Significance
Transformation from immature to a fully reproductive adult form is an essential process during the development of higher organisms. In insects, transition from juvenile to adult stages is triggered by the decline of the juvenile hormone, but the molecular mechanisms underlying the dramatic morphological and physiological changes remain poorly understood. Here, we report that a single factor, E93, controls juvenile-to-adult transition in hemimetabolous and holometabolous insects, thus acting as the universal adult specifier in winged insects. Interestingly, we find that E93 not only promotes adult metamorphosis but also represses the expression of the antimetamorphic genes Krüppel-homolog 1 and Broad-Complex, ensuring the proper juvenile–adult transition. This study represents a significant step toward defining the molecular mechanisms underlying insect metamorphosis.
Keywords: insect metamorphosis, insect hormone, evolution of metamorphosis
Abstract
All immature animals undergo remarkable morphological and physiological changes to become mature adults. In winged insects, metamorphic changes either are limited to a few tissues (hemimetaboly) or involve a complete reorganization of most tissues and organs (holometaboly). Despite the differences, the genetic switch between immature and adult forms in both types of insects relies on the disappearance of the antimetamorphic juvenile hormone (JH) and the transcription factors Krüppel-homolog 1 (Kr-h1) and Broad-Complex (BR-C) during the last juvenile instar. Here, we show that the transcription factor E93 is the key determinant that promotes adult metamorphosis in both hemimetabolous and holometabolous insects, thus acting as the universal adult specifier. In the hemimetabolous insect Blattella germanica, BgE93 is highly expressed in metamorphic tissues, and RNA interference (RNAi)-mediated knockdown of BgE93 in the nymphal stage prevented the nymphal–adult transition, inducing endless reiteration of nymphal development, even in the absence of JH. We also find that BgE93 down-regulated BgKr-h1 and BgBR-C expression during the last nymphal instar of B. germanica, a key step necessary for proper adult differentiation. This essential role of E93 is conserved in holometabolous insects as TcE93 RNAi in Tribolium castaneum prevented pupal–adult transition and produced a supernumerary second pupa. In this beetle, TcE93 also represses expression of TcKr-h1 and TcBR-C during the pupal stage. Similar results were obtained in the more derived holometabolous insect Drosophila melanogaster, suggesting that winged insects use the same regulatory mechanism to promote adult metamorphosis. This study provides an important insight into the understanding of the molecular basis of adult metamorphosis.
The transformation from immature stages to a fully reproductive adult form is a fundamental hormonally dependent process during the development of higher organisms. Insects have been very useful model organisms to uncover the mechanisms underlying this complex transformation as all winged insects undergo metamorphosis to reach adulthood. Particularly interesting is that this transformation can be completed through two different metamorphic strategies, either directly (hemimetaboly) or through an intermediate metamorphic–pupal stage (holometaboly). Hemimetabolous insects, such as true bugs, cockroaches, or locusts, hatch from the egg resembling miniature adults, with external wing pads that encase the wing primordia, and metamorphose into adults with functional wings and genitalia during the last juvenile (also called nymphal) instar. In contrast, the larvae of holometabolous insects, like beetles, butterflies, or flies, undergo a complete morphological transformation to form the adult. The reorganization is so dramatic in these insects that a two-stage metamorphic process bridged by the intermediate pupal stage is required to fully transform the crawling larva into a winged adult (1).
Despite dramatic differences between hemimetaboly and holometaboly, both types of metamorphosis require a temporally regulated balance between cell death of nymphal/larval tissues and growth and morphogenesis of adult ones (2). Two hormones control this precise balance, the steroid 20-hydroxyecdysone (20E) and the sesquiterpenoid juvenile hormone (JH), produced by the prothoracic glands and corpora allata glands, respectively (3–5). Whereas periodic pulses of 20E promote every developmental transition, the presence or absence of JH determines the nature of each transition. Thus, JH acting through its receptor Methoprene-tolerant prevents adult differentiation during the preultimate immature stages by inducing the expression of the antimetamorphic transcription factor gene Krüppel-homolog 1 (Kr-h1) (6). Upon entering into the last juvenile stage, the last nymphal instar of hemimetabolous insects or the pupae of holometabolous insects, the disappearance of JH and the down-regulation of the Kr-h1 gene expression permit the metamorphic transformations resulting in the adult (7, 8). In holometabolous insects, also required is the repression of the pupal-specifier factor Broad-Complex (BR-C) during the pupal stage to allow a proper pupal–adult transition (6).
The role and mode of action of JH has been extensively studied since V. B. Wigglesworth discovered its antimetamorphic function in the 1930s (6, 9, 10). However, the molecular mechanism underlying the switch from juvenile to adult genetic programs in holometabolous and hemimetabolous insects is not completely understood. In the present study, we identified the transcription factor E93 as the key factor that promotes adult metamorphosis in Blattella germanica, as a model of hemimetaboly, and the holometabolous Tribolium castaneum and Drosophila melanogaster. We show that, despite the different developmental strategies between hemimetabolous and holometabolous insects, E93 is highly up-regulated during the metamorphic stages in both types of insects and that this stage-specific activation is required for adult metamorphosis to occur. On the basis of our findings, we propose that E93 functions as the universal adult specifier in insects.
Results and Discussion
E93 Is Up-Regulated in Metamorphic Tissues During the Nymphal–Adult Transition and Promotes Adult Differentiation in the Hemimetabolous B. germanica.
