Significance
MicroRNAs (miRNAs) comprise a large family of small RNA molecules that regulate gene expression in diverse biological pathways of both plants and animals. We used a combination of proteomic and functional analyses of proteins associated with the miRNA pathway to identify the ubiquitin E3 ligase tripartite motif 65 (TRIM65) and five other genes implicated in regulation of miRNA activity in human cells. Biochemical analysis established that TRIM65 forms stable complexes with trinucleotide repeat containing six (TNRC6) proteins and that these molecules colocalize in processing body-like structures. Gain of function and RNAi analyses reveal that TRIM65 regulates miRNA-driven suppression of mRNA translation by targeting TNRC6 proteins for ubiquitination and degradation. Thus, TRIM65 represents a new negative regulator of miRNA activity.
Keywords: RNA-induced silencing complex, protein interaction networks, GW proteins
Abstract
MicroRNAs (miRNAs) are small evolutionarily conserved regulatory RNAs that modulate mRNA stability and translation in a wide range of cell types. MiRNAs are involved in a broad array of biological processes, including cellular proliferation, differentiation, and apoptosis. To identify previously unidentified regulators of miRNA, we initiated a systematic discovery-type proteomic analysis of the miRNA pathway interactome in human cells. Six of 66 genes identified in our proteomic screen were capable of regulating lethal-7a (let-7a) miRNA reporter activity. Tripartite motif 65 (TRIM65) was identified as a repressor of miRNA activity. Detailed analysis indicates that TRIM65 interacts and colocalizes with trinucleotide repeat containing six (TNRC6) proteins in processing body-like structures. Ubiquitination assays demonstrate that TRIM65 is an ubiquitin E3 ligase for TNRC6 proteins. The combination of overexpression and knockdown studies establishes that TRIM65 relieves miRNA-driven suppression of mRNA expression through ubiquitination and subsequent degradation of TNRC6.
MicroRNAs (miRNAs) are small noncoding RNAs that regulate the translation and stability of mRNA in animals and plants (1, 2). The canonical biogenesis of miRNAs starts with a hairpin-like primary miRNA (primiRNA), typically a product of RNA polymerase II (3, 4). In the nucleus, the DROSHA/DGCR8 microprocessor complex recognizes and cleaves the primiRNA hairpin, which leads to release of a precursor miRNA (premiRNA) hairpin that is ∼55–70 nt in length (5–7). The premiRNA is exported to the cytoplasm by a complex of Exportin 5 and RAN-GTP (8, 9). In the cytoplasm, the premiRNA terminal loop is cleaved by DICER in collaboration with TARBP2, yielding ∼22-nt RNA duplexes. One strand of the duplex is preferentially incorporated into the RNA-induced silencing complex (RISC), where the miRNA and mRNA interact (3). In the RISC, miRNA targets mRNA for translational repression, deadenylation, or degradation (10–12).
The RISC minimally consists of two core protein components, Argonaute (AGO) and trinucleotide repeat containing six (TNRC6; also known as GW182) proteins or their paralogs, which are key factors for function of the RISC. These proteins localize in specialized cytoplasmic foci known as mRNA processing bodies (P-bodies) (13, 14). P-bodies also contain effector molecules that facilitate mRNA degradation, including decapping enzymes (DCP1 and DCP2) required for miRNA-mediated gene silencing (15) and the CCR4–NOT deadenylase complex, which removes poly(A) from messages destabilized by miRNA (15).
The core proteins that participate in miRNA biogenesis and regulation have been identified (6, 16–20), but the global organization and coordination of this system are incompletely understood. To explore the miRNA protein–protein interaction network systematically, we initiated a global proteomic analysis of the miRNA pathway interactome (MPI) in human cells. Analysis of 40 MPI-associated baits revealed connections with 363 proteins, forming a framework of 499 unique protein interactions. RNAi screening of 66 previously unidentified proteins associated with the MPI identified tripartite motif 65 (TRIM65) and five other regulators of miRNA activity. TRIM65 is an ubiquitin E3 ligase that colocalizes with TNRC6 paralogs and triggers proteasome-dependent degradation of TNRC6 proteins. TRIM65 represents a previously unidentified member of the miRNA pathway, which negatively regulates the miRNA-guided mRNA silencing machinery.
Results
Mapping the MPI.
A proteomic search strategy was used to identify protein components putatively associated with the miRNA processing machinery. Forty proteins with known or suspected involvement in canonical miRNA biogenesis and regulation were chosen as baits. Each bait was tagged with the FLAG epitope and stably expressed in HEK 293 cells. Affinity purification by elution from anti-FLAG beads coupled with MS (AP-MS) was used to identify proteins linked to the MPI (21). Controls for excluding nonspecific binding proteins (NSBPs) include eluates from FLAG-GFP–transfected and nontransfected HEK 293 cells. Protein complexes were retrieved from two independent biological experiments. Furthermore, each affinity-purified sample was divided into two aliquots, which were analyzed by liquid chromatography-tandem MS on different days. In total, 80 complexes were purified and 160 samples were examined.
