One and 2 h following nebulization, infants were tested in an imitation recognition task in which a human experimenter imitated all of an infant’s mouth movements for 2 min, followed by 2 min of still face (i.e., neutral face), while trying to maintain eye contact with the infant. This paradigm was selected because of previous findings that monkeys recognize when they are being imitated (44) and display affiliation toward social partners who imitate them (45). We collected saliva samples 2 and 4 h after the end of nebulization to measure salivary oxytocin and cortisol levels (see SI Methods for details).
Results
Exogenous Oxytocin Elevated Salivary Oxytocin and Decreased Salivary Cortisol.
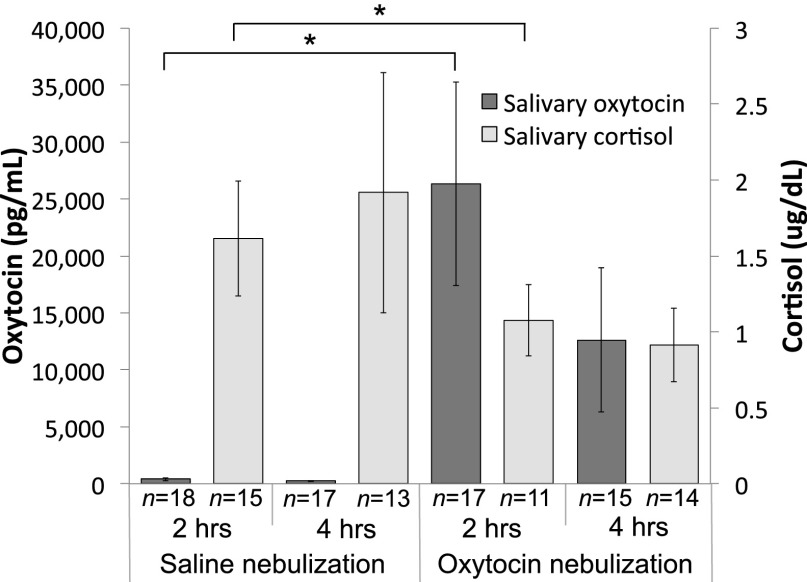
All analyses initially included the variable of sex (male, female), but there were no main effects or interactions (values of P > 0.05); therefore, we excluded this variable. We then analyzed infants’ saliva to assess peripheral oxytocin levels (46). We carried out two paired-samples t tests, one at each postnebulization time (2, 4 h), for both salivary oxytocin and cortisol, to compare the levels in each condition (saline control, oxytocin) (Fig. 1). There was higher salivary oxytocin in the oxytocin condition [mean (M) = 26,995 pg/mL, SD = 37,976] than the saline condition (M = 396 pg/mL, SD = 145) at 2 h postnebulization [t(14) = 1.83, P = 0.013, d = 0.73], but no significant difference in the conditions at 4 h postnebulization [t(13) = 1.86, P = 0.086]. Salivary cortisol was lower in the oxytocin condition (M = 1.04 μg/dL, SD = 0.25) than in the saline condition (M = 1.49 μg/dL, SD = 0.38) at 2 h postnebulization [t(7) = 2.48, P = 0.042, d = 0.88], but there was no difference 4 h postnebulization [t(11) = 1.12, P = 0.287] (Fig. 1).
Fig. 1.
Saliva analysis for oxytocin and cortisol levels, 2 and 4 h after nebulization with either saline control or oxytocin. For salivary oxytocin (light bars; left axis), there was a main effect of condition (Oxytocin > Saline Control) [F(1,13) = 8.95, P = 0.010, ηp2 = 0.408]. For salivary cortisol (dark bars; right axis), there was an effect at 2 h postnebulization (saline control > oxytocin) [t(8) = 2.97, P = 0.018, d = 0.99], but no effect at 4 h postnebulization [t(12) = 1.28, P = 0.225]. Error bars reflect SEM. *P < 0.05.
We next examined whether there was any association between the nebulized amount of oxytocin delivered and infants’ behaviors, salivary oxytocin, or cortisol levels. There were no significant associations (values of P > 0.05) indicating that variations in delivery dosages were not large enough to produce differences in our behavioral or physiological measures.
Oxytocin Increased Infants’ Facial Gestures.
