Significance
A pool of latently infected resting CD4+ T cells in patients on antiretroviral therapy is a major barrier to a cure for HIV-1. Clinical trials are underway to assess whether small molecules can “kick” HIV-1 out of latency, but the potency of such latency reversing agents has not been evaluated at the level of single proviruses. Using a unique quantitative system, we found that the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid), which is being investigated clinically, did not induce unspliced cellular viral RNA or lead to virion production from the large majority of proviruses in resting CD4+ T cells. Our results demonstrate that more potent interventions will likely be necessary to reduce the size of the latent reservoir.
Keywords: HIV-1 persistence, HIV-1 eradication, HIV-1 cure, fractional provirus expression
Abstract
Reversal of proviral latency is being pursued as a curative strategy for HIV-1 infection. Recent clinical studies of in vivo administration of the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA; vorinostat) show increases in unspliced cellular HIV-1 RNA levels in resting CD4+ T cells. A critical unknown, however, is the proportion of latent proviruses that can be transcriptionally reactivated by SAHA or T-cell activation. In this study, we quantified the fraction of HIV-1 proviruses in resting CD4+ T cells from patients on suppressive antiretroviral therapy that were reactivated ex vivo with SAHA or antibodies to CD3/CD28. At concentrations of SAHA achieved clinically, only 0.079% of proviruses in resting CD4+ T cells were reactivated to produce virions, compared with 1.5% of proviruses in cells treated with anti-CD3/CD28 antibodies after correcting for spontaneous virion production in the medium control. A significant positive correlation (ρ = 0.67, P < 0.001) was found between levels of virions in the supernatant and unspliced cellular HIV-1 RNA following anti-CD3/CD28 treatment, but not following SAHA treatment (ρ = 0.21, P = 0.99). These results reveal that the majority of HIV-1 proviruses are not reactivated by current therapeutic approaches and that more effective means of reversing proviral latency will likely be required to deplete HIV-1 reservoirs.
Antiretroviral therapy (ART) for HIV-1 infection suppresses viral replication but is not curative. Assays of infectious virus recovery from quiescent CD4+ T cells isolated from patients on ART have revealed the existence of a reservoir of latent, replication competent HIV-1 with a half-life of 44 mo (1–4). In addition, low-level plasma viremia persists indefinitely on ART (5, 6), and the level of virus in plasma rebounds following cessation of ART (7, 8). New therapeutic approaches are required to eliminate both persistent low-level viremia and the latent proviral reservoir. A “kick and kill” approach has been proposed in which latency reversing agents, administered in conjunction with ART, will “kick” proviruses out of latency, followed by a “kill” of the infected cells through viral cytopathic effects or immune-mediated cytotoxicity.
Histone deacetylase inhibitors (HDACi) have been proposed as latency reversing agents, and single-dose or multidose administration of suberoylanilide hydroxamic acid (SAHA; vorinostat) in vivo was shown to increase expression of unspliced cellular HIV-1 RNA in resting CD4+ T (rCD4) cells in patients on suppressive ART (9, 10). Although three- to fivefold increases in cellular HIV-1 RNA were observed (9), the fraction of latent HIV-1 proviruses that were reactivated by SAHA was not quantified. It is possible that SAHA transcriptionally reactivated many latent proviruses, or alternatively reactivated only a minority of latent proviruses. These two alternatives have very different implications in terms of the impact SAHA could have on the latent reservoir.
Results
To evaluate the magnitude of latency reversal, we isolated rCD4 cells by negative selection from 180-mL blood draws from 13 patients on suppressive ART for an average of 8 y, all with plasma HIV-1 RNA <20 copies per mL by a Food and Drug Administration-approved assay (Roche Taqman 2.0; Tables 1 and 2). Of the 13 patients studied, 11 had persistent low-level viremia (mean 2.6 copies per mL) detectable by reverse transcriptase quantitative PCR (RT-qPCR) with single copy sensitivity (Table 2) (11). We achieved >95% purity of rCD4 cells from all patients, with <0.1% of isolated rCD4 cells expressing the activation markers CD69, CD25, and HLA-DR. The mean total HIV-1 DNA level in the purified rCD4 cells was 1,440 copies per 106 cells as measured by qPCR (Table 2) (11). In a subset of the patients, flow cytometry was used to evaluate the memory phenotypes of the isolated rCD4 cells by the surface expression of CD3, CD4, CD45RA, CCR7, and CD27 (Tables S1 and S2) as described (12). This analysis revealed that on average, 57% of the rCD4 cells isolated were naïve T cells, 25% were central memory T cells, and the remaining cells consisted of relatively minor populations of transitional and effector memory cells and terminally differentiated T cells (Fig. S1).