For data processing, we compared AP-MS data from the MPI with our laboratory database containing 158 FLAG-tagged protein complexes from stably transfected HEK 293 cells, including 55 proteins involved in innate immunity (21). We identify high-confidence interacting proteins (HCIPs) by calculation of z-scores based on total spectral counts (protein abundance). In addition, we consider prey occurrence (the uniqueness of a protein in our database) and reproducibility in our algorithm. We set high stringency thresholds: The z-score is set at ≥3 when prey occurrence is <5%, the z-score is set at ≥10 when prey occurrence is ≥ 5%, and the cutoff for reproducibility is fixed at ≥50% (appearance in at least two of four MS runs for the same bait). We identified 363 HCIPs making a protein interactome of 499 unique interactions and forming major clusters corresponding to the core components, DROSHA, DICER, and AGO (Fig. 1A and Dataset S1). In comparison to the Biological General Repository for Interaction Datasets (BioGRID), IntAct, and Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) protein interaction databases and curated literature, we found 55 known interactions and 444 interactions that were not present in these databases (Dataset S2). Thus, this proteomic analysis expands the number of candidate proteins linked to the miRNA pathway.
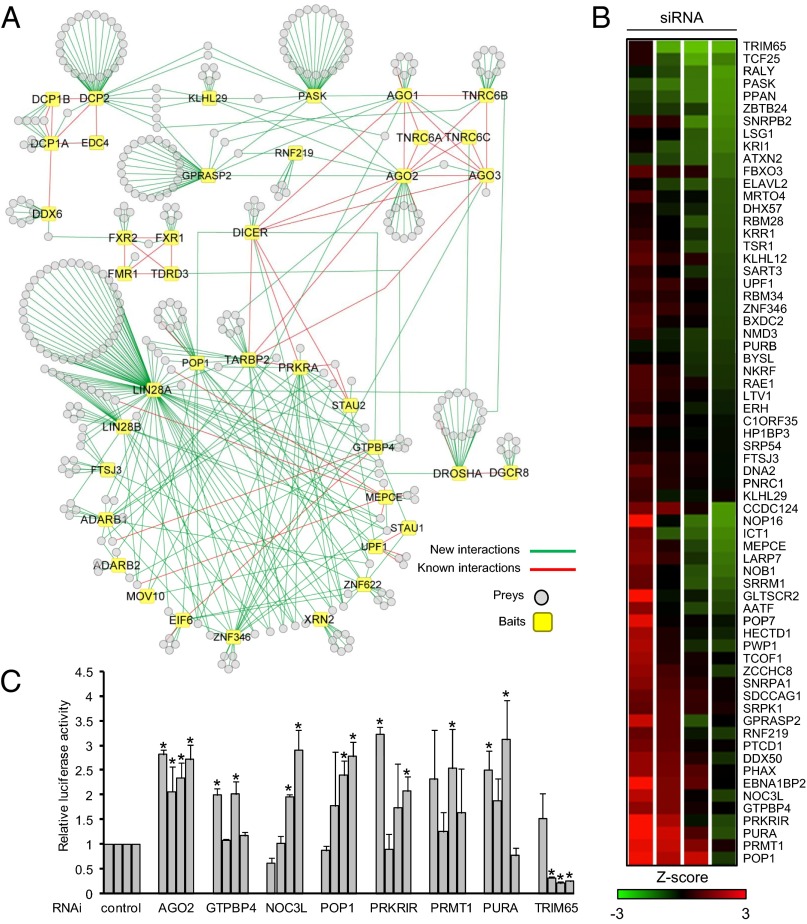
Fig. 1.
Overview and RNAi analysis of MPI. (A) Baits and HCIPs are shown as squares and circles, respectively. The red line indicates a previously known interaction, and the green line indicates a previously unidentified interaction. Identification of HCIPs is provided in Dataset S1. (B) Heat map screening of siRNAs on a let-7a reporter (let7 A) in HeLa cells. Sixty-six HCIP RNAi oligos (four siRNAs per gene) were transfected into HeLa cells along with a let-7a reporter and control firefly luciferase. Log transformation of normalized values, (Renilla luciferase value)/(firefly luciferase value), was calculated for z-score analysis. For statistical analysis, the data were combined with our laboratory database containing results from 483 other siRNAs tested for let-7a reporter activity in HeLa cells. (C) To validate the screening assay, HeLa cells were treated with the indicated siRNA along with let-7a reporter and control firefly luciferase. RNAi efficiency is presented in Fig. S2 B–G. Each bar represents a different siRNA sequence. Data depict the mean ± SD of triplicate samples. *P < 0.05.
Validation and RNAi Analysis of the MPI.
Coimmunoprecipitation (co-IP) was used to confirm previously unreported protein interactions. Bait and HCIPs were tagged with different epitopes and coexpressed in HEK 293 cells. In total, 93% (50 of 54) of previously unknown interactions that were tested by co-IP were validated (Fig. S1 A–H and Dataset S2). Proteomic analysis established 16 reciprocal protein interactions. Thus, combining previously established, co-IP, and reciprocal interactions, 24% (121 of 499) of protein interactions have been validated in the MPI (Fig. S2A and Dataset S2).
RNAi knockdown was used to screen the effects of HCIP on let-7a miRNA function. HeLa cells were transfected with a plasmid expressing Renilla luciferase mRNA with a 3′ UTR containing two copies of a let-7a miRNA binding site, along with a firefly luciferase control (22). Using this let-7a reporter, we systematically examined the function of 66 HCIPs that lacked known roles in miRNA-mediated translational repression. RNAi depletion (four short interfering RNAs per gene) was used to screen functional activity. We focused on HCIPs with multiple interactions in the MPI or with enzymatic activity. Z-scores ≥2 or ≤−2 were considered significant hits. Genes with two or more significant hits were selected as candidate regulators of the miRNA pathway. In total, depletion of six identified genes (GTPBP4, NOC3L, POP1, PRKRIR, PRMT1, and PURA) increases reporter activity, whereas knockdown of only one gene (TRIM65) reduces reporter activity (Fig. 1B).