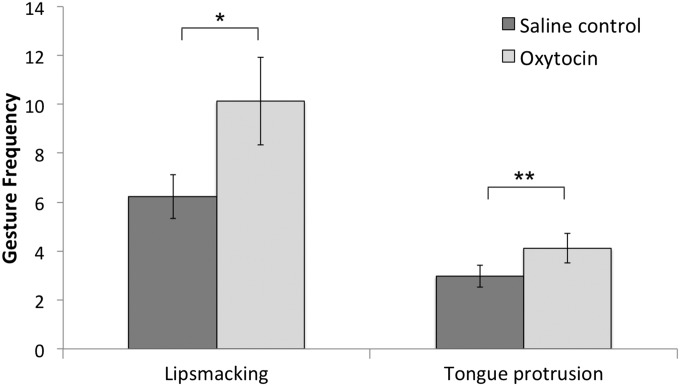
We analyzed 12 behavioral measures (Table 1) with repeated-measures ANOVAs (Tables S1–S3) to determine whether each behavior varied across condition (saline control, oxytocin), time postnebulization (1 or 2 h after nebulization), and experimental period (imitation, still face). In terms of oxytocin effects, only facial gestures—LPS and TP—occurred more frequently in the oxytocin condition relative to the saline condition (values of P < 0.05) (Fig. 2). There were no other main effects or interactions for the variable of condition for any of the other behaviors (values of P > 0.05).
Fig. 2.
Infants’ lip smacking (LPS) and tongue protrusion (TP) gesture rates in the imitation recognition task (imitation and still-face periods combined), nebulized with either saline or oxytocin. These data are averaged across time postnebulization (1 and 2 h after nebulization). There was a main effect of condition (oxytocin > saline control) [F(1,26) = 5.96, P = 0.022, ηp2 = 0.186]. Error bars reflect SEM. *P = 0.014, **P = 0.029.
Time Spent in Close Proximity to Caregiver Was Positively Associated with Salivary Oxytocin.
We examined whether there were any associations between infants’ behaviors and their salivary oxytocin levels. We focused specifically on the 2-h postnebulization time, since we had both the behavioral and salivary oxytocin measure at this time. We carried out a series of four linear correlations on each of the 12 behaviors—examining both conditions (saline control, oxytocin) and both stimulus periods (imitation, still face)—using a Bonferroni correction (P < 0.05/4 = 0.0125). In the oxytocin condition, during the imitation period, there was a positive correlation between salivary oxytocin and time spent in close proximity to the caregiver [r(14) = 0.642, P = 0.007] (Table S4). There were no other correlations (values of P > 0.0125).
Neonatal Imitation Predicted Oxytocin-Induced Increases in Affiliative Behaviors.
Finally, we examined whether infants’ facial imitation performance in the first week of life was related to their behavioral and physiological reactivity to oxytocin. We assessed infants’ strength of LPS imitation in the first week of life (SI Methods). To assess the oxytocin-related behavioral changes, we calculated the difference in the frequency or duration of each behavior (Table 1) in oxytocin and saline conditions (i.e., frequency in saline condition subtracted from frequency in oxytocin condition). Oxytocin-associated difference scores greater than zero reflect increases in behaviors, and scores below zero reflect decreases in behaviors. We first examined the 2-min imitation period 2 h after nebulization. There were positive correlations between LPS imitative skill and three affiliative social behaviors: LPS gestures [r(26) = 0.649, P < 0.001], time spent looking at the caregiver [r(24) = 0.719, P < 0.001], and time spent in close proximity to the caregiver [r(26) = 0.498, P = 0.008] (Fig. 3). There were negative correlations between LPS imitative skill and two behaviors—self-clasping [r(26) = 0.430, P = 0.025] and sleeping [r(26) = −0.573, P = 0.002]—and a positive correlation between LPS imitation skill and locomotion [r(26) = 0.472, P = 0.013]. There were no significant correlations between imitative skill and oxytocin-associated difference scores, in the still-face periods, or in the sessions 4 h after nebulization (values of P > 0.10). There were also no associations between infants’ imitative skills and their salivary oxytocin or cortisol levels in either the oxytocin or saline conditions (values of P > 0.10).
Fig. 3.