Table 1.
Characteristics of study subjects
| Patient ID no. | Sex | Age | Current CD4+ count, cells/mm3 | Current antiretroviral therapy | Years plasma HIV-1 RNA <50 copies per mL |
| 1 | Female | 56 | 846 | EFV, FTC, TDF | 12 |
| 2 | Male | 57 | 563 | AZT, ETV, FTC, RAL, TDF | 4 |
| 3 | Female | 45 | 477 | DRV/r, FTC, TDF | 2 |
| 4 | Female | 59 | 825 | EFV, FTC, TDF | 7 |
| 5 | Female | 55 | 1,373 | EFV, FTC, TDF | 12 |
| 6 | Male | 57 | 774 | EFV, FTC, TDF | 3 |
| 7 | Male | 53 | 1,111 | 3TC, ABC, AZT, NVP | 10 |
| 8 | Male | 50 | 380 | EFV, FTC, TDF | 11 |
| 9 | Male | 65 | 748 | EFV, FTC, TDF | 4 |
| 10 | Male | 57 | 934 | EFV, FTC, TDF | 16 |
| 11 | Male | 51 | 534 | 3TC, ABC, RAL | 16 |
| 12 | Male | 43 | 367 | 3TC, ABC, RAL | 3 |
| 13 | Female | 51 | 1,131 | FTC, RAL, TDF | 2 |
| Mean ± SD | — | 54 ± 6 | 774 ± 309 | — | 8 ± 5 |
3TC, lamivudine; ABC, abacavir; AZT, zidovudine; DRV/r, ritonavir boosted darunavir; EFV, efavirenz; ETV, etravirine; FTC, emtricitabine; NVP, nevirapine; RAL, raltegravir; TDF, tenofovir disoproxil fumarate.
Table 2.
Quantification of residual plasma viremia and total HIV-1 DNA in the rCD4 cell population
| Patient ID no. | Plasma HIV-1 RNA by TaqMan, copies per mL | Residual Plasma HIV-1 RNA by SCA, copies per mL | Total HIV-1 DNA in rCD4 cells, copies per 106 cells |
| 1 | TND | <0.25 | 136 |
| 2 | <20 | 1.5 | 1,458 |
| 3 | <20 | <0.25 | 1,110 |
| 4 | TND | 1.8 | 1,551 |
| 5 | TND | 7.2 | 2,678 |
| 6 | <20 | 6.0 | 887 |
| 7 | <20 | 2.2 | 350 |
| 8 | TND | 1.3 | 978 |
| 9 | TND | 2.8 | 1,957 |
| 10 | TND | 1.8 | 2,849 |
| 11 | <20 | 2.1 | 2,132 |
| 12 | TND | 3.1 | 1,503 |
| 13 | <20 | 3.3 | 1,128 |
| Mean ± SD | — | 2.6 ± 2.0 | 1,440 ± 811 |
SCA, single copy assay; TND, target not detected by Roche COBAS TaqMan HIV-1 test v2.0.
To quantify the fraction of proviruses that can be reactivated to produce virions or unspliced cellular HIV-1 RNA, rCD4 cells were plated in serial threefold dilutions (667,000–304 cells per well) and cultured with efavirenz (300 nM) in the medium to prevent the spread of virus to other cells. The number of proviruses seeded in each well was estimated from the number of HIV-1 DNA copies per million rCD4 cells determined by qPCR, assuming negligible unintegrated HIV-1 DNA in patients after long-term suppressive ART (13, 14). The number of wells positive for virion-associated HIV-1 RNA in the supernatant at each cell dilution after 7 d of culture was determined by automated RT-qPCR that detects HIV-1 long terminal repeat (LTR) and gag sequences with a quantification limit of 20 copies per sample (Roche TaqMan v 2.0). Control experiments showed that >95% of HIV-1 nucleic acid in supernatants from cells treated with anti-CD3/CD28 antibodies could be pelleted by centrifugation at 24,000 × g for 1 h and was resistant to DNase I treatment. A maximum likelihood estimate (MLE) (15) was then applied to determine the fraction of proviruses that were reactivated to produce virions or cellular unspliced HIV-1 RNA (Fig. 1).
Fig. 1.