To validate the screening results, a side-by-side comparison was performed with siRNAs against AGO2 as a positive control and the seven candidate genes identified in the primary screen. With the exception of PRMT1, triplicate experiments confirmed two or more significant RNAi hits for six genes (NOC3L, POP1, PRKRIR, PURA, TRIM65, and GTBP4) that modulate let-7a reporter activity in HeLa cells (Fig. 1C). RNAi activity in the reporter assays correlated with their efficiency in inhibiting protein or RNA expression (Fig. S2 B–G). Knockdown of four genes (NOC3L, POP1, PURA, and TRIM65) also regulated let-7a reporter activity in A549 cells (Fig. S2H). Although depletion of GTPBP4 and PRKRIR increased let-7a reporter levels in HeLa cells, it did not significantly regulate reporter activity in A549 cells (Fig. S2H). In summary, a sampling of HCIPs (66 of 363) identified six genes capable of regulating miRNA activity, two of which are cell type-dependent.
TRIM65 Interaction and Colocalization with TNRC6.
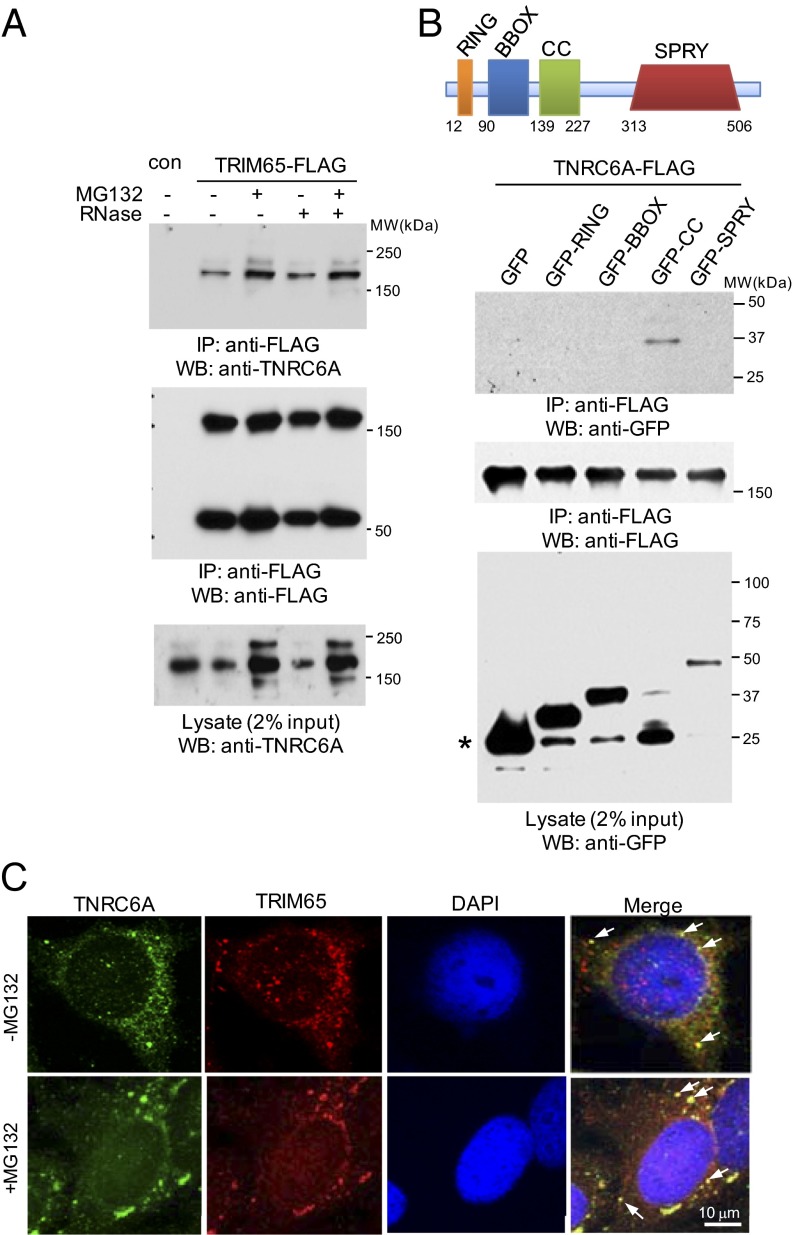
The E3 ubiquitin ligase TRIM65 associates with TNRC6B in the MPI. Therefore, we examined the interactions of TRIM65 with the family of TNRC6 proteins (TNRC6A, TNRC6B, and TNRC6C). Endogenous TNRC6A bound to transiently (Fig. S3A) and stably expressed FLAG-TRIM65, and the interaction was enhanced by MG132 proteasome inhibitor (Fig. 2A). Given the ability of TNRC6 to bind RNA, we examined the effect of RNase treatment on TNRC6–TRIM65 interactions. Depletion of RNA had little effect on TNRC6–TRIM65 associations, indicating the interaction is not bridged by RNA (Fig. 2A). TRIM65 also bound ectopically expressed TNRC6B and TNRC6C when coexpressed in HEK 293 cells (Fig. S3B). To determine if TRIM65 was also associated with other RISC components, we looked for co-IP between TRIM65 and AGO1 or AGO2 proteins. TRIM65 selectively interacts with TNRC6 and fails to coimmunoprecipitate with AGO proteins (Fig. S3 A and C). These data suggest direct protein interactions between TRIM65 and TNRC6 proteins.