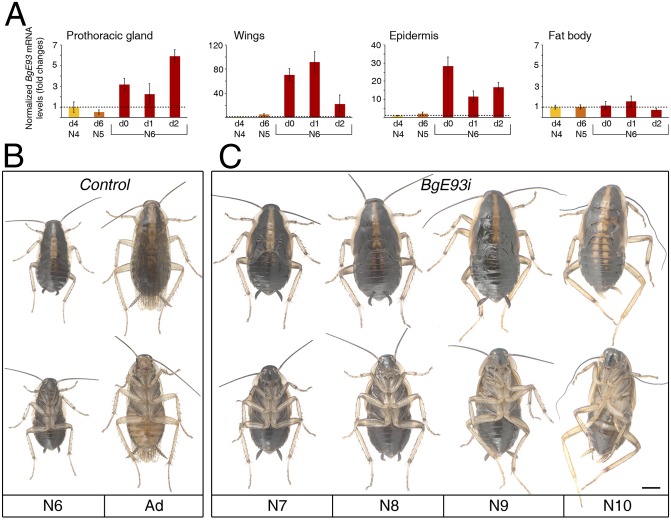
As a first step toward the characterization of the molecular mechanisms underlying adult differentiation, we studied the hemimetabolous insect B. germanica because hemimetabolous insects exhibit a simpler metamorphosis than holometabolous insects. This cockroach progresses through six nymphal instars (N1–N6) before molting into the adult. The metamorphic transition that occurs during the last nymphal instar (N6) is mainly characterized by changes in cuticle pigmentation and the appearance of genitalia and functional wings (Fig. S1 A–H). During this transition, the prothoracic gland, which is responsible for the synthesis of ecdysteroids, degenerates, and the corpora allata cells switch from the proliferative phase to the adult-specific nonproliferative state (11) (Fig. S1 I–N). Recently, we have shown that a complex 20E-triggered hierarchy of nuclear receptors controls these metamorphic transformations in B. germanica (11, 12). The same gene cascade, however, is also present during the previous nymphal–nymphal transitions (12), implying that stage-specific factors modify the responsiveness of target genes during the metamorphic transition. Because metamorphosis requires tissue death and remodeling, we speculated that both types of metamorphic changes could be regulated by the same factor(s) in a coordinated manner. We searched for such a determinant in B. germanica by looking for genes that control apoptosis in degenerating tissues (prothoracic gland) and that were also highly expressed in tissues undergoing morphogenetic transformations (wings and epidermis) during N6. Using this approach, we identified the BgE93 gene (Fig. S2), the ortholog of D. melanogaster helix-turn-helix transcription factor Eip93F (13, 14), as it was strongly up-regulated in all metamorphic tissues during the N6 instar whereas no significant changes were observed in nonmetamorphic tissues, such as the fat body (Fig. 1A and Fig. S3 A and B).
Fig. 1.
BgE93 is required for adult differentiation in the hemimetabolous insect B. germanica. (A) BgE93 expression, normalized to BgActin5C (qRT-PCR), in metamorphic (prothoracic gland, wings, and epidermis) and nonmetamorphic (fat body) tissues during the last three nymphal instars (N4, N5, and N6). Fold changes are relative to the expression of BgE93 in N4 nymphs, arbitrarily set to 1. Error bars represent SEM (n = 3). (B and C) Effect of RNAi of BgE93 on adult metamorphosis. Newly emerged N5 nymphs were injected with dsBgE93 (BgE93i) or with dsMock (Control) and left until the next molts. (B) Dorsal and ventral views of a N6 Control nymph and a winged adult (Ad). (C) Dorsal and ventral views of supernumerary BgE93i nymphs (from N7 to N10). (Scale bar: C, 2 mm.)
To examine the role of BgE93 in B. germanica metamorphosis, we used systemic RNA interference (RNAi) to knockdown BgE93 during the last N6 instar. Injection of BgE93 dsRNA into newly emerged penultimate N5 instar (BgE93i) caused a 95% reduction in the BgE93 transcript level (Fig. S3 C–F). All BgE93i animals (n = 150) molted to normal N6 nymphs but subsequently failed to metamorphose into adults and instead repeated the nymphal molt to a succeeding supernumerary N7 instar (Fig. 1 B and C). Instead of the normal adult phenotype with brown cuticle and fully developed and articulated wings, supernumerary N7 BgE93i animals had all of the external characteristics of a nymph: namely, black cuticle, two thick stripes of black melanin in the pronotum, nymphal cerci, and, most notably, external wing pads with no evidence of morphological transformation of the wing epithelial cells (Fig. S4 A–A″). Consistently, the prothoracic gland of BgE93i nymphs did not show any sign of degeneration, and the corpora allata cells did not stop dividing (Fig. S4 B–C″). Furthermore, the duration of the N6 that is 48 h longer than previous stages in untreated insects was shortened in BgE93i ones. Unable to molt into adults, all of the supernumerary BgE93i nymphs continuously molted to new supernumerary nymphal instars to finally reach giant N10, when they terminally arrested development due to problems in the shedding of the exuvia (Fig. 1C). Overall, our data therefore identify BgE93 as the critical stage-specific factor that promotes adult metamorphosis in the hemimetabolous B. germanica.
BgE93 Represses BgKr-h1 and BgBr-C During the Last Nymphal Instar of B. germanica.