Illustration and explanation of the experimental system used to quantify fPVE. (A) Purified rCD4 cells are serially diluted and HIV-1 DNA copy number (per million rCD4 cells) is determined by qPCR to estimate the number of proviruses seeded per well. After treatment with a reactivating agent (or medium control), wells positive for HIV-1 virions in the supernatant are identified by RT-qPCR (Roche Taqman v2.0). The same experimental setup can be used to determine the fraction of proviruses that have been reactivated to produce cellular unspliced HIV-1 RNA by extracting total nucleic acid from cultured cells and quantifying HIV-1 RNA levels per well by RT-qPCR. (B) The number of positive wells from the hypothetical experiment in A was then tabulated, and a generalized linear model with a binomial distribution and a log link function was applied to determine the maximum likelihood estimate of fPVE. In the hypothetical example above, 1.5% of proviruses in rCD4 cells were reactivated to produce virions.
The MLE incorporates data from the full dynamic range of the dilution culture assay to calculate the population parameter of interest (i.e., fractional provirus expression; fPVE). This calculation is mathematically accomplished by calculating the value of fPVE that maximizes the likelihood of producing the experimentally observed results. Essentially, the MLE uses the data from each replicate well over all of the dilutions to determine the fraction of proviruses that were most likely reactivated to produce the experimentally observed dataset. The parametric MLE based on a binomial distribution is statistically superior to likelihood methods that determine the cell dilution at which 50% of the wells are expected to be positive (ED50), such as the Spearman–Karber method (15).
To assess maximal fPVE achieved with T-cell activation, we reversed latency ex vivo in rCD4 cells by signaling through the T-cell receptor associated CD3 and the costimulatory receptor CD28 with anti-CD3/CD28 antibodies. To determine the fraction of proviruses that could be reactivated with SAHA, we treated cells for 7 d with 0.5 μM SAHA, which is similar to the unbound serum concentration of SAHA achieved with FDA-approved dosing (∼0.4 μM; Zolinza Package Insert, Merck and Co.). Treatment of purified rCD4 cells ex vivo for 7 d of culture with anti-CD3/CD28 led to a surface phenotype of CD25+/HLA-DR+ in >94% of cells, indicating cellular activation, whereas treatment with 0.5 μM SAHA did not up-regulate surface activation markers. Total HIV-1 DNA levels increased 27-fold on average after 7 d of treatment with anti-CD3/CD28 antibodies, consistent with proliferation of infected cells. Total HIV-1 DNA levels were approximately twofold higher on average following treatment with SAHA. Viability was assessed after 7 d of culture, and SAHA-treated wells were found to have comparable viability (>90%) compared with medium control, whereas anti-CD3/CD28 treated wells were found to have 3.4-fold higher viability because of increased cell number from proliferation associated with T-cell receptor activation.
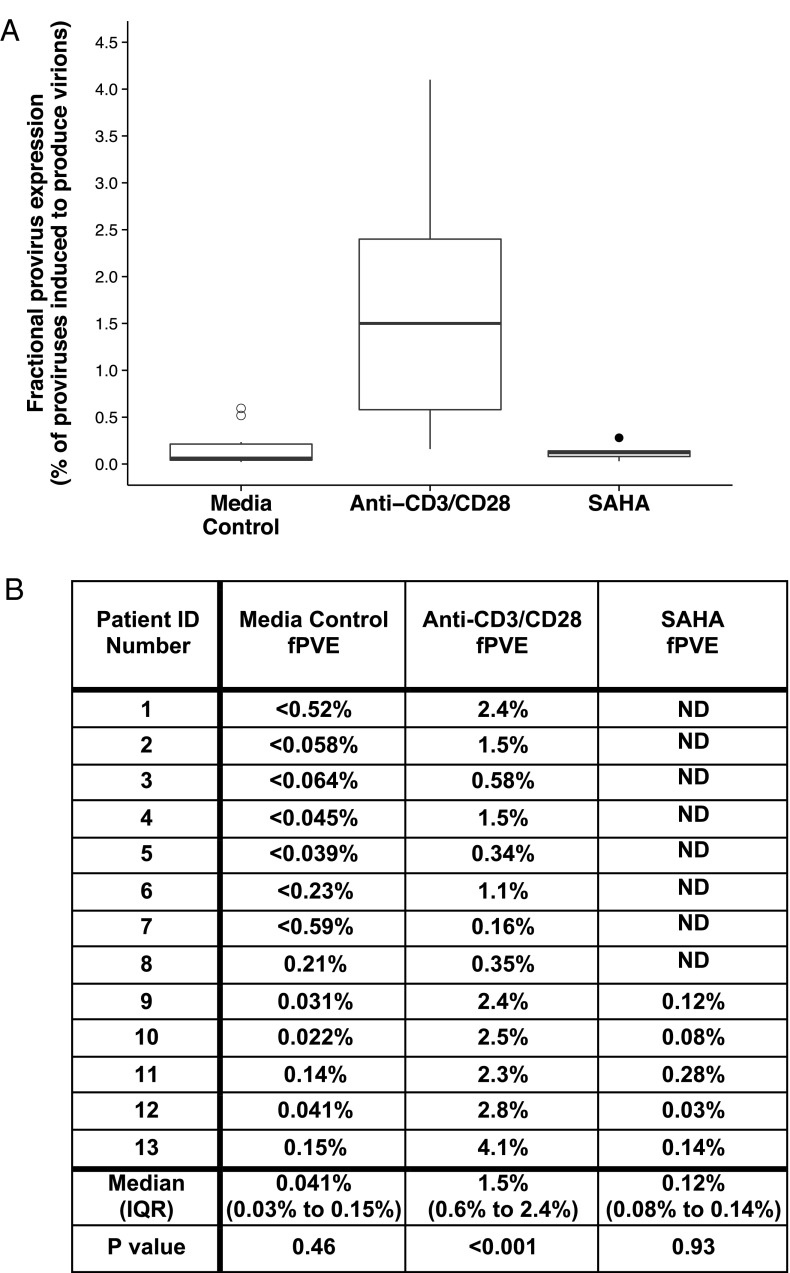