Fig. 2.
TRIM65 interacts and colocalizes with TNRC6. (A) HEK 293 cells stably expressing TRIM65-FLAG were treated with 10 μM protease inhibitor MG132 or DMSO for 4 h. Cell lysates were collected and treated with 100 μg/mL RNase A. Immunoprecipitation (IP) and immunoblotting were performed using the indicated antibodies. con, control; WB, Western blot. (B) GFP and various TRIM65 domains fused with GFP were transfected with TNRC6A-FLAG into HEK 293 cells. Cell lysates were coimmunoprecipitated with anti-FLAG antibody and blotted with the indicated reagents. The asterisk indicates GFP protein size. (C) HeLa cells were first treated with 10 μM MG132 or DMSO for 4 h and then fixed before incubation with anti-TNRC6A and anti-TRIM65 antibodies, followed by detection with secondary antibodies. DAPI was used to detect nuclei. Arrows highlight colocalization.
The tripartite N-terminal portion of TRIM65 contains the RING (Really Interesting New Gene), BBOX (B-Box–type zinc finger), and CC (Coiled Coil) domains characteristic of TRIM family members. TRIM65 also contains a SPRY (SPla domain of the ryanodine receptor) domain within the C-terminal segment (Fig. 2B). TNRC6A and TNRC6B were cotransfected with GFP or a GFP-tagged TRIM65 domain. Domain mapping experiments demonstrate that the CC domain is sufficient for TNRC6 association (Fig. 2B and Fig. S3D). To investigate the relationship among AGO, TNRC6, and TRIM65, we fractionated cell lysates from cells stably expressing FLAG-TRIM65 by centrifugation over a 15–55% sucrose gradient. Consistent with a previous reports (19, 23), AGO and TNRC6 sedimented across a broad range of densities, which are referred to as complexes 1–3 (Fig. S4A). FLAG-TRIM65 is primarily located in complex 1, a low-sucrose density fraction. Digestion of sucrose gradient fractions with a serine/threonine phosphatase, calf-intestinal alkaline phosphatase, suggests the presence of phosphorylated TRIM65 (Fig. S4B). In addition to TRIM65 monomers, more slowly migrating forms were observed by SDS/PAGE under reducing conditions. The TRIM65 oligomers cosediment with the monomers. Co-IP suggests that TNRC6A interacts with both TRIM65 monomers and oligomers (Fig. 2A and Fig. S4C). In contrast, FXR1 and PABP1 did not sediment with TRIM65 but were found in complexes 3 and 2/3, respectively (Fig. S4A). Coexpression of HA- and FLAG-tagged TRIM65 demonstrates the capacity for TRIM65 self-association (Fig. S4D). The data suggest multiple AGO–TNRC6 subcomplexes, at least one of which contains TRIM65 and TNRC6A.
TNRC6 family proteins are enriched in cytoplasmic foci known as P-bodies (14, 24). When expressed alone, GFP-TRIM65 formed speckles in the cytoplasm (Fig. S5A). A high degree of overlap was observed between GFP-TRIM65 and endogenous TNRC6A foci (Fig. S5B). Endogenous TRIM65 is also found in punctate cytosolic structures that partially colocalize with endogenous TNRC6A in HeLa and HEK 293 cells (Fig. 2C and Fig. S5C). After treatment with MG132, these P-body–like structures appear enlarged and concentrated (Fig. 2C and Fig. S5C). Taken together, TRIM65 interacts with TNRC6 and colocalizes with TNRC6 in P-body–like structures.
TRIM65 Regulates TNRC6 Ubiquitination and Stability.