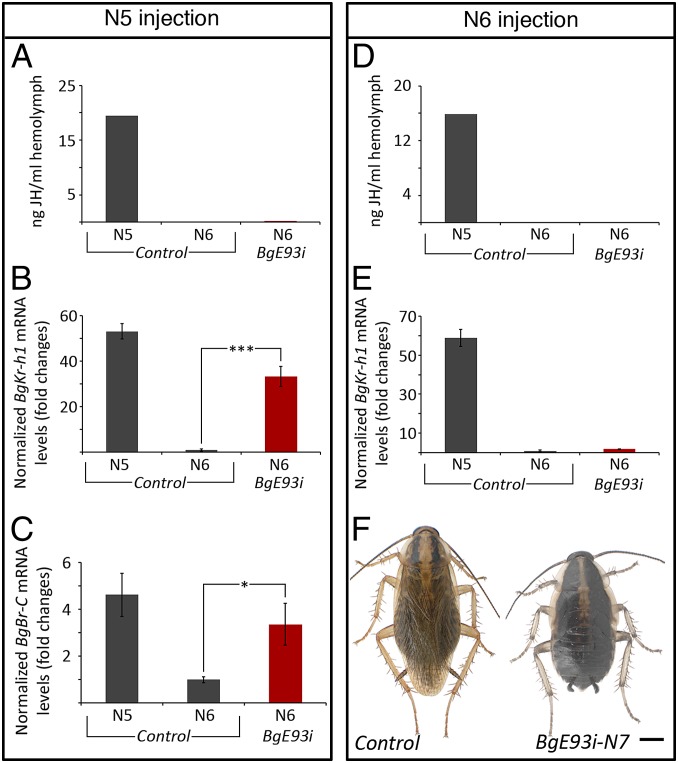
Adult differentiation requires the disappearance of JH and its target gene Kr-h1 during the last nymphal instar of hemimetabolous insects (15, 16). To examine whether the failure of BgE93i nymphs to molt into adults is the result of elevated levels of JH and the BgKr-h1 gene transcript during N6, we injected BgE93 dsRNA into early penultimate N5 instar and measured JH titer and BgKr-h1 expression in BgE93i nymphs during the following N6 stage. As shown in Fig. 2A, the disappearance of JH occurred normally in BgE93i nymphs, indicating that BgE93 is not involved in the decline of the JH titer. However, BgKr-h1 expression during N6 was not properly down-regulated in these animals (Fig. 2B), indicating that the decline of BgKr-h1 transcription in the final nymphal instar is controlled by BgE93 rather than from the disappearance of JH. The expression patterns of BgE93 and BgKr-h1 are consistent with the repressive role of BgE93 as the up-regulation of BgE93 at the onset of N6 precedes by 24 h the down-regulation of BgKr-h1 (Fig. S5). This finding prompted us to explore the possibility that the BgE93i phenotypes might stem from the sustained expression of BgKr-h1 in N6. If this hypothesis were correct, then depleting BgE93 in 1-d-old N6 nymphs, when JH is absent and BgKr-h1 expression has been already repressed by BgE93, would be sufficient to redirect the molt to adult differentiation. Consequently, we injected N6 nymphs with BgE93 dsRNA 24 h after ecdysis (N6-BgE93i) and confirmed that, under this RNAi treatment, JH, BgKr-h1, and BgE93 remained low during N6 in these animals (Fig. 2 D and E and Fig. S3G). Interestingly, all of the N6-BgE93i nymphs also failed to metamorphose into adults and instead molted into perfect new supernumerary N7 nymphal instar (Fig. 2F), indicating that the repetition of the nymphal molt in the absence of BgE93 was not due to the sustained BgKr-h1 gene expression. Taken together, these results clearly demonstrate that BgE93 is the crucial factor that controls the developmental switch from nymph to adult in the hemimetabolous B. germanica and that its metamorphosis-promoting effect is not exclusively channeled through the repression of BgKr-h1 during the last nymphal instar, as adult metamorphosis is completely blocked in BgE93i nymphs irrespective of the presence or absence of BgKr-h1. Furthermore, we also show that the absence of JH and BgKr-h1 during the final nymphal instar is not sufficient to cause the formation of the adult.
Fig. 2.
BgE93 represses BgKr-h1 and BgBr-C expression during the last nymphal instar of B. germanica. (A–C) Newly emerged penultimate N5 nymphs were injected with dsBgE93 (BgE93i) or with dsMock (Control) to ensure the reduction of BgE93 at the onset of N6. (A) JH levels, (B) BgKr-h1 expression, and (C) BgBr-C expression, relative to BgActin5C, measured by qRT-PCR in wings from 5-d-old N5 and 5-d-old N6 nymphs. Fold changes are relative to N6 control nymphs, arbitrarily set to 1. Error bars represent SEM (n = 5). Asterisks indicate differences statistically significant as follows: *P ≤ 0.05; ***P ≤ 0.001 (t test). (D–F) Twenty-four-hour-old N6 nymphs were injected with dsBgE93 to knockdown BgE93 expression after the natural decline of JH and BgKr-h1 or with dsMock as control. (D) JH levels and (E) BgKr-h1 expression relative to BgActin5C measured by qRT-PCR in wings from 3-d-old N5 and 3-d-old N6 nymphs. Fold changes are relative to N6 control nymphs, arbitrarily set to 1. Error bars represent SEM (n = 5). (F) Dorsal view of adult control and a supernumerary BgE93i N7 nymph upon injection of dsBgE93 in 1-d-old N6 nymphs. (Scale bar: F, 2 mm.)
Br-C is the second transcription factor that is involved in regulation of metamorphosis of hemimetabolous insects. RNAi experiments showed that B. germanica BgBr-C is required for progressive growth of wings to reach the correct size and form at the end of the nymphal development, a function that is conserved in the hemimetabolous species Oncopeltus fasciatus and Pyrrhocoris apterus (15, 17). BgBr-C mRNA is present in all nymphal stages, but its level declines in the final N6 nymphal instar paralleling BgKr-h1 mRNA levels (16, 18), just after BgE93 up-regulation (Fig. S5). Given this similarity, we therefore asked whether BgE93 also controls BgBr-C repression during the last nymphal instar, and, for that, we measured BgBr-C levels in BgE93i animals in N6. Notably, as Fig. 2C shows, BgE93 depletion prevented the down-regulation of BgBr-C, as was the case with BgKr-h1. So far, several studies show that Kr-h1 and Br-C are induced by JH in hemimetabolous insects, leading to the assumption that the natural fall of JH at the onset of the final nymphal instar is responsible for the down-regulation of Kr-h1 and Br-C (15, 17, 19). Importantly, our results show that this idea is not correct as BgKr-h1 and BgBr-C repression largely depends on BgE93 rather than on the normal decline of JH levels observed in the wild-type shift from N5 to N6.
The Antimetamorphic Effect of Methoprene Is Not Mediated by BgE93 Repression.
