Primate model of hantavirus pulmonary syndrome
Studies of the pathology and progression of hantavirus pulmonary syndrome (HPS), an acute respiratory disease, have been hampered by the lack of a primate model that experiences severe respiratory response to the disease, similar to that observed in humans. Based on available observations, researchers had previously concluded that the progression of the disease stemmed from an overactive host immune response. To develop a primate model of disease progression, David Safronetz et al. (pp. 7114–7119) inoculated 17 rhesus macaques with Sin Nombre virus that had previously infected deer mice. Seven macaques developed severe respiratory disease, including coughing, rapid breathing, and chest crackles, within 3 weeks of infection, similar to human incubation periods for the disease. The authors monitored the progression of the disease in the macaques and observed that a combination of virus replication, abnormalities in blood cells, and uncontrolled host inflammatory responses contributed to HPS development. The results suggest that using rhesus macaques as a primate model for HPS might allow further research into the rare but fatal disease and may aid the development of primate models for other serious diseases, according to the authors. — P.G.
Experience and gene expression
A recently discovered DNA modification, 5-hydroxymethylcytosine (5-hmC), is produced from methylated DNA by enzymes in the ten-eleven translocation (Tet) family. Previous studies have shown that 5-hmC accumulates with age and is highly enriched in the adult brain, but the functional significance of the accrual and the roles of individual Tet proteins remain unclear. Xiang Li et al. (pp. 7120–7125) report that Tet3—a highly expressed protein in the adult cortex known to hydroxylate 5-mc to 5-hmC—mediates rapid behavioral adaptation in mice. The authors trained mice to inhibit previously learned fears, a behavioral process known as fear extinction, then performed 5-hmC capture and high-throughput DNA sequencing to study the genome-wide distribution of 5-hmC in the infralimbic prefrontal cortex of the brain. The findings reveal that intergenic accumulation of 5-hmC not only serves as a functional intermediate of DNA demethylation but creates a unique epigenetic state conducive to experience-dependent gene expression. The 5-hmC modification is known to shape neuronal differentiation and aid the reprogramming of pluripotent stem cells. According to the authors, the findings suggest that Tet3-mediated accumulation of 5-hmC in the adult cortex, triggered by extinction learning, is also required to establish the epigenetic states that promote gene expression and allow for rapid behavioral adaptation. — A.G.
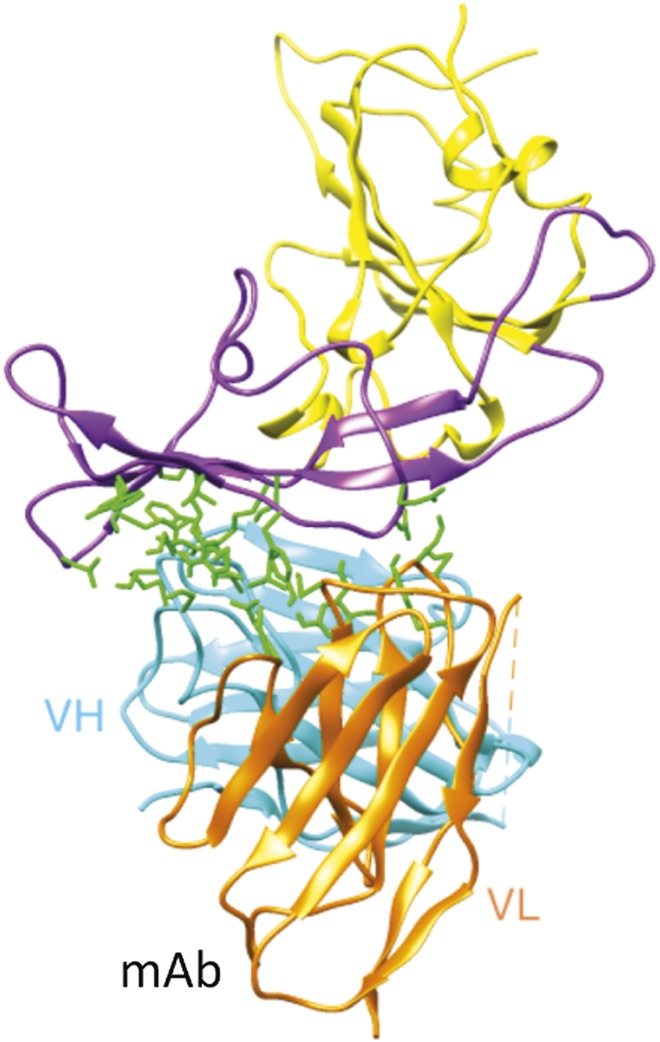
Human antibodies against MERS-CoV
Interaction of MERS-CoV receptor binding domain (purple) and MERS-CoV monoclonal antibody.