Using the MLE, a mean of 1.5% of proviruses in rCD4 cells produced detectable levels of virion-associated HIV-1 RNA after 7 d of treatment with anti-CD3/CD28 antibodies (Fig. 2). By comparison, a mean of 0.12% of proviruses produced detectable virion-associated HIV-1 RNA after 7 d of treatment with SAHA (Fig. 2). The average ratio of fPVEs (SAHA:anti-CD3/CD28 antibodies) was 0.050, revealing that SAHA reactivated only a very small fraction of maximal proviral expression achieved with anti-CD3/CD28 antibodies. Importantly, fPVE for virion production was also assessed from replicates of untreated wells at each dilution. This analysis of the medium control wells showed that on average, 0.041% of proviruses produced virions in the absence of any stimulation. Notably, the fPVE from untreated rCD4 cells was greater than the fPVE from SAHA-treated cells in 2 of 5 patients in whom SAHA was evaluated. After correcting for fPVE from medium control wells (i.e., wells without treatment), the mean fPVE for SAHA was 0.079% and, for anti-CD3/CD28 antibodies, remained 1.5%.
Fig. 2.
Only a small fraction of HIV-1 proviruses can be reactivated to produce virions during 7 d of culture with SAHA (0.5 μM) or anti-CD3/CD28 antibodies. The ratio of SAHA fPVE to anti-CD3/CD28 fPVE was 0.050 on average. Using a linear mixed effects model (34), anti-CD3/CD28 treatment significantly increased fPVE (P < 0.001), whereas neither medium control nor SAHA had a significant impact on fPVE. To determine mean fPVE values, the mean spontaneous fPVE (i.e., fPVE from medium control wells) was subtracted from the mean SAHA or anti-CD3/CD28 treated fPVE revealing that an average of 0.079% of proviruses can be reactivated to produce virions following SAHA treatment, and 1.5% of proviruses can be reactivated following anti-CD3/CD28 treatment. Open symbols represent samples below the limit of detection of fPVE for virion production. Symbols shown are values that fall outside 1.5 times the interquartile range of the boxplots.
Next, we evaluated the fraction of proviruses that were reactivated to produce unspliced cellular HIV-1 RNA pol sequences following treatment with anti-CD3/CD28 or SAHA in a subset of donors. HIV-1 RNA was isolated from serially diluted cells following treatment with anti-CD3/CD28 or SAHA, and wells were scored as positive or negative for cell-associated HIV-1 RNA as determined by RT-qPCR with single copy sensitivity. By the MLE method, we found that 6.8% and 8.2% of proviruses from donors 4 and 5, respectively, were reactivated by anti-CD3/CD28 antibodies to produce unspliced cellular HIV-1 RNA (Table 3). These fPVE values for unspliced cellular HIV-1 RNA are 2.4-fold and 2.0-fold higher, respectively, than the fraction of proviruses that were activated to produce virions by cells from the same two donors. Following treatment with SAHA, 0.09% and 0.19% of proviruses were reactivated to produce unspliced cellular HIV-1 RNA in cells from the same two donors, which is 3.1-fold and 1.4-fold higher, respectively, than the fraction of proviruses activated to produce virions with SAHA. Medium control wells showed that the fraction of proviruses expressing unspliced HIV-1 RNA following SAHA treatment was not greater than medium control in one of the two patients (Table 3). Sufficient numbers of cells were not available to assess fPVE for cellular HIV-1 RNA from the other patients included in this study.