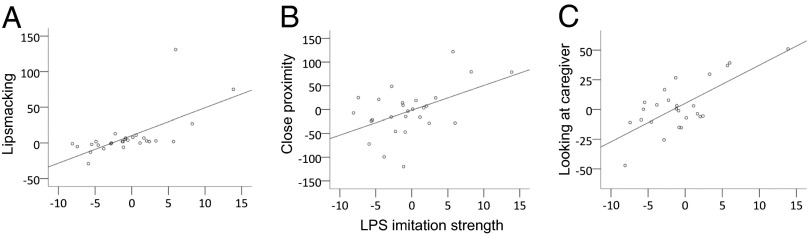
Associations between imitative skill and behavioral changes due to oxytocin. Scatter plots reflect the association between the strength of infants’ lip smacking (LPS) imitation in the first week of life (x axis) and the change in behaviors as a consequence of oxytocin administration (y axis: saline control condition subtracted from oxytocin condition; 0 indicates no changes as a consequence of oxytocin) during the imitation recognition task in the second week of life, for (A) the frequency of LPS, (B) time (in seconds) in close proximity to the caregiver, and (C) time (in seconds) looking at the caregiver.
Discussion
In the present study, exogenous oxytocin had positive effects on 7- to 14-d-old macaques’ social behaviors: infants produced more facial gestures when nebulized with oxytocin compared with saline. Synchronous communicative exchanges between infants and caregivers, such as these, are important for healthy development (for a review, see ref. 47), so increasing such exchanges could be beneficial to infants (48). This finding has several implications for possible early interventions promoting social behaviors in children whose social skills could be compromised, such as in autism (49).
The positive effect of oxytocin on social behavior found in the present study is likely the consequence of the action of oxytocin, or of its metabolites, on the central nervous system (CNS). In fact, nebulizing oxytocin in macaque neonates increased salivary oxytocin 2 h after nebulization. Although we were unable to directly assess oxytocin in the CNS, this finding of elevated salivary oxytocin is consistent with studies in adult macaques that reported elevated CSF oxytocin levels 35–120 min after aerosolized oxytocin delivery (4, 50). It is also consistent with studies in rodents reporting that intranasal oxytocin increases extracellular concentrations of oxytocin in behaviorally relevant brain regions, peaking 30–60 min after administration (51), and studies of elevated oxytocin in human CSF 75 min following intranasal administration (52). Although the relation between central and peripheral oxytocin levels has not been fully elucidated (e.g., ref. 53), elevated oxytocin in CSF is likely to reflect transnasal penetration into the CNS (4, 52), verifying that nebulization of oxytocin was successful. Unlike other routes of administration (e.g., intranasal, intravenous), nebulizing oxytocin may allow oxytocin molecules to be absorbed into the CNS through the nasal mucosa (54, 55), making it one of the most effective routes of administration (50). Elevated oxytocin levels in both the CNS and periphery are most likely due to the direct effects of exogenous peptide administration, although it is impossible to rule out a contribution from endogenous oxytocin release (50). Finally, functional magnetic resonance imaging (fMRI) studies have demonstrated that intranasal oxytocin produces changes in the activity of several brain areas in humans and macaques (49, 56, 57), thus supporting other findings of the CNS effects of this neuromodulator (58).
Cortisol levels were lower in the oxytocin condition compared with the saline condition at 2 h after nebulization, consistent with previous studies in adults (e.g., ref. 59). This finding may indicate that oxytocin had some anxiolytic effects, although behaviorally no such effects were detected. It is worth noting, however, that both salivary oxytocin and cortisol levels did not remain altered 4 h after nebulization, suggesting that, in newborns, the effects were not as long lasting as in human adults [e.g., lasting more than 7 h (60)]. Lower doses of oxytocin may have shorter effect durations; however, in the present study, our shorter effect durations are unlikely to be due to lower doses, as the dose was comparable to that in previous studies.
In contrast to our predictions, however, oxytocin did not appear to increase infants’ visual attention to social partners, as reported in adult monkeys (4). It is possible that, although oxytocin did not impact the overall amount of visual attention to the social partner, it may have affected the distribution of infants’ attention to caregivers; namely, attention to the most relevant aspects of the face, such as the eyes (61), or attention to particular types of expressions (6). It would also be interesting to examine whether oxytocin would influence infants’ attention to eyes, as it does in adults [e.g., humans (61); macaques (5)], especially given that attention to the eyes in early infancy may be a key signature of neurodevelopmental disorders, such as autism (22).