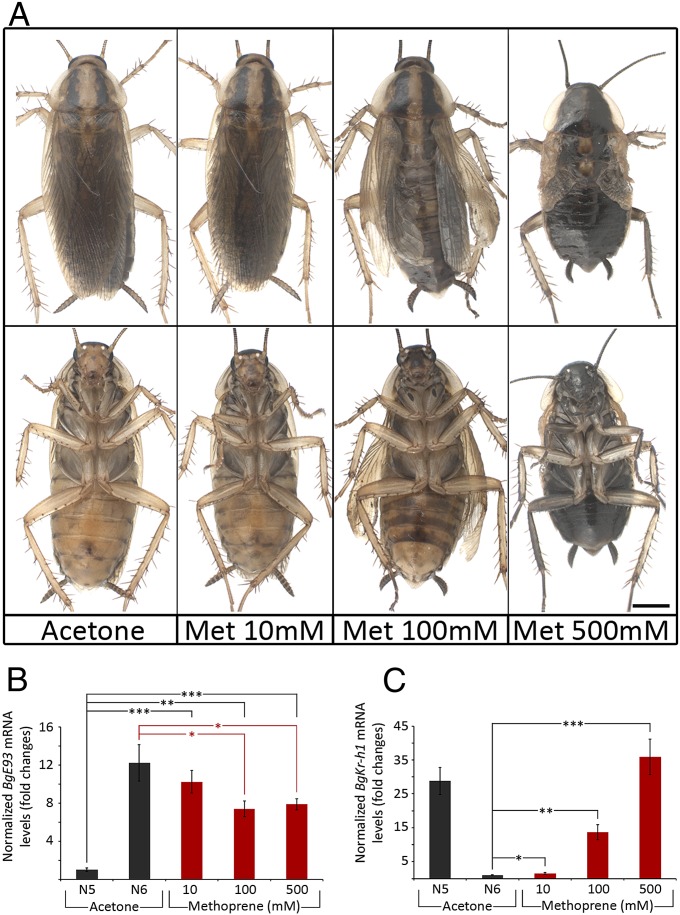
The up-regulation of BgE93 at the onset of the final nymphal instar of B. germanica coincides with the disappearance of JH. This inverse relationship, together with the antimetamorphic effect of ectopic JH during final nymphal instar in hemimetabolous insects (14, 15), suggests a possible effect of JH upon BgE93 expression. To check this hypothesis, we first tested whether the JH-mimic methoprene would prevent adult metamorphosis in B. germanica. As Fig. 3A shows, when mid-penultimate N5 instar nymphs were treated with 10 nmol methoprene, all animals developed into normal adults after two molts. When 100 nmol methoprene were applied, slight nymphal characteristics such as patches of dark coloration were visible on the ventral abdomen of otherwise normal adults. In contrast, after exposure to 500 nmol, adult differentiation was strongly prevented, and the animals molted into an almost perfect supernumerary N7 instar (Fig. 3A, Right). We next examined the expression of BgE93 in these animals during the N6 stage and found that, although a significant reduction in BgE93 mRNA levels was observed, BgE93 was still strongly up-regulated regardless of the concentration of methoprene used (Fig. 3B). Therefore, these data show that the critical stage-specific induction of BgE93 in N6 is controlled in a JH-independent manner although the disappearance of JH is also required for the full up-regulation of this gene.
Fig. 3.
The antimetamorphic effect of methoprene is not mediated by BgE93 repression. (A) Dorsal (Upper) and ventral (Lower) views of adult animals resulting from acetone- and methoprene-treated mid-N5 nymphs showing that the severity of the juvenilization correlated with the levels of BgKr-h1 expression during N6, irrespective of the levels of BgE93. (B and C) Expression levels of (B) BgE93 and (C) BgKr-h1, relative to BgActin5C (qRT-PCR), were measured in wings of 3-d-old N5 (acetone-treated only) and 1-d-old N6 nymphs (acetone- and methoprene-treated). Fold changes are relative to acetone-treated N5 nymphs in B and acetone-treated N6 nymphs in C, arbitrarily set to 1. Error bars represent SEM (n = 5). Asterisks indicate differences statistically significant as follows: *P ≤ 0.05; **P ≤ 0.005, and ***P ≤ 0.001 (t test). (Scale bar: A, 2 mm.)
The results above demonstrate that topical application of methoprene blocks metamorphosis despite an elevated level of BgE93. To address a possible mechanism of methoprene action, we examined its effect on the expression of the antimetamorphic BgKr-h1 gene as this transcription factor is activated by JH in all insects analyzed to date (20, 21). We found a clear methoprene dose-dependent increase in BgKr-h1 transcript levels (Fig. 3C). Comparing BgKr-h1 and BgE93 transcript levels with the phenotypes of the methoprene-treated animals, it is interesting to note that adult metamorphosis was blocked only when the increase of BgKr-h1 transcript levels was comparable with that normally seen during the penultimate N5 nymphal instar of B. germanica, irrespective of BgE93 transcript levels (Fig. 3C). These results suggest that the BgE93-dependent metamorphic differentiation is prevented in the presence of high levels of BgKr-h1, indicating that two requirements are essential to promote adult differentiation during the last nymphal instar, the up-regulation of the BgE93 transcript level and the repression of the BgKr-h1 gene expression. In this regard, our data suggest that the very low levels of BgKr-h1 mRNA during N6 are due to the strong up-regulation of BgE93 and to the critical decline of the JH titer. This JH decline, which is not sufficient for the down-regulation of BgKr-h1 (Fig. 2 A and B), is presumably required to avoid the detrimental reinduction of BgKr-h1 during the last nymphal instar.
Overall, our data demonstrate that BgE93 acts as the adult specifier during the last nymphal instar of the hemimetabolous B. germanica by promoting the metamorphic transition to the adult and also by repressing BgKr-h1 expression, thus eliminating the antimetamorphic effect of the latter and, in doing so, ensuring the proper nymphal–adult transition when the appropriate stage of development is achieved.
The Role of E93 as the Adult Specifier Is Conserved in the Basal Holometabolous T. castaneum.