Table 3.
Quantification of the fraction of proviruses that can be induced to produce unspliced cellular HIV-1 RNA
| Patient ID no. | Medium control fPVE, % | Anti-CD3/CD28 fPVE, % | SAHA fPVE, % | SAHA fPVE:Anti-CD3/CD28 fPVE |
| 4 | 0.37 | 6.8 | 0.093 | 0.014 |
| 5 | 0.054 | 8.2 | 0.19 | 0.023 |
| Mean ± SD | 0.21 ± 0.22 | 7.5 ± 0.96 | 0.14 ± 0.066 | 0.018 ± 0.0067 |
Previous work has shown that the production of cellular HIV-1 RNA from rCD4 cells in patients on suppressive ART does not imply the production of virions in the absence of antigenic stimuli (16). We therefore evaluated the relationship between unspliced cellular HIV-1 RNA and virion production following anti-CD3/CD28 or SAHA treatment in cells from 5 donors by quantifying virion-associated HIV-1 RNA and unspliced HIV-1 RNA from the same wells (Fig. 3). With anti-CD3/CD28 treatment, a highly significant positive correlation was evident between the levels of unspliced cellular HIV-1 RNA and virion production (ρ = 0.67, P < 0.001). By contrast, there was no significant correlation between unspliced cellular HIV-1 RNA and virion production in SAHA-treated cells (ρ = 0.21, P = 0.99). This finding may help to explain the lack of a consistent increase in low-level plasma viremia despite three- to fivefold increased in cellular HIV-1 RNA levels following in vivo SAHA administration (9).
Fig. 3.
Correlations between unspliced cellular HIV-1 RNA and virion production following treatment with anti-CD3/CD28 antibodies or SAHA. Virion-associated HIV-1 RNA levels were quantified from the supernatants of treated wells, and unspliced HIV-1 RNA was quantified from the cells in the same wells. Each data point is representative of one pair of virion and cellular RNA levels from one well. Several wells were selected at each dilution. (A) Treatment with anti-CD3/CD28 resulted in a significant positive correlation between unspliced cellular HIV-1 RNA and virion production in the same wells over 7 d of culture [ρ = 0.67, Spearman rank correlation based on resampling methods (30); P < 0.001, repeated measures analysis using generalized estimating equations]. (B) Treatment with SAHA led to unspliced cellular HIV-1 RNA production, but this increase in HIV-1 transcription did not correlate with virion production in the supernatant over 7 d [ρ = 0.21, Spearman rank correlation based on resampling methods (30); P = 0.99, repeated measures analysis using generalized estimating equations]. Negative values for virion-associated HIV-1 RNA and unspliced cellular HIV-1 RNA were set at 50% of the limit of quantification (10 copies and 1.5 copies, respectively, for each assay). Samples that were undetectable by either or both measures are shown as open symbols.
Discussion
Using a unique system to quantify fractional provirus expression, we found that only 1.5% of proviruses could be reactivated to produce virions following T-cell activation and that SAHA reactivated only 0.079% of proviruses, which was not significantly higher than medium control. By measuring the fraction of proviruses that were reactivated to produce cellular HIV-1 RNA, we found SAHA treatment led to the marginal activation of cellular HIV-1 RNA transcription compared with anti-CD3/CD28 treatment. Finally, we found that there was a strong correlation between levels of virion production and unspliced cellular HIV-1 RNA following T-cell activation, but not following SAHA treatment.
Recent work by Ho et al. (17) sought to evaluate whether a single round of T-cell activation reactivates all intact proviruses. Their work revealed that 12% of noninduced proviruses were functionally intact, with the remaining 88% having lethal mutations and/or deletions. The noninduced, but intact, proviruses have the potential to be reactivated in vivo and, thus, represent a larger barrier to HIV-1 eradication than previously recognized. Our work extends these finding by providing direct quantification of all proviral reactivation and reveals that that the large majority of proviruses (98.5%) are not induced to produce virions with T-cell activation. Taken together, these two studies identify a large proviral reservoir that cannot be readily activated and that is a major barrier to achieving an HIV-1 cure through the kick and kill strategy.
The finding that SAHA reactivates far fewer proviruses than maximal T-cell activation differs from previously reported results of viral outgrowth assays using a p24 endpoint with higher input cell numbers (18). However, the findings in this study are in agreement with a study in which virion production after SAHA treatment was not higher than virion production in the medium control (19). Quantification of the levels of infectious virus reactivated by anti-CD3/CD28 or SAHA was not performed in this study, but the finding that SAHA reactivates only a small proportion of the proviruses compared with anti-CD3/CD28 suggests that SAHA treatment is unlikely to eliminate much of the replication competent proviral reservoir.