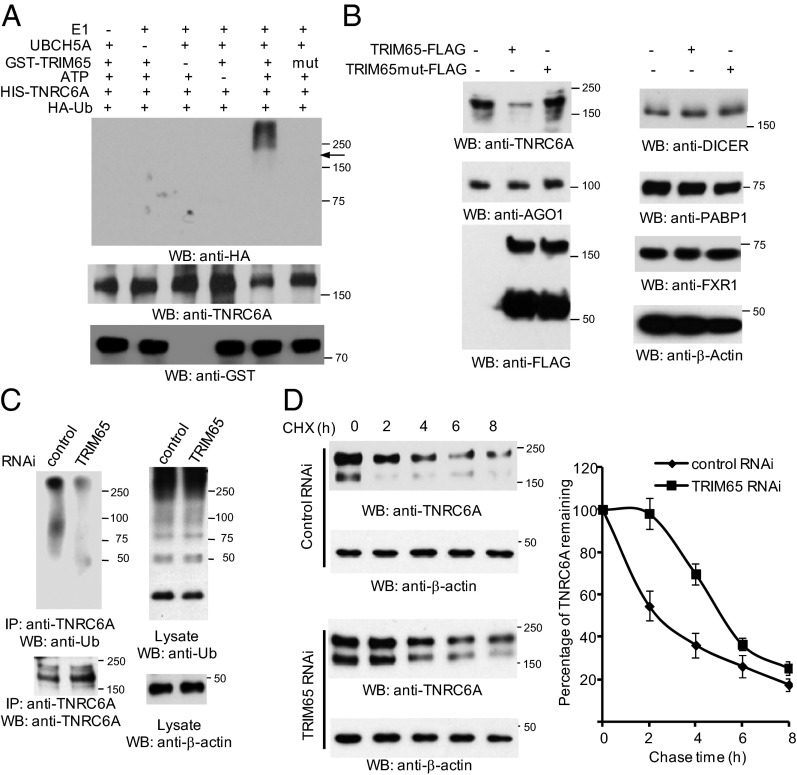
We hypothesized that TNRC6 proteins were substrates of the TRIM65 E3 ubiquitin ligase. In vitro ubiquitination assays demonstrate bacterial-derived TRIM65-GST effectively delivers ubiquitin to TNRC6A. In contrast, the TRIM65 ligase mutant containing a disrupted RING domain failed to induce ubiquitination (Fig. 3A). To examine the role of TRIM65 in the ubiquitination of endogenous TNRC6A, TRIM65 and its ligase-defective mutant were transfected into HeLa cells treated with the MG132 proteasome inhibitor. As reported previously (25), TNRC6A was constitutively ubiquitinated, which may be due to endogenous TRIM65 activity (Fig. S6A). Overexpression of TRIM65 enhanced the in vivo ubiquitination of TNRC6A, whereas the TRIM65 mutant had no substantial effect on TNRC6A ubiquitination (Fig. S6A).
Fig. 3.
TRIM65 regulates ubiquitination and stability of TNRC6. (A) In vitro ubiquitination of TNRC6A by TRIM65 plus E1, E2 (UBCH5A, also known as UBE2D1), ATP, and HA-Ub. GST-tagged TRIM65 and mutant TRIM65 were purified from bacteria. His-tagged TNRC6A protein was purified from HEK 293 cells through denatured washings. After an in vitro ubiquitination reaction, the mixture was incubated with nickel resins. The resins were washed under denatured conditions, and eluates were blotted with anti-HA antibody. The arrow indicates approximate TNRC6A position. (B) TRIM65 and the ligase mutant were transfected into HeLa cells. Cells were collected and blotted with the indicated antibodies. (C) Control and TRIM65 siRNA (RNAi sequence #4, see Supporting Information) were transfected into HeLa cells. Knockdown efficiency is shown in Fig. S2E. Cells were treated with 10 μM MG132 for 4 h before harvest. Cell lysates were immunoprecipitated with anti-TNRC6A antibody and immunoblotted as indicated. (D) TRIM65 siRNA and control siRNA were transfected into HEK 293 cells. After 48 h, cells were equally aliquoted into 24-well plates and treated with 50 μg/mL cycloheximide (CHX). Whole-cell lysates were harvested at the indicated times. Quantitative analysis of Western blots by densitometry is shown in the graph. Values are means ± SD of data from three separate experiments.
We next investigated the effect of TRIM65 overexpression on the stability of TNRC6 proteins. Consistently, TRIM65 induced endogenous TNRC6A protein degradation in HeLa cells when proteasome inhibitor was absent. In contrast, TNRC6A protein levels showed no substantial change when cells were transfected with the TRIM65 ligase mutant (Fig. 3B). TNRC6B or TNRC6C protein levels were also reduced when coexpressed with TRIM65 in HEK 293 cells (Fig. S6B). The effects on TNRC6 were specific, because the levels of other proteins in the RISC machinery were not affected (Fig. 3B). To corroborate the role of TRIM65 in the ubiquitination of endogenous TNRC6A, we used siRNA to knock down TRIM65. TNRC6A ubiquitination was reduced after silencing TRIM65 (Fig. 3C). To assess the role of the proteasome in TNRC6A degradation, cells were transfected with control vector or TRIM65 and MG132 was added for various times. Accumulation of endogenous TNRC6A was observed after addition of MG132 in the presence or absence of exogenous TRIM65 (Fig. S6C). Next, cells were treated with the translation inhibitor cycloheximide to examine the role of TRIM65 in TNRC6A stability further. After TRIM65 knockdown, TNRC6A t1/2 was prolonged (Fig. 3D). The effect of TRIM65 knockdown on TNRC6 protein levels appears specific, because the levels of other RISC components are unchanged (Fig. S6D). To determine the effects of TRIM65 protein on TNRC6A and AGO2 mRNA expression, we examined relative mRNA expression levels after TRIM65 overexpression or depletion. Neither overexpression nor knockdown of TRIM65 regulated TNRC6A and AGO2 mRNA expression (Fig. S6E). Taken together, the data indicate TRIM65 is a cognate ubiquitin E3 ligase that selectively targets TNRC6 for ubiquitination and subsequent protein degradation.
TRIM65 Relieves miRNA-Mediated mRNA Repression.