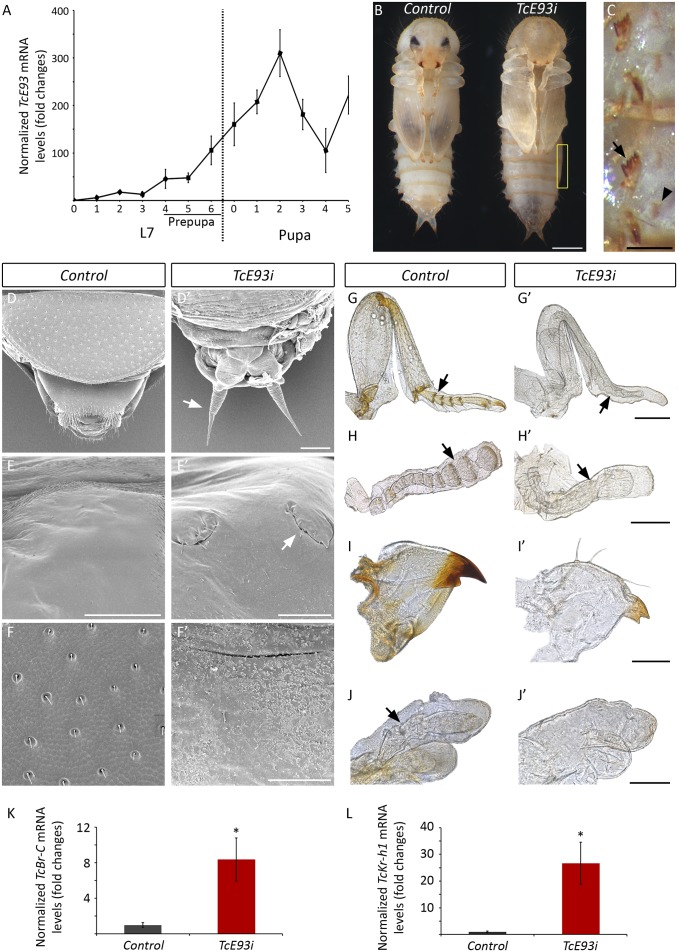
Next, we asked whether the essential role of E93 in adult differentiation might be common to holometabolous insects, which arose from hemimetabolan ancestors some 350 million years ago (1, 22) and turned to the coleopteran T. castaneum as a model of holometabolan development. In contrast to hemimetabolous insects, transformation from immature wingless larvae to winged adults in holometabolous insects requires a transitory stage known as the pupa, which can be considered as the last immature stage (Fig. S6). The genome of T. castaneum contains one E93 homolog (TcE93) that is highly up-regulated during the end of the last larval stage (prepupal stage) and the entire pupal period (Fig. 4A). To assess the functional role of TcE93, we injected TcE93 dsRNA into midfinal instar larvae, which resulted in 98% reduction of TcE93 transcript abundance during the pupal period (Fig. S7A). The majority of the TcE93i larvae (84%; n = 69) pupated normally, although with reduced elytra and wings (Fig. S7 C–F). Consistent with the function as the adult specifier, depletion of TcE93 prevented adult morphogenesis as the TcE93i pupae produced a supernumerary second pupa instead of an adult at the end of the pupal stage (Fig. 4 B and C). A closer examination of the supernumerary pupae after removal of the apolysed pupal cuticle revealed a new cuticle with all of the pupal-specific structures, such as a second set of urogomphi, abdominal gin traps, and a surface with the characteristic pupal-like microsculpture (Fig. 4 D–F′). In addition, the appendage metamorphic changes normally occurring at the pupal stage, which include segmentation, pigmentation, and major changes in shape, were also prevented (Fig. 4 G–J′). Confirming that the adult genetic program has not been installed in the TcE93i pupae, the activation of the adult-specific cuticle gene TcCPR27 normally occurring during the pupal–adult molt (23) was completely abrogated (Fig. S7B).
Fig. 4.
Loss of TcE93 blocks adult differentiation and produces a supernumerary pupa in the holometabolous insect T. castaneum. (A) Developmental expression profile of TcE93 relative to TcRpL32 (qRT-PCR) from last larval stage (L7) and the pupal period. Fold changes are relative to TcE93 expression in 0-d-old L7 larvae, arbitrarily set to 1. Error bars represent SEM (n = 3). (B and C) Effect of RNAi of TcE93 on adult metamorphosis. Mid-L7 larvae were injected with dsTcE93 (TcE93i) or with dsMock (Control). (B) Ventral view of a 5-d-old control and TcE93i pupae. (C) High magnification of the abdomen of TcE93i pupa (rectangle in B) showing ectopic gin traps (arrowhead) underneath of the ones (arrow) from the external cuticle. (D–J′) Adult metamorphic transformations were blocked in TcE93i pupae. (D–F′) Scanning electron microscopy photographs of (D′, E′, and F′) 5-d-old TcE93i pupae compared with (D, E, and F) a 5-d-old control pupae, after removal of the apolysed first pupal cuticle in both cases, showing (arrow in D′) supernumerary pupal urogomphi, (arrow in E’) supernumerary pupal gin traps, and (F′) the pupal-like cuticle surface with the absence of the typical adult microsculpture and rounded pits with sensillae (F). (G–J′) Comparison of the external morphology of appendages in (G–J) pharate control adults and (G′–J′) supernumerary Tc-E93i pupae 5 d after pupation. (G and G′) Hindlegs, (H and H′) antennae, (I and I′) mandible, and (J and J′) maxilla. Arrows indicate the degree of adult segmentation and pigmentation. (K and L) TcE93 represses the expression of the pupal specifier TcBr-C and TcKr-h1 during the pupal stage. (K) TcBr-C and (L) TcKr-h1 mRNA levels were measured by qRT-PCR in control and TcE93i animals 2 d after pupation. Transcript abundance values are normalized against TcRpL32 transcript. Fold changes are relative to TcKr-h1 and TcBr-C expression in control pupae, arbitrarily set to 1. Error bars in K–L indicate the SEM (n= 5). Asterisks indicate differences statistically significant as follows: *P ≤ 0.05 (t test). (Scale bars: B, 0.5 mm; C, D, D′, E, I, I′, J, and J′, 100 µm; E′, F, and F′, 50 µm; G, G′, H, and H′, 200 µm.)