The Middle East Respiratory Syndrome coronavirus (MERS-CoV) has claimed 80 lives and infected 184 people in 10 countries in the Middle East, Europe, and North Africa, as of February 2014. Bats and dromedary camels might transmit the virus, which can also spread among humans, albeit at moderate rates and through unclear mechanisms. Because regulatory hurdles can hamper the timely collection of patient blood samples for the identification of anti-MERS-CoV protective antibodies, Xian-Chun Tang et al. (pp. E2018–E2026) used a technique based on a large, nonimmune antibody-phage library to identify seven human neutralizing antibodies that recognize at least three distinct epitopes at the interface of the viral spike protein and its human cellular receptor. The authors report that all seven antibodies neutralized MERS-CoV infection in laboratory tests, with one antibody, 3B11, showing the highest binding affinity and neutralization activity. The authors explored how the virus adapted to the antibodies and examined the effect of viral “escape mutations” on the antibodies’ efficacy. Most of the escape viruses triggered by a given antibody epitope group failed to confer resistance to a different antibody epitope group, and most escape mutations hobbled viral fitness. According to the authors, the vulnerable spike protein–cellular receptor interface might serve as a target for protective vaccines, and 3B11 might serve as a potent ingredient in a therapeutic antibody cocktail. — P.N.
Plant odors and pest defense

Common cutworm feeds on the leaves of a tomato plant.
Damaged plants emit volatile organic compounds (VOCs), odors that can signal other plants to prepare for impending tissue damage. Koichi Sugimoto et al. (pp. 7144–7149) investigated the mechanism by which plants collect VOC signals from neighboring plants undergoing an insect attack and use the signals to fortify defenses. The authors placed tomato plants infested with common cutworms next to uninfested tomato plants and analyzed metabolite production. The authors report that the uninfested plants produced higher levels of a single compound, (Z)-3-hexenylvicianoside (HexVic), when grown next to infested plants than did uninfested control plants grown next to other uninfested plants. Common cutworms raised on a diet containing HexVic experienced lower survival rates and weight gain, compared with cutworms fed a normal diet, suggesting a role for HexVic in herbivore defense. An analysis of the VOCs released by infested tomato plants revealed that the green leaf alcohol (Z)-3-hexenol was a primary component. In addition, uninfested plants exposed to airborne (Z)-3-hexenol converted the chemical to HexVic. A field experiment confirmed that uninfested plants grown next to infested plants produced more HexVic than uninfested plants grown next to other uninfested plants. According to the authors, the results demonstrate a mechanism of plant defense in which insect-infested plants signal neighboring healthy plants with VOCs that the healthy plants may use to produce insecticidal toxins. — J.P.J.
HIV-1 coreceptor ratios and susceptibility to infection
To initiate an infection, the HIV-1 viral envelope glycoprotein, gp120, forms a complex with the target host cell receptor, CD4, and a coreceptor, either CXCR4 or CCR5. The most commonly transmitted viral variant, R5, targets CCR5-expressing immune cells concentrated at the mucosa; a switch to the X4 variant accompanies infection of CXCR4-expressing Th2 cells in the blood and the development of AIDS. Laura Martínez-Muñoz et al. (pp. E1960–E1969) investigated the influence of CCR5 expression on the interaction of host cells with X4 virus. By generating fusions of CCR5 and CXCR4 coreceptors to fluorophores to detect energy transfer between proximate molecules, the authors found that CCR5 bound to CD4-CXCR4 heterodimers on the cell surface, altering their conformation and disrupting the gp120 binding site, without affecting binding of CXCR4 to its ligand. Expression of plasmid-encoded CCR5 reduced susceptibility to infection with X4 HIV-1 in both cultured and primary CD4+ T-cell lines; conversely, lowering the level of cell-surface CXCR4 in CCR5-expressing T cells increased susceptibility to infection with an R5 strain. HIV-1 coreceptor ratios might modulate viral pathogenesis, suggesting a potential avenue for blocking X4 HIV-1 infection using compounds that mimic the CCR5-induced conformational change in CD4-CXCR4, according to the authors. — C.B.