We also assessed whether variation in salivary oxytocin levels were associated with variation in infants’ affiliative behaviors. Salivary oxytocin was positively correlated with time spent in close proximity to the caregiver, consistent with our prediction that elevated oxytocin increases affiliation. Similarly, in adult humans, oxytocin increases affiliation (62), and in animals, oxytocin increases social approach (63).
Although these results provide clear support for the proposition that oxytocin increases affiliation, interpretations of these findings at a neurophysiological level remain speculative. It is possible that oxytocin reduced anxiety, resulting in an increased propensity to socially engage with the caregiver. Several studies have shown that oxytocin has stress-buffering effects that are manifested behaviorally in terms of reduced anxiety and increased affiliative motivation (42, 64) and physiologically in terms of reduced activity of the hypothalamic-pituitary adrenal (HPA) axis (41, 65). The present finding of decreased salivary cortisol following oxytocin administration is consistent with these results, as well as reports that oxytocin locally affects several hypothalamic nuclei, e.g., through dendritic release (66). However, in the present study, there were no oxytocin-related behavioral anxiolytic effects, that is, no changes in self-directed behaviors commonly associated with stress in macaques [e.g., scratching (43)]. The incongruity between a significant oxytocin-related decrease in cortisol in the absence of a change in stress/anxiety-related behaviors could be explained if the endocrine effect was independent of stress. Indeed, the testing situation was likely not stressful for the infants because they interacted with human caregivers regularly and the task took place in their familiar home incubator. Alternatively, it is possible that oxytocin had anxiolytic effects on the infants, but those effects were observable only in salivary cortisol because the behavioral measures used were insensitive for detecting changes in stress levels.
Another possibility is that increases in prosocial behavior as a consequence of oxytocin were not due to anxiolytic actions, but rather were a consequence of oxytocin’s actions on brain regions involved in social processing and the control of social behavior (42, 67). In support of this, neuroimaging studies in humans demonstrate that intranasal oxytocin modulates activity of the amygdala and cingulate cortex (see ref. 67 for a review). A recent fMRI study in monkeys showed that intranasal administration of oxytocin reduced the effects of facial expression valence (e.g., threatening and neutral facial stimuli) on the activity of the amygdala, the medial prefrontal cortex, and the inferotemporal cortex (57), similar to effects found in humans (68). Further evidence comes from fMRI studies demonstrating that intranasal oxytocin produces changes in the activity of several brain areas in human adults with Asperger syndrome (56) and children with autism (49). Specifically, oxytocin increased brain activity for socially meaningful stimuli and attenuated activity for nonsocial stimuli (49), thus supporting other findings of the CNS effects of this neuromodulator (58). Further studies are clearly necessary to further test oxytocin’s effects on various brain regions.
Finally, individual differences in infants’ LPS imitative skill predicted their sensitivity to exogenous oxytocin. Stronger LPS imitation in the first week was associated with increased affiliative behaviors in the second week—LPS, time spent looking at a caregiver, and time in close proximity to a caregiver—as a consequence of oxytocin administration. In previous studies, we reported that the early capacity of imitation in newborn monkeys might be an index of the maturation of voluntary skilled movements as well as of cognitive and social skills (35, 38). Although indirectly, these data suggest that early social competencies are linked to brain networks involved in motor control and in regulating socioemotional responses. Infants who are more sensitive and responsive to social cues from birth are also more sensitive to the prosocial effects of oxytocin, possibly due to oxytocin-associated amplification of intrinsic social motivation (2, 4). Infants’ imitative skills were not, however, associated with salivary oxytocin or cortisol levels, in either the oxytocin or control conditions, which indicates that, although affiliative behaviors were altered by oxytocin as a function of imitative skill, physiological responses—at least in our salivary measures—were not different as a function of imitative skill. This suggests that salivary oxytocin and cortisol may be more influenced by exogenous than endogenous levels of these hormones. Alternatively, these data may indicate that, although salivary oxytocin may be a fairly accurate measurement of peripheral oxytocin, these levels may not necessarily indicate the precise quantity of oxytocin absorbed in CNS, and therefore this noninvasive assessment may lack the high resolution necessary for identifying potential interactions between endogenous and exogenous levels.