In T. castaneum, adult metamorphosis requires the disappearance of the TcKr-h1 and TcBr-C transcripts during the pupal period (20, 21, 24). In a consistent manner, JH treatment at the onset of the pupal stage of T. castaneum causes the reexpression of TcKr-h1 and TcBr-C and the block of adult morphogenesis (18, 20, 21). Having established that BgE93 represses BgKr-h1 and BgBr-C expression in the final nymphal instar of B. germanica, we next tested whether a similar relation occurs in T. castaneum. We found that mRNA levels of TcKr-h1 and TcBr-C were maintained at high levels in TcE93i pupae (Fig. 4 K and L), clearly indicating that TcE93 is necessary for the down-regulation of TcKr-h1 and TcBr-C during the pupal stage of T. castaneum. Collectively, our findings indicate that the function and the regulatory mechanisms underlying E93-mediated adult differentiation are evolutionary conserved between hemimetabolous and holometabolous insects. In this regard, it is important to note that, unlike a highly conserved role of Kr-h1 as the JH-dependent repressor of metamorphosis in hemimetabolous and holometabolous insects, the role of Br-C is dramatically different in these insect groups. Whereas Br-C does not play major metamorphic effects in hemimetabolous insects, being mainly limited to wing size control (15, 17, 19), it exerts a crucial stage-specific metamorphic role as the pupal specifier in all holometabolous insects studied so far (18, 20, 24). Previous work has shown that the evolution of the metamorphic functions of Br-C has been achieved, in part, through marked changes in its expression. Although Br-C is expressed during embryogenesis and throughout nymphal development in hemimetabolous insects, its expression is confined to the prepupal period of the final larval instar in holometabolous insects (15, 17, 19, 20). In contrast to these heterochronic changes of Br-C expression, its E93-dependent down-regulation during the metamorphic stage is a conserved trait of hemimetaboly and holometaboly. Based on this conservation, it is tempting to speculate that the cooption of Br-C for specifying the pupal state in holometabolous insects could have been favored, in addition to the heterochronic changes of Br-C expression, by the conserved transcriptional relationship between E93 and Br-C during the last metamorphic transition already present in hemimetabolous insects.
E93 Is also Required for Adult Differentiation in the Derived Holometabolous D. melanogaster.
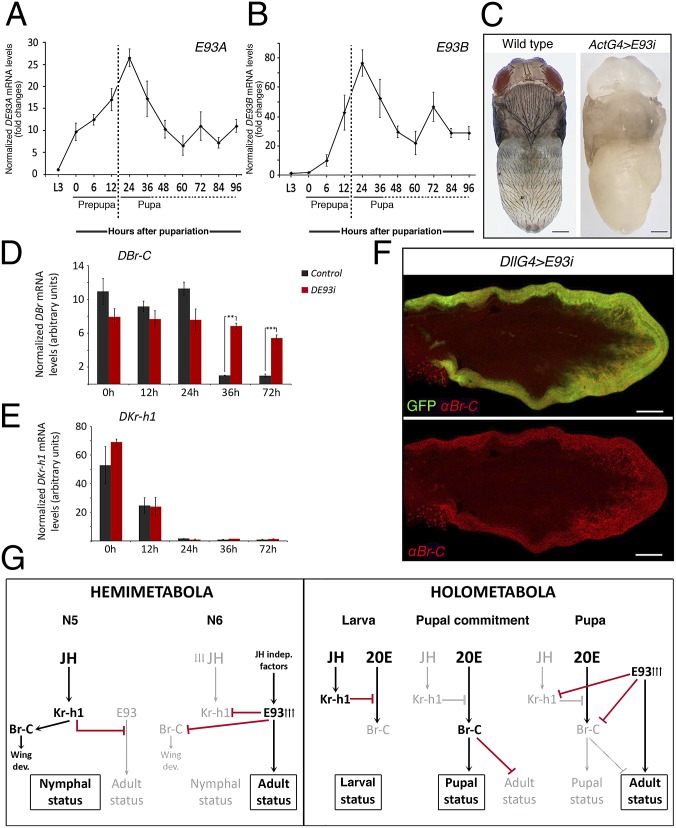
Finally, we have found that the role of E93 is also conserved in the highly derived holometabolous insect D. melanogaster. Although first identified as a specific regulator of cell death during the prepupal stage (13, 14), DE93 has recently been found to be a more general temporal regulator of gene expression during the metamorphosis of the fly (25, 26). Consistent with its role as adult specifier, we observed that DE93 expression is restricted to the metamorphic prepupal and pupal stages (Fig. 5 A and B). Likewise, to confirm that silencing DE93 blocks adult differentiation, we next assayed the effects of down-regulating DE93 with an RNAi transgene driven by the ubiquitous Actin-Gal4 driver. These flies with reduced levels of the DE93 transcript (70% reduction in DE93A and 80% in DE93B) pupated normally but died at the end of the pupal stage with an overall impairment of adult differentiation (Fig. 5C and Figs. S8 and S9 E and F). In accordance with this result, we found that the expression level of the pupal gene DEdg78E was unaffected whereas the induction of adult-specific DAcp65A gene was abrogated in DE93-depleted flies (Fig. S9 A–D). Moreover, the absence of DE93 during the pupal stage of D. melanogaster also prevented the critical down-regulation of DBr-C that is required for adult differentiation to occur (Fig. 5 D and F). In contrast to T. castaneum, the absence of DE93 did not prevent the decline of DKr-h1 expression during the pupal stage, likely due to the uniqueness of D. melanogaster development (Fig. 5E). Indeed, it has been shown that the repression of DKr-h1 that is required for the neural maturation and remodeling during the metamorphic stage of the fly is controlled by the conserved homeobox protein Orthodenticle acting together with the ecdysone receptor (27).