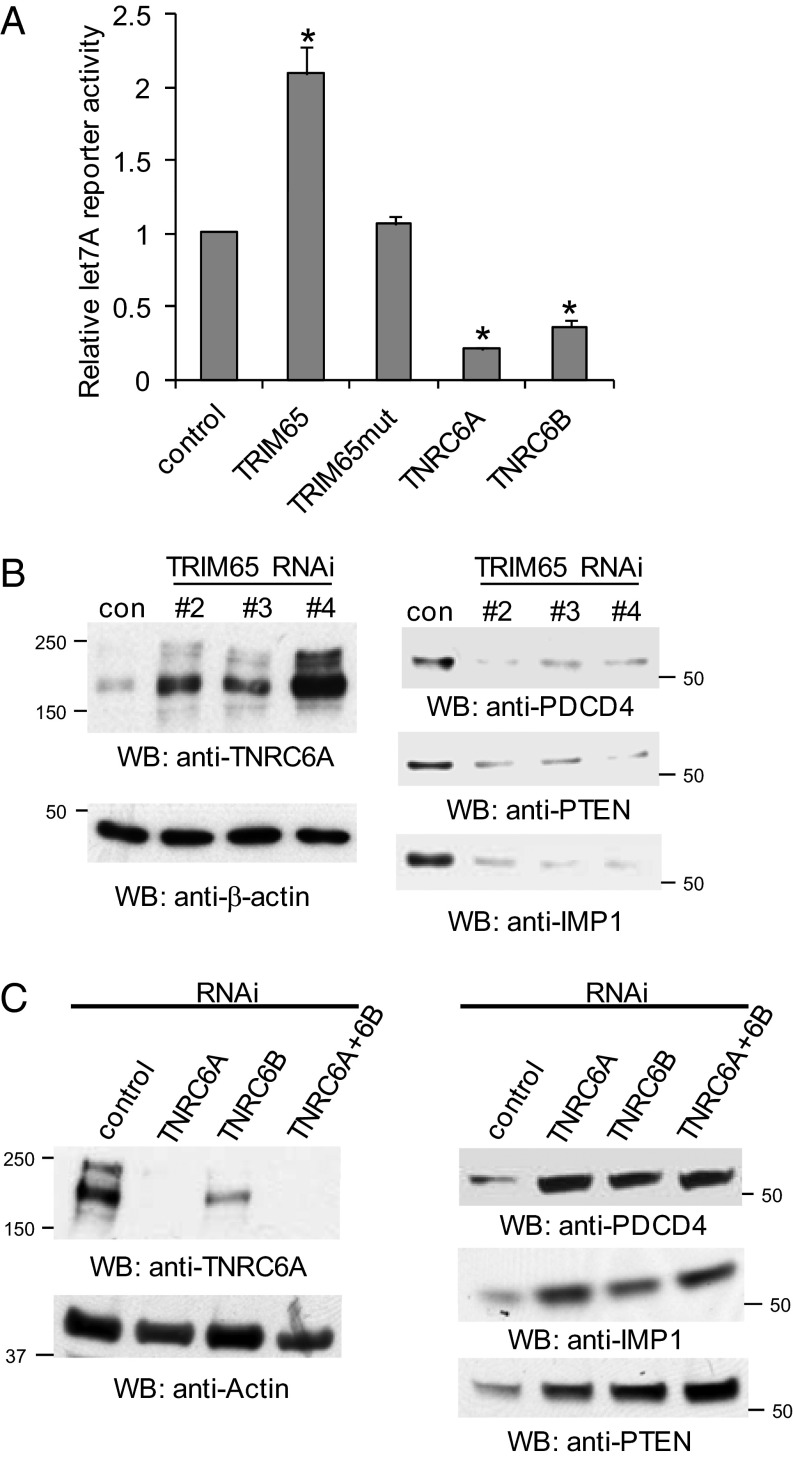
We next examined the effect of overexpression of TRIM65 on let-7a miRNA reporter activity. Ectopic expression of wild-type TRIM65, but not the mutant, increased reporter activity (Fig. 4A). Because TNRC6 degradation is associated with increased miRNA reporter activity, we reasoned that overexpression of TNRC6 would have the opposite effect. As predicted, overexpression of TNRC6A and TNRC6B decreased reporter activity (Fig. 4A). We next assessed the effect of TRIM65 depletion on endogenous miRNA-targeted mRNA expression. Three siRNAs were used to silence TRIM65 (Fig. S2E). Two miR-21–targeted genes, PDCD4 and PTEN (26, 27), and one let-7–targeted gene, IMP1 (28), were examined after knockdown of TRIM65. Depletion of TRIM65 resulted in increased TNRC6A protein levels and concomitant reduction of PDCD4, PTEN, and IMP1 protein levels (Fig. 4B). Real-time PCR quantification was used to monitor transcription rates after Actinomycin D treatment. The data suggest mRNA t1/2 is not altered by silencing TRIM65 (Fig. S7 A–D). Conversely, knockdown of TNRC6A and/or TNRC6B leads to increases of PDCD4, PTEN, and IMP1 expression (Fig. 4C and Fig. S7E). Collectively, the data suggest that TRIM65 ubiquitination of TNRC6 results in TNRC6 degradation, and consequently relieves miRNA-mediated translational repression.
Fig. 4.
TRIM65 relieves miRNA repression. (A) TNRC6A, TNRC6B, TRIM65, and its mutant were transfected into HeLa cells along with let-7a Renilla luciferase reporter and control firefly luciferase. Data depict the mean ± SD of triplicate samples. *P < 0.05. (B) Knockdown of TRIM65 represses endogenous miRNA targets at the protein level. Control and three validated TRIM65 siRNAs (Fig. S2E) were transfected into HeLa cells. TNRC6A, PTEN, IMP1, PDCD4, and actin protein levels were examined by Western blot. (C) Depletion of TNRC6A and/or TNRC6B relieved the suppression of miRNA-targeted gene expression. Control and TNRC6A and/or TNRC6B siRNAs were transfected into HeLa cells. TNRC6A, PTEN, IMP1, PDCD4, and actin protein levels were examined by Western blot. The efficiency of TNRC6B RNAi is illustrated in Fig. S7E.
Discussion
Here, we provide an extensive map describing a physical and functional protein interaction network associated with miRNA biogenesis and regulation. AP-MS was used to assemble a network of 499 unique interactions between 40 baits and 363 HCIPs. AP-MS has been widely used to map protein interaction networks of mammalian signaling pathways. As with any screening approach, the results must be interpreted with care and validated with more rigid experiments. About 11% of proteins (40 of 363) in the MPI harbor RNA-binding domains. Therefore, some protein interactions may depend on RNA to bridge or stabilize protein complexes (29). Conversely, some protein interactions may go undetected by AP-MS because of low levels of prey expression or cell-type dependence. Interactions may also be omitted due to the high stringency of our algorithm, which is designed to help exclude NSBPs. Although ribosomal proteins are known to be involved in the miRNA pathway (30), they are filtered out from the MPI because of pervasive associations with RNA-binding proteins. Thus, the MPI does not represent a complete interaction network. However, it is important to emphasize that 89% of the MPI interactions were not previously reported, including six genes with demonstrated functional activity in regulating miRNA activity. Thus, the MPI represents a resource for the study of molecular mechanisms controlling miRNA biogenesis or regulation and offers opportunities to integrate the miRNA silencing machinery with other cellular processes.
TNRC6 proteins are critical cofactors of AGO proteins and serve as key components of the RISC silencing machinery. The molecular mechanisms by which TNRC6 proteins contribute to translational repression remain incompletely understood. Furthermore, little is known about how TNRC6 proteins are regulated. It has been shown that TNRC6A undergoes ubiquitination and inhibition of HSP90 activity diminishes TNRC6A levels (25, 31), but the link between TNRC6A ubiquitination and proteasome-mediated degradation has not been established. We demonstrate that TRIM65 regulates TNRC6 activity at the posttranscriptional level by targeting TNRC6 proteins for ubiquitination and subsequent degradation. We conclude that TRIM65 is an ubiquitin E3 ligase for TNRC6 based on several lines of evidence: TRIM65 associates with TNRC6 through its CC domain; TRIM65 cosediments and colocalizes with TNRC6 proteins in P-body–like structures; TRIM65 is able to ubiquitinate TNRC6 in vitro and in vivo; overexpression of TRIM65, but not its ligase-deficient mutant, leads to TNRC6 degradation and let-7a reporter induction; and depletion of TRIM65 stabilizes and increases TNRC6A protein levels, and consequently enhances miRNA-mediated suppression of endogenous gene expression. Overall, TRIM65 acts as an E3 ligase for TNRC6 ubiquitination and degradation.
TNRC6 proteins participate in the downstream effector steps controlling miRNA-mediated gene silencing (32, 33). Despite TNRC6 redundancy, depleting TNRC6A, TNRC6B, or TNRC6C partially relieves the silencing of miRNA targets in human cells (11, 13, 14, 19, 34, 35). TNRC6 proteins bridge and interact with RISC components through distinct domains. Their aminoterminal domain binds to AGO proteins, whereas a bipartite silencing domain in the TNRC6 middle and carboxyl-terminal regions interacts with PABP1. TNRC6 presumably promotes translational repression and mRNA degradation by coupling AGO proteins with the PABP1 and CCR4/NOT deadenylase complexes. TRIM65 degradation of TNRC6 proteins does not result in noticeable degradation of other components in the RISC. Although we, as well as others, have noted constitutive ubiquitination of TNRC6 (25), the physiologically relevant cues that regulate TNRC6 protein stability and TRIM65 E3 ligase activity remain enigmatic.
Here, we demonstrate a central role for the E3 ligase TRIM65 in regulating miRNA activity. Apart genome-wide association studies demonstrating that dementia-associated, age-related white matter lesions of the brain are linked to a locus near TRIM65 (36, 37), little has been reported about TRIM65 function. To our knowledge, this is the first report demonstrating TRIM65 E3 ligase activity. TRIM65 targeted TNRC6 proteins for ubiquitin-dependent degradation, releasing miRNA-mediated mRNA repression. However, such activity must be carefully regulated to permit physiological miRNA function. The molecular mechanisms underlying TRIM65 E3 ligase function are incompletely understood. The roles of TRIM65 phosphorylation or oligomerization in regulating TNRC6 functional activity remain under further investigation, because such modifications have the potential for positively or negatively regulating the miRNA pathway. An understanding of the signals and mechanisms that control TNRC6 homeostasis and the interrelationship with TRIM65 E3 ligase activity is needed for improved understanding of miRNA in health and disease.
TRIM proteins represent one of the oldest and largest families of ubiquitin E3 ligases. In mammals, TRIM32 ubiquitinates PIASy, leading to its relocalization to the P-body (38). TRIM32 also directly associates with AGO proteins and activates miRNAs (39). The Caenorhabditis elegans TRIM-NHL protein (NHL-2) localizes to the core RISC in the P-body and functions as a cofactor to enhance miRNA-mediated gene silencing (40). In contrast to the above positive regulators, TRIM71 (also known as Lin41) associates with and ubiquitinates AGO proteins (41). However, the functional consequences of TRIM71 ubiquitination of AGO are unclear (41–44). Other TRIM proteins that interact with AGOs to regulate miRNAs include the Drosophila TRIM members Mei-P26 and BRAT (45). We now identify an additional TRIM protein critical for regulating miRNA function. Given the fundamental importance of miRNA-guided regulation of gene expression in nearly all cellular pathways, it is not surprising that multiple layers of miRNA control exist. TRIM65 serves as a rheostat for modulating TNRC6 stability, and thereby provides a mechanism for regulating miRNA biology.
Materials and Methods
Cell Lines and Constructs.
HEK 293, HeLa, and A549 cells were purchased from the American Type Culture Collection. To establish stable cell lines, bait cDNAs were transfected into HEK 293 cells using Lipofectamine 2000 (Invitrogen). Two days after transfection, cells were treated with hygromycin or puromycin for 14 d. Single colonies were picked and expanded in six-well plates. Western blotting was used to select clones for AP-MS. The DROSHA stable HEK 293 cell line was a gift from R. Gregory (Children’s Hospital, Boston).
Human TRIM65 was cloned from T98G glioblastoma cells (American Type Culture Collection). The first and second Cys (Cys12 and Cys15) in the TRIM65-RING domain were mutated to Ala to destroy E3 ligase activity. AGO2-HA-FLAG, TNRC6A-HA-FLAG, TNRC6B-HA-FLAG, TNRC6C-HA-FLAG, and DGCR8-HA-FLAG were kind gifts from T. Tuschl (The Rockefeller University, New York, NY). The let-7a reporter (let7 A) and DICER plasmids were generously provided by P. Sharp (Massachusetts Institute of Technology, Cambridge, MA) and P. Provost (Université Laval, Quebec City, Canada), respectively. Affinity-purified Flag eluates revealed the dominant presence of bait protein by MS analysis.
Affinity Protein Purification.
A total of 2 × 108 cells were lysed in 10 mL of lysis TAP buffer [50 mM Tris⋅HCl (pH 7.5), 10 mM MgCl2, 100 mM NaCl, 0.5% Nonidet P-40, 10% (vol/vol) glycerol, phosphatase inhibitors, and protease inhibitors]. Supernatants were collected and precleared with 50 μL of protein A/G resin. After shaking for 1 h at 4 °C, resin was removed by centrifugation. Cell lysates were added to 20 μL of anti-FLAG M2 resin (Sigma) and incubated on a shaker. After 12 h, anti-FLAG M2 resin was washed three times (total of 15 min) with 10 mL of TAP buffer. After removing the wash buffer, the resin was transferred to a spin column (Sigma) and incubated with 40 μL of 3× FLAG peptide (Sigma) for 1 h at 4 °C on a shaker. Eluates were collected by centrifugation and stored at −80 °C.
RNAi Perturbation.
Dharmacon siRNA oligos were transfected using Lipofectamine 2000 according to the manufacturer’s protocol.
Statistics.
The experimental data were grouped by prey, followed by a calculation of mean total spectral count and SD for all bait–prey associations. The results were used to derive a z-score for each bait–prey interaction. The z-scores were then used to derive a P value for each interaction using the pnorm function of R, which calculates the P value by integrating over the area of the normal distribution from negative infinity to the z-score.
Supplementary Material
Acknowledgments
We thank Drs. Phillip Sharp (Massachusetts Institute of Technology), Thomas Tuschl (The Rockefeller University), Narry Kim (Seoul National University), and Patrick Provost (Université Laval) for constructs; Richard Gregory (Children’s Hospital, Boston) for the DROSHA HEK 293 cell line; and the Taplin Mass Spectrometry Facility (Harvard University) for services. S.L. is a John and Virginia Kaneb fellow. This work was supported by National Institutes of Health Grants AI089829 and AI099860.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322545111/-/DCSupplemental.
References
- 1.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czech B, Hannon GJ. Small RNA sorting: Matchmaking for Argonautes. Nat Rev Genet. 2011;12(1):19–31. doi: 10.1038/nrg2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghildiyal M, Zamore PD. Small silencing RNAs: An expanding universe. Nat Rev Genet. 2009;10(2):94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han J, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125(5):887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 6.Gregory RI, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432(7014):235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 7.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 8.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303(5654):95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 9.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karginov FV, et al. Diverse endonucleolytic cleavage sites in the mammalian transcriptome depend upon microRNAs, Drosha, and additional nucleases. Mol Cell. 2010;38(6):781–788. doi: 10.1016/j.molcel.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meister G, et al. Identification of novel argonaute-associated proteins. Curr Biol. 2005;15(23):2149–2155. doi: 10.1016/j.cub.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 12.Shin C, et al. Expanding the microRNA targeting code: Functional sites with centered pairing. Mol Cell. 2010;38(6):789–802. doi: 10.1016/j.molcel.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakymiw A, et al. Disruption of GW bodies impairs mammalian RNA interference. Nat Cell Biol. 2005;7(12):1267–1274. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, et al. A role for the P-body component GW182 in microRNA function. Nat Cell Biol. 2005;7(12):1261–1266. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behm-Ansmant I, et al. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20(14):1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 17.Chendrimada TP, et al. MicroRNA