Oxytocin clearly exerts its effects on several cortical network and subcortical structures that regulate social behavior, as previously suggested. However, other networks could also be involved. Indeed, the mirror neuron system has been shown to be central in cortical action-perception mechanisms involved in social perception and imitation (69, 70). Although speculative, the heightened oxytocin sensitivity of infants with higher imitative skills suggests that the social brain network linking action and perception may be one target of this neuropeptide. Specifically, we hypothesize that the mirror neuron system is a potential candidate for mediating some of the social effects of oxytocin. Recently, it was reported that, during the observation and imitation of facial gestures, such as LPS, specific brain rhythms (the mu rhythm) are desynchronized (71). These EEG changes may reflect the activity of cortical parietal-frontal networks, which are part of the mirror neuron system (72). Critically, in human adults, the administration of oxytocin modulates the mu rhythm (73), providing preliminary support for the hypothesis that this network may be a target of oxytocin. Further research on whether and how oxytocin influences the mirror neuron system is warranted.
In addition, the administration of oxytocin in young populations may produce different effects than in adults, depending on the maturation of brain structures that mediate responses to social stimuli [e.g., potential dysregulation of HPA axis with chronic administration (65)]. Similarly, chronic administration of oxytocin in animals [e.g., pigs (65), mice (74), voles (75)] appears to reduce social behavior relative to acute administration, which generally increases social behaviors. Caution is therefore warranted with chronic administration, particularly in developing populations, which may be more vulnerable (e.g., ref. 49; for a review, see ref. 53). However, further investigation is required to understand the complex nature of the interaction between the general effects of oxytocin and its action on different brain areas involved in the perception and production of social behavior (e.g., amygdala, cingulate cortex, and parietal-premotor cortex).
To our knowledge, this study provides the first evidence that administration of oxytocin in newborns increases positive social behaviors. Further research on endogenous oxytocin levels in infants is another promising direction, as it may be possible to gain similar benefits to that of exogenous oxytocin through social interactions, including gentle touch and face-to-face interactions (for a review, see ref. 76). A more thorough understanding of oxytocin and its behavioral consequences may help in designing potential therapeutic tools to prevent the detrimental risks of disrupted early caregiver–infant interactions (e.g., institutionalized infants, infants whose mothers suffer from depression), or to ameliorate skills in young populations whose social behaviors are impaired due to biological preconditions (e.g., infants diagnosed with autism spectrum disorder). In this context, oxytocin has significant appeal as an innovative adjunctive therapy for autism spectrum disorder (11–13) and has shown promise as an early intervention (e.g., refs. 15–17). Although much remains unknown about the influence of exogenous oxytocin on infants, the present study presents promising results, suggesting potential social benefits. The lasting effects of these benefits and the extent to which there are individual differences in these benefits, however, are topics that warrant further exploration.
Methods
Subjects.
We tested 28 infant rhesus macaque monkeys (Macaca mulatta), 16 males and 12 females, on neonatal imitation every other day between 1 and 8 d of age (see SI Methods for details) and on imitation recognition between 7 and 14 d of age (M = 9.84 d, SD = 1.78). All infants were full-term and healthy at time of testing. We tested one additional infant but excluded him from analysis due to ill health at the time of testing. On the day of birth, infants were separated from their mothers and raised in a neonatal primate nursery facility (see SI Methods for details). The Animal Care and Use Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) approved this study.
Neonatal Imitation Testing Procedure.
Infants were tested three times per day on up to 4 separate days within the first week of life. During the baseline period, a human experimenter presented a still face or still disk, followed by one of three stimulus periods: LPS, TP, and Disk Control. Total frequencies of LPS and TP were recorded from videotaped sessions by an experimenter blind to the experimental condition, and were averaged across all testing days. We computed an imitation index to capture the strength of infants’ neonatal imitation performance (SI Methods).
Oxytocin Administration Procedure.