Fig. 5.
DE93 is necessary for proper differentiation of adult characters and for the repression of the pupal specifier DBr-C in the derived holometabolous insect D. melanogaster. (A and B) Developmental expression profile of the two D. melanogaster DE93 isoforms, (A) DE93A and (B) DE93B, relative to DRpL32 (qRT-PCR) from last larval stage (L3) and the prepupal and pupal periods. Fold changes are relative to DE93 expression in L3, arbitrarily set to 1. Error bars represent SEM (n = 3). (C) Dorsal views of wild-type and ActG4>DE93i animals 96-h after puparium formation (APF). (D and E) Expression levels of (D) DBr-C and (E) DKr-h1 relative to DRpL32 in wild-type (in black) and ActG4>DE93i pupae (in red) (qRT-PCR). Fold changes are relative to 72-h APF wild-type pupae, arbitrarily set to 1. Error bars represent SEM (n = 5). Asterisks indicate differences statistically significant as follows: **P ≤ 0.005; ***P ≤ 0.0005 (t test). (F) Wing from 30-h APF pupa expressing a DE93-RNAi construct and GFP (Upper) under the control of Distal-less (Dll)-Gal4 driver. DBr-C expression is maintained in absence of DE93 in the distal part of the wing (red, Lower). (Scale bars: C, 0.3 mm; F, 200 µm.) (G) Model depicting the regulatory activity of E93 in controlling adult differentiation and repressing the expression of Kr-h1 (except in D. melanogaster) and Br-C in hemimetabolous and holometabolous insects. Black arrows represent inductive effects, and red lines represent repressive effects. Gray colors denote genes and transcriptional regulatory events that are absent during each particular period.
Conserved Function of E93 in Hemimetabolous and Holometabolous Insects.
Our study has demonstrated that, despite the evolutionary distance and the differences in the developmental strategies of reaching adulthood, E93 is the universal adult specifier that acts as the “master factor” of adult metamorphosis in hemimetabolous and holometabolous insects. Based on our results, we thus propose the following model for adult metamorphosis in hemimetabolous and holometabolous insects (scheme in Fig. 5G): (i) During the preterminal nymphal stages in hemimetabolous insects, the JH-effector Kr-h1 maintains the nymphal status of the animal by preventing the adult-promoting activity of E93. At the onset of the last nymphal instar, increasing levels of E93 down-regulate Kr-h1 expression and induce adult differentiation. Coupling the adult promoting activity with the repression of the antimetamorphic factor Kr-h1 in a single regulatory factor ensures that a proper nymphal–adult transition takes place during the last nymphal instar. E93 also represses transcription of Br-C during the last nymphal instar of hemimetabolous insects although Br-C has not been linked with metamorphosis in these insects. Concurrently with E93 up-regulation, the hemolymph JH titer drops to undetectable levels, preventing Kr-h1 reinduction. It has been proposed that the acquisition of a metamorphosing “critical weight” triggers the dramatic decline in the JH titer (28). Given that the fall of the JH titer occurs simultaneously with the strong up-regulation of E93, we hypothesize that a similar critical weight mechanism may control the latter event. (ii) In contrast to hemimetabolous insects, holometabolan metamorphosis takes place in two different stages, first to pupa and then to adult. The transition to pupal stage is mainly controlled by the pupal specifier Br-C after the decline of the JH hemolymph titer and the Kr-h1 transcript in the last larval instar (29). Upon pupation, and similar to the last nymphal instar in hemimetabolous insects, E93 induction promotes adult differentiation and also ensures the repression of the antimetamorphic factors Br-C and Kr-h1 (with the exception of D. melanogaster), thus allowing adult morphogenesis to take place.
Similar to the last juvenile stages in insects, mammalian puberty, including that of humans, is characterized by a number of hormonal events, resulting in the attainment of adult reproductive capacity. Two mammalian E93 orthologs, ligand-dependent corepressor (LCoR) and its paralog LCoR-like, have been identified (30, 31). LCoR is widely expressed in fetal and adult tissues and has been reported to interact with a series of ligand-bound steroid nuclear receptors, including the thyroid hormone receptor, a protein that controls metamorphosis in amphibians (30, 32). Given the conservation of the E93/LCoR factors, the interesting possibility remains to be explored that such factors could be involved in the regulation of the adult genetic program during the passage of puberty in mammals or the amphibian metamorphosis.
In conclusion, our study has clearly demonstrated that E93 is the universal adult specifier in hemimetabolous and holometabolous insects. The present work thus represents a significant step toward defining the molecular mechanisms underlying insect metamorphosis. Furthermore, it contributes to our understanding of evolutionary conservation of the molecular program determining the developmental transition to adulthood in animals.
Materials and Methods
Specimens of B. germanica were obtained from a colony reared in the dark at 30 ± 1 °C and 60–70% relative humidity. Wild-type T. castaneum strain and the enhancer-trap line pu11 (obtained from Y. Tomoyasu, Miami University, Oxford, OH) were reared on organic wheat flour containing 5% nutritional yeast and maintained at 29 °C in constant darkness. Flies were raised on standard D. melanogaster medium at 25 °C, unless otherwise required. Details on the metamorphic characteristics of each insect strain, as well as other experimental methods, are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Vienna Drosophila RNAi Center, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank for flies and antibodies; C. de Miguel for technical assistance with T. castaneum injections; and A. Casali, J. Casanova, S. Araujo, L. A. Baena, K. Campbell, M. Furriols, M. Llimargas, and J. P. Vincent for helpful suggestions on the manuscript. E.U. was supported by a predoctoral fellowship from Consejo Superior de Investigaciones Cientificas and C.M. by a Juan de la Cierva fellowship. This work was funded by the Spanish Ministerio de Ciencia e Innovacion [Projects BFU2009-10571 (to D.M.) and BFU2009-08748 (to X.F.-M.)].