To fully reverse latency, several key transcriptional blocks present in rCD4 cells likely need to be overcome. In vivo HIV-1 latency is thought to be predominantly established by the return of a productively infected activated CD4+ cell to a resting memory state (20). Alternatively, preactivation latency has also been described, where HIV-1 DNA can be integrated into quiescent rCD4 cells via interactions of chemokines with CCR7, CXCR3, and CCR6 in the absence of productive infection (21). Independently of whether productive infection has occurred, the G0 resting state in rCD4 cells contributes to the inhibition of HIV-1 transcription and translation through a variety of mechanisms, including the sequestration of transcription factors NF-κB and NFAT (22, 23); association of P-TEFb with the inhibitory 7SK snRNA complex (24, 25); and association of the BAF complex and histone deacetylases with the HIV long terminal repeat (26, 27). In addition to transcriptional blocks, posttranscriptional nuclear sequestration of the spliced tat and rev HIV-1 mRNAs in latently infected rCD4 cells prevent productive infection (28). Furthermore, miRNAs that are enriched in rCD4 cells compared with activated CD4 cells inhibit HIV-1 protein translation by targeting HIV-1 mRNA (29). The situation is very complex, and a variety of these factors are likely to be operating in different latently infected cells in the same patient. HDACi antagonize one of many factors that promote proviral latency; additional latency mechanisms likely explain why a small fraction of proviruses can be activated by SAHA to produce virions compared with T-cell activation.
The ability of SAHA to increase levels of HIV-1 RNA transcription in rCD4 cells in vivo (9) was an important first step toward latency reversal. Our ex vivo study shows that a small but quantifiable fraction of proviruses can be reactivated with SAHA in a subset of patients. However, the lack of correlation between cellular levels of unspliced HIV-1 RNA and virion production following SAHA treatment suggests that SAHA does not reverse all latency mechanisms in most latently infected rCD4 cells.
In summary, we have described a quantitative system with which putative latency reversing agents can be evaluated for their ability to reactivate proviruses among the total population of latent proviruses. Quantifying the fraction of proviruses that can be induced will be essential for evaluating the potency of the proximal kick step in the kick and kill approach to reducing latent HIV-1 reservoirs. Identifying strategies to increase fractional proviral expression without inducing global T-cell activation is critical to achieving progress toward a cure of HIV-1 infection.
Materials and Methods
Isolation of rCD4 Cells from Patients on ART.
Large volume (∼180-mL) phlebotomy was obtained from patients on suppressive ART for a minimum of 2 y (Table 1). All patients gave written informed consent, and the blood donation protocol was approved by the University of Pittsburgh Institutional Review Board. Peripheral blood mononuclear cells (PBMC) were isolated by ficoll-hypaque density gradient centrifugation. Next, total CD4+ T cells were isolated from PBMC by negative selection by using the CD4+ T Cell Isolation Kit II from Miltenyi Biotec. rCD4 cells were isolated from total CD4+ T cells by negative selection using anti-CD69, anti-CD25. and anti–HLA-DR antibodies from Miltenyi Biotec to remove activated cells. Isolated rCD4 cells were cryopreserved and stored for downstream evaluation of purity by flow cytometry.
Flow Cytometric Analysis.
Cells were analyzed for surface expression of T-cell and activation markers by using CD3-V450, CD4-APC-H7, CD69-APC, CD25-PE, and HLA-DR-PerCP-Cy5.5 to evaluate the purity of isolated rCD4 cells. To assess the T-cell memory phenotypes of the rCD4 cells, cells were analyzed for surface expression of CD3-V450, CD4-APC-H7, CD45RA-APC, CCR7-FITC, and CD27-PE as described (12). All antibodies were obtained from BD Biosciences. All cells were fixed before analysis by using BD Cytofix buffer, and an LSRII cytometer with FACSDiva software (BD Biosciences) was used to acquire and analyze the results.
Endpoint Dilution Cultures To Quantify Fractional Proviral Expression.
Purified rCD4 cells were serially diluted threefold from 667,000 cells per well to 304 cells per well and seeded into individual wells of 48-well plates (12 replicates at each dilution). Ten replicates at each dilution were treated with either anti-CD3/CD28 antibody-coated microbeads or 0.5 μM SAHA, and two replicates per dilution were not treated. Cells were cultured for 7 d in RPMI medium 1640 without phenol red containing 10% (vol/vol) fetal bovine serum, 0.6% penicillin/streptomycin, and 300 nM efavirenz to prevent subsequent rounds of HIV-1 replication. Viability was assessed after 7 d of culture by using the CellTiter-Fluor Cell Viability Assay as described by the manufacturer.