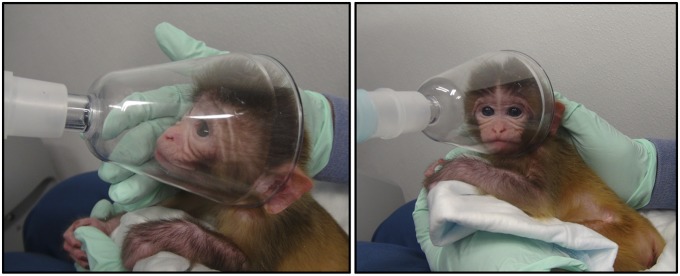
We tested infants on 2 separate days, once between days 7 and 11 (M = 8.9 d, SD = 1.4) and a second time between days 8 and 14 (M = 10.8 d, SD = 1.6). On each test day, we administered either oxytocin or a saline control solution. The order of conditions was counterbalanced across subjects. Experimenters who collected the data and coded the videos were blind to the experimental condition (i.e., which solution the infant received) and to the imitator status of infants. We sought to deliver 25 international units (IU) per infant (1.25 mL at 20 IU/mL; Bimeda-MTC Animal Health) of oxytocin, or 1.25 mL of a sterile saline solution, via nebulizing it into the nose and mouth continuously for 7 min, using a Pari Baby Nebulizer (Fig. 4). Nebulization amounts were monitored by measuring any remaining solution; infants received between 0.8 and 2.2 mL of oxytocin [M = 1.30 mL (26 IU), SD = 0.32 mL (6.4 IU)] and 0.9–2 mL of saline (M = 1.46 mL, SD = 0.27). This dose is comparable to that given to newborn pigs [24 IU (65)] and adult macaques [25–48 IU (4–6)]. During nebulization, infants were cradled in the arms of a trained experimenter, and a small nebulization mask was gently held over the nose and mouth. Infants displayed no signs of distress during nebulization.
Fig. 4.
Infant during nebulization procedure.
Saliva Collection Procedure, Purification, and Analysis.
After nebulization, infants were placed back in their incubator. We collected saliva samples from a subsample of infants (n = 19) 2 and 4 h after the end of nebulization. We collected infants’ saliva by allowing infants to chew on flavored dental cotton rope (SI Methods).
Imitation Recognition Testing Procedure.
One and 2 h after the end of the nebulization, we carried out an imitation recognition task, which consisted of imitating all of the infant’s mouth movements for 2 min, followed by 2 min of still face (i.e., neutral face). The model remained stationary just outside of the infant’s incubator and attempted to maintain eye contact with the infant for the entire test. The time period between the two tests was ∼1 h (M = 58 min, SD = 6 min). Imitation recognition tests were videotaped (Sony Digital Video Camcorder HDR-CX560V), and infants’ behaviors were coded off-line, frame by frame (30 frames per s). Fourteen behaviors were scored (Table 1), including four social behaviors: two facial gestures, LPS and TP (Fig. S1), the duration of time infants looked at the human caregiver, and the duration of time the infant spent in close proximity to the human caregiver. We additionally scored nonsocial behaviors common in newborn macaques, including the frequency of scratching and yawning, and the duration of time sucking hands/feet, self-clasping, interacting with the surrogate, resting, locomotion, and exploring the environment. One observer coded all sessions. To ensure reliability, this observer was compared with three additional observers who together randomly selected and coded all behaviors in 21–24% of the videos (SI Methods).
Supplementary Material
Acknowledgments
We thank Ruth Woodward and Angela Ruggiero for technical assistance administering oxytocin and collecting saliva. We thank Lydia Martin, Grace Maloney, and Chris Catalfamo for behavioral reliability coding. Thanks to Nathan Fox for feedback on an earlier version of this paper. The Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NICHD Grant P01HD064653 (to P.F.F.), and National Institutes of Health Grant RR11122 (to M.A.N.) supported this research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402471111/-/DCSupplemental.
References
- 1.Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Horm Behav. 2012;61(3):410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Chang SW, Platt ML. Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Res. 2013 doi: 10.1016/j.brainres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solyst JA, Buffalo EA (2013) Intranasal oxytocin modulates attention to social stimuli in rhesus macaques. Poster presented at the Society for Neuroscience Meeting, San Diego, CA. Available at www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=6ffd0310-e813-4f40-8a42-04f3d7cf0983&cKey=de9d1507-0c26-4d57-b693-518e344e1bdc&mKey=%7b8D2A5BEC-4825-4CD6-9439-B42BB151D1CF%7d.
- 4.Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci USA. 2012;109(3):959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebitz RB, Watson KK, Platt ML. Oxytocin blunts social vigilance in the rhesus macaque. Proc Natl Acad Sci USA. 2013;110(28):11630–11635. doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parr LA, Modi M, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys’ attention to negative facial expressions. Psychoneuroendocrinology. 2013;38(9):1748–1756. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curley JP, Keverne EB. Genes, brains and mammalian social bonds. Trends Ecol Evol. 2005;20(10):561–567. doi: 10.1016/j.tree.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32(4):426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37(3):438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Bakermans-Kranenburg MJ, van IJzendoorn MH. Sniffing around oxytocin: Review and meta-analysis of trials in healthy and clinical groups with implications for pharmacotherapy. Transcult Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollander E, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61(4):498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm Behav. 2012;61(3):340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andari E, et al. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107(9):4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guastella AJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Kosaka H, et al. Long-term oxytocin administration improves social behaviors in a girl with autistic disorder. BMC Psychiatry. 2012;12(110):1–4. doi: 10.1186/1471-244X-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachibana M, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013;23(2):123–127. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- 18.Anagnostou E, et al. Intranasal oxytocin in the treatment of autism spectrum disorders: A review of literature and early safety and efficacy data in youth. Brain Res. 2014 doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 19.Young LJ. When too much of a good thing is bad: Chronic oxytocin, development, and social impairments. Biol Psychiatry. 2013;74(3):160–161. doi: 10.1016/j.biopsych.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Dadds MR, et al. Nasal oxytocin for social deficits in childhood autism: A randomized controlled trial. J Autism Dev Disord. 2014;44(3):521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- 21.Stavropoulos KK, Carver LJ. Research review: Social motivation and oxytocin in autism—implications for joint attention development and intervention. J Child Psychol Psychiatry. 2013;54(6):603–618. doi: 10.1111/jcpp.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–31. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ainsworth MDS, Bell SM. Mother-infant interaction and the development of competence. In: Connolly K, Bruner J, editors. The Growth of Competence. New York: Academic; 1974. pp. 97–118. [Google Scholar]
- 24.Schmid B, et al. Quality of early mother-child interaction associated with depressive psychopathology in the offspring: A prospective study from infancy to adulthood. J Psychiatr Res. 2011;45(10):1387–1394. doi: 10.1016/j.jpsychires.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Child Psychiatry. 1978;17(1):1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- 26.Atzil S, Hendler T, Feldman R. The brain basis of social synchrony. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenzi D, et al. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cereb Cortex. 2009;19(5):1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- 28.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48(3-4):262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisman O, et al. Oxytocin shapes parental motion during father-infant interaction. Biol Lett. 2013;9(6):20130828. doi: 10.1098/rsbl.2013.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin administration, salivary testosterone, and father-infant social behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49(3):47–52. doi: 10.1016/j.pnpbp.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Apter-Levi Y, Zagoory-Sharon O, Feldman R. Oxytocin and vasopressin support distinct configurations of social synchrony. Brain Res. 2013 doi: 10.1016/j.brainres.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 32.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18(11):965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 33.Clark CL, et al. Neonatal CSF oxytocin levels are associated with parent report of infant soothability and sociability. Psychoneuroendocrinology. 2013;38(7):1208–1212. doi: 10.1016/j.psyneuen.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61(3):380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari PF, Paukner A, Ionica C, Suomi SJ. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr Biol. 2009;19(20):1768–1772. doi: 10.1016/j.cub.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrari PF, et al. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4(9):e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrari PF, et al. Interindividual differences in neonatal imitation and the development of action chains in rhesus macaques. Child Dev. 2009;80(4):1057–1068. doi: 10.1111/j.1467-8624.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson EA, Paukner A, Sclafani V, Suomi SJ, Ferrari PF. Lipsmacking imitation skill in newborn macaques is predictive of social partner discrimination. PLoS One. 2013;8(12):1–6. doi: 10.1371/journal.pone.0082921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson EA, Paukner A, Suomi SJ, Ferrari PF. Visual attention during neonatal imitation in newborn macaque monkeys. Dev Psychobiol. 2013;56(4):864–870. doi: 10.1002/dev.21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simpson EA, Murray L, Paukner A, Ferrari PF (2014) The mirror neuron system as revealed through neonatal imitation: Presence from birth, predictive power, and evidence of plasticity. Philos Trans R Soc Lond B Biol Sci 369(1644):20130289. [DOI] [PMC free article] [PubMed]