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database [accession no. HF678196 (BgE93)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401478111/-/DCSupplemental.
References
- 1.Gilbert LI, Tata JR, Atkinson BG. Metamorphosis. San Diego: Academic Press; 1996. [Google Scholar]
- 2.Buszczak M, Segraves WA. Insect metamorphosis: Out with the old, in with the new. Curr Biol. 2000;10(22):R830–R833. doi: 10.1016/s0960-9822(00)00792-2. [DOI] [PubMed] [Google Scholar]
- 3.Dubrovsky EB. Hormonal cross talk in insect development. Trends Endocrinol Metab. 2005;16(1):6–11. doi: 10.1016/j.tem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Truman JW, Riddiford LM. Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol. 2002;47:467–500. doi: 10.1146/annurev.ento.47.091201.145230. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu Rev Entomol. 2013;58:497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 7.Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen Comp Endocrinol. 2012;179(3):477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Truman JW, Riddiford LM. The morphostatic actions of juvenile hormone. Insect Biochem Mol Biol. 2007;37(8):761–770. doi: 10.1016/j.ibmb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Wigglesworth VB. The function of the corpus allatum in the growth and reproduction of Rhodnius prolixus (Hemiptera) Q J Microsc Sci. 1936;s2-79:91–121. [Google Scholar]
- 10.Wigglesworth VB. Memoirs: The physiology of ecdysis in Rhodnius prolixus (Hemiptera). II. Factors controlling moulting and “metamorphosis.”. Q J Microsc Sci. 1934;S2-77:191–222. [Google Scholar]
- 11.Mané-Padrós D, et al. The hormonal pathway controlling cell death during metamorphosis in a hemimetabolous insect. Dev Biol. 2010;346(1):150–160. doi: 10.1016/j.ydbio.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Mané-Padrós D, Borràs-Castells F, Bellés X, Martín D. Nuclear receptor HR4 plays an essential role in the ecdysteroid-triggered gene cascade in the development of the hemimetabolous insect Blattella germanica. Mol Cell Endocrinol. 2012;348(1):322–330. doi: 10.1016/j.mce.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Baehrecke EH, Thummel CS. The Drosophila E93 gene from the 93F early puff displays stage- and tissue-specific regulation by 20-hydroxyecdysone. Dev Biol. 1995;171(1):85–97. doi: 10.1006/dbio.1995.1262. [DOI] [PubMed] [Google Scholar]
- 14.Lee CY, et al. E93 directs steroid-triggered programmed cell death in Drosophila. Mol Cell. 2000;6(2):433–443. doi: 10.1016/s1097-2765(00)00042-3. [DOI] [PubMed] [Google Scholar]
- 15.Konopova B, Smykal V, Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE. 2011;6(12):e28728. doi: 10.1371/journal.pone.0028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozano J, Bellés X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci Rep. 2011;1:163. doi: 10.1038/srep00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J-H, Lozano J, Bellés X. Broad-complex functions in postembryonic development of the cockroach Blattella germanica shed new light on the evolution of insect metamorphosis. Biochim Biophys Acta. 2013;1830(1):2178–2187. doi: 10.1016/j.bbagen.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y, Truman JW, Riddiford LM. The role of Broad in the development of Tribolium castaneum: Implications for the evolution of the holometabolous insect pupa. Development. 2008;135(3):569–577. doi: 10.1242/dev.015263. [DOI] [PubMed] [Google Scholar]
- 19.Erezyilmaz DF, Riddiford LM, Truman JW. The pupal specifier broad directs progressive morphogenesis in a direct-developing insect. Proc Natl Acad Sci USA. 2006;103(18):6925–6930. doi: 10.1073/pnas.0509983103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konopova B, Jindra M. Broad-Complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development. 2008;135(3):559–568. doi: 10.1242/dev.016097. [DOI] [PubMed] [Google Scholar]
- 21.Minakuchi C, Namiki T, Shinoda T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev Biol. 2009;325(2):341–350. doi: 10.1016/j.ydbio.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Truman JW, Riddiford LM. The origins of insect metamorphosis. Nature. 1999;401(6752):447–452. doi: 10.1038/46737. [DOI] [PubMed] [Google Scholar]
- 23.Arakane Y, et al. Formation of rigid, non-flight forewings (elytra) of a beetle requires two major cuticular proteins. PLoS Genet. 2012;8(4):e1002682. doi: 10.1371/journal.pgen.1002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parthasarathy R, Tan A, Bai H, Palli SR. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech Dev. 2008;125(3-4):299–313. doi: 10.1016/j.mod.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mou X, Duncan DM, Baehrecke EH, Duncan I. Control of target gene specificity during metamorphosis by the steroid response gene E93. Proc Natl Acad Sci USA. 2012;109(8):2949–2954. doi: 10.1073/pnas.1117559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jafari S, et al. Combinatorial activation and repression by seven transcription factors specify Drosophila odorant receptor expression. PLoS Biol. 2012;10(3):e1001280. doi: 10.1371/journal.pbio.1001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fichelson P, Brigui A, Pichaud F. Orthodenticle and Kruppel homolog 1 regulate Drosophila photoreceptor maturation. Proc Natl Acad Sci USA. 2012;109(20):7893–7898. doi: 10.1073/pnas.1120276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nijhout HF. Stretch-induced moulting in Oncopeltus fasciatus. J Insect Physiol. 1979;25:277–281. [Google Scholar]
- 29.Zhou X, Riddiford LM. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129(9):2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes I, et al. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol Cell. 2003;11(1):139–150. doi: 10.1016/s1097-2765(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 31.Soranzo N, et al. Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet. 2009;5(4):e1000445. doi: 10.1371/journal.pgen.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, et al. Ligand-dependent corepressor acts as a novel corepressor of thyroid hormone receptor and represses hepatic lipogenesis in mice. J Hepatol. 2012;56(1):248–254. doi: 10.1016/j.jhep.2011.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.