Quantification of Virion HIV-1 RNA in Culture Supernatant.
Culture supernatants were collected after 7 d, diluted twofold, and HIV-1 RNA was measured by RT-qPCR (Roche Taqman 2.0) with a quantification limit of 20 copies per sample. Control experiments showed that >95% of HIV-1 RNA in supernatants could be pelleted by centrifugation at 24,000 × g for 1 h and were resistant to DNase I treatment.
Quantification of Residual Plasma Viremia.
Residual plasma HIV-1 RNA was quantified as reported with a two-step RT-qPCR assay targeting the integrase region of pol (11). The limit of detection for HIV-1 RNA was one copy per reaction.
Quantification of Total HIV-1 DNA.
Total HIV-1 DNA levels in purified rCD4 cells on day 0 and 7 of culture were quantified as reported (11). Preliminary experiments confirmed previous findings that 2-LTR episomal HIV-1 DNA contributed negligibly to total HIV-1 DNA from patients on long-term suppressive ART (13, 14).
Quantification of Unspliced Cellular HIV-1 RNA.
The isolation of cellular nucleic acid was based on reported methods (31). Total nucleic acids were isolated by first adding 100 μL of proteinase K and guanidinium hydrochloride (3 M guanidinium hydrochloride, 50 mM Tris⋅HCl at pH 7.6, 1 mM calcium chloride, and 1 mg/mL proteinase K), sonicating the sample for 10 s, and incubating at 42 °C for 1 h. Following the incubation, 400 μL of guanidinium thiocyanate and glycogen (6 M guanidinium thiocyanate, 50 mM Tris⋅HCl at pH 7.6, 1 mM EDTA, and 600 μg/mL glycogen) were added followed by incubation at 42 °C for 10 min. Next, 500 μL of 100% isopropanol was added, and the sample was centrifuged at 21,000 × g for 10 min at room temperature. The supernatant was then decanted, and the pellet was washed with 750 μL of 70% ethanol. The sample was then spun again at 21,000 × g for 10 min, the supernatant was removed, and the pellet was dried for 10 min. After drying, the pellet was resuspended in 36 μL of Roche DNase incubation buffer, sonicated again, and split into two aliquots. One aliquot was frozen for quantification of HIV-1 DNA, and the other was treated for 20 min with 20 units of Roche recombinant RNase-free DNase I. After 20 min, RNA was precipitated by adding 200 μL of guanidinium thiocyanate described above without glycogen and 250 μL of 100% isopropanol, washed with 70% ethanol, and resuspended in 70 μL of 5 mM Tris at pH 8.0, 1 μM DTT, and 1,000 units/mL of recombinant RNasin. Cell-associated HIV-1 RNA was then quantified by using a modification of the two-step RT-qPCR assay for residual viremia (11). The cDNA synthesis step was modified by using 0.75 μg of random hexamers and 100 units of reverse transcriptase per reaction, and incubating for 30 min at 90 °C following cDNA synthesis. Real-time PCR was then performed by using the described primers and probe targeting the integrase region of pol (11). This described primer/probe pair also amplifies vif mRNA, which constitutes 0.21% of the 4.0-kb family of spliced mRNAs in T cells (32), and, thus, does not contribute significantly to quantification of unspliced HIV-1 RNA. The cellular gene IPO8 was quantified as an internal standard for cellular RNA recovery by using forward primer 5′-GCTCTGATAACTGTGCAG-3′, reverse primer 5′-CAGTGTGTACACCTCCTG-3′, and probe 5′-[6FAM]TGCTGTCCTCTGATCCTCGC[TAMRA]-3′. IPO8 is stably expressed in both rCD4 and activated T cells (33). This assay has the ability to detect one cell producing HIV-1 RNA in a background of 2 million HIV-1 negative cells and has a limit of quantification of three copies.
Statistical Analysis.
A maximum likelihood estimate (MLE) was used to determine the fraction of proviruses that were reactivated to produce virions or unspliced cellular HIV-1 RNA compared with all proviruses detected by qPCR. A generalized linear model with a binomial distribution and a log link function was used to calculate MLEs in R (34). A linear mixed effects model using the nlme package (35) in R was used to evaluate differences in fPVE for virion production between treatment groups. Spearman rank correlation based on resampling methods was used to determine correlation coefficients as described (30), and P values for correlations were determined by using repeated measures analysis with generalized estimating equations. Samples below the limit of detection were imputed as half the limit of detection for statistical analysis. P values of <0.05 were considered statistically significant. Figures were generated by using the ggplot2 package (36) in R.