- 41.Legros JJ, Chiodera P, Geenen V, Smitz S, von Frenckell R. Dose-response relationship between plasma oxytocin and cortisol and adrenocorticotropin concentrations during oxytocin infusion in normal men. J Clin Endocrinol Metab. 1984;58(1):105–109. doi: 10.1210/jcem-58-1-105. [DOI] [PubMed] [Google Scholar]
- 42.Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm Behav. 2012;61(3):392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schino G, Troisi A, Perretta G, Monaco V. Measuring anxiety in nonhuman primates: Effect of lorazepam on macaque scratching. Pharmacol Biochem Behav. 1991;38(4):889–891. doi: 10.1016/0091-3057(91)90258-4. [DOI] [PubMed] [Google Scholar]
- 44.Paukner A, Anderson JR, Borelli E, Visalberghi E, Ferrari PF. Macaques (Macaca nemestrina) recognize when they are being imitated. Biol Lett. 2005;1(2):219–222. doi: 10.1098/rsbl.2004.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paukner A, Suomi SJ, Visalberghi E, Ferrari PF. Capuchin monkeys display affiliation toward humans who imitate them. Science. 2009;325(5942):880–883. doi: 10.1126/science.1176269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weisman O, Zagoory-Sharon O, Feldman R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology. 2012;37(9):1582–1586. doi: 10.1016/j.psyneuen.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Feldman R. Parent-infant synchrony biological foundations and developmental outcomes. Curr Dir Psychol Sci. 2007;16(6):340–345. [Google Scholar]
- 48.Landa RJ, Holman KC, O’Neill AH, Stuart EA. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: A randomized controlled trial. J Child Psychol Psychiatry. 2011;52(1):13–21. doi: 10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon I, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci USA. 2013;110(52):20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Modi ME, et al. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. 2014 doi: 10.1016/j.psyneuen.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Striepens N, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3(3440):1–5. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79(3):383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 54.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Guastella AJ, et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38(5):612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 56.Domes G, et al. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74(3):164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 57. Liu N, et al. (2013) Oxytocin modulates fMRI responses to facial expression in macaques. Poster presented at the Society for Neuroscience Meeting, San Diego, CA. Available at www.abstractsonline.com/plan/ViewAbstract.aspx?cKey=ee05ff23-5954-4af9-b857-2fc0bfa086b2&mID=3236&mKey=8d2a5bec-4825-4cd6-9439-b42bb151d1cf&sKey=cf68d068-d586-418e-ab49-1c71424dd808.
- 58.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76(1):142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 59.Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: A dose-response study. Psychoneuroendocrinology. 2013;38(3):399–407. doi: 10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 60.van Ijzendoorn MH, Bhandari R, van der Veen R, Grewen KM, Bakermans-Kranenburg MJ. Elevated salivary levels of oxytocin persist more than 7 h after intranasal administration. Front Neurosci. 2012;6(174):174. doi: 10.3389/fnins.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 62.Insel TR. Oxytocin—a neuropeptide for affiliation: Evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17(1):3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- 63.Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51(1):18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- 64.Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rault JL, et al. Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol Behav. 2013;112-113:40–48. doi: 10.1016/j.physbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7(2):126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 67.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 68.Domes G, et al. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 69.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 70.Ferrari PF, Bonini L, Fogassi L. From monkey mirror neurons to primate behaviours: Possible “direct” and “indirect” pathways. Philos Trans R Soc Lond B Biol Sci. 2009;364(1528):2311–2323. doi: 10.1098/rstb.2009.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrari PF, et al. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. J Cogn Neurosci. 2012;24(5):1165–1172. doi: 10.1162/jocn_a_00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J Neurosci. 2011;31(40):14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perry A, et al. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35(10):1446–1453. doi: 10.1016/j.psyneuen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Huang H, et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39(5):1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bales KL, et al. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74(3):180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crockford C, Deschner T, Ziegler TE, Wittig RM. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: A review. Front Behav Neurosci. 2014;8:68. doi: 10.3389/fnbeh.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.