Supplementary Material
Acknowledgments
We thank the volunteers for participating in this study. Funding was provided by the Pitt AIDS Research Training Program 5 T32 AI065380-08 and Science Applications and International Corporation (SAIC) Contract 25XS119 through the National Cancer Institute. J.M.C. was a research professor of the American Cancer Society with support from the F. M. Kirby Foundation.
Footnotes
Conflict of interest statement: J.W.M. is a consultant for Gilead Sciences and RFS Pharma and owns shares of RFS Pharma.
See Commentary on page 6860.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402873111/-/DCSupplemental.
References
- 1.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 2.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 3.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 4.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 5.Maldarelli F, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3(4):e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer S, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davey RT, Jr, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96(26):15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamlyn E, et al. INSIGHT SMART and SPARTAC Investigators Plasma HIV viral rebound following protocol-indicated cessation of ART commenced in primary and chronic HIV infection. PLoS ONE. 2012;7(8):e43754. doi: 10.1371/journal.pone.0043754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Archin NM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elliott J, et al. (2013) Paper presented at the 20th annual Conference on Retroviruses and Opportunistic Infections, Altanta, GA, 4 March.
- 11.Cillo AR, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Defic Syndr. 2013;63(4):438–441. doi: 10.1097/QAI.0b013e31828e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besson GJ, McMahon D, Maldarelli F, Mellors JW. Short-course raltegravir intensification does not increase 2 long terminal repeat episomal HIV-1 DNA in patients on effective antiretroviral therapy. Clin Infect Dis. 2012;54(3):451–453. doi: 10.1093/cid/cir721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi RT, et al. AIDS Clinical Trials Group (ACTG) A5244 Team No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59(3):229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers LE, McQuay LJ, Hollinger FB. Dilution assay statistics. J Clin Microbiol. 1994;32(3):732–739. doi: 10.1128/jcm.32.3.732-739.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun TW, et al. Gene expression and viral prodution in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc Natl Acad Sci USA. 2003;100(4):1908–1913. doi: 10.1073/pnas.0437640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archin NM, et al. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25(2):207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blazkova J, et al. Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2012;206(5):765–769. doi: 10.1093/infdis/jis412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siliciano RF, Greene WC. HIV latency. Cold Spring Harb Perspect Med. 2011;1(1):a007096. doi: 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron PU, et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci USA. 2010;107(39):16934–16939. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams SA, et al. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25(1):139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113(1):58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, et al. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997;11(20):2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Contreras X, Barboric M, Lenasi T, Peterlin BM. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog. 2007;3(10):1459–1469. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdin E, Paras P, Jr, Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12(8):3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rafati H, et al. Repressive LTR nucleosome positioning by the BAF complex is required for HIV latency. PLoS Biol. 2011;9(11):e1001206. doi: 10.1371/journal.pbio.1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lassen KG, Ramyar KX, Bailey JR, Zhou Y, Siliciano RF. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog. 2006;2(7):e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13(10):1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 30.Follmann D, Proschan M, Leifer E. Multiple outputation: Inference for complex clustered data by averaging analyses from independent data. Biometrics. 2003;59(2):420–429. doi: 10.1111/1541-0420.00049. [DOI] [PubMed] [Google Scholar]
- 31.Venneti S, et al. Longitudinal in vivo positron emission tomography imaging of infected and activated brain macrophages in a macaque model of human immunodeficiency virus encephalitis correlates with central and peripheral markers of encephalitis and areas of synaptic degeneration. Am J Pathol. 2008;172(6):1603–1616. doi: 10.2353/ajpath.2008.070967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ocwieja KE, et al. Dynamic regulation of HIV-1 mRNA populations analyzed by single-molecule enrichment and long-read sequencing. Nucleic Acids Res. 2012;40(20):10345–10355. doi: 10.1093/nar/gks753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledderose C, Heyn J, Limbeck E, Kreth S. Selection of reliable reference genes for quantitative real-time PCR in human T cells and neutrophils. BMC Res Notes. 2011;4:427. doi: 10.1186/1756-0500-4-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. R Core Team (2013) in R, A Language and Environment for Statistical Computing, Available at www.R-project.org.
- 35. Pinheiro J, Bates D, DebRoy S, Sarkar D, and the R Development Core Team. (2013) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-113.
- 36. Wickham H (2009) in ggplot2: Elegant Graphics for Data Analysis (Springer, New York), http://had.co.nz/ggplot2/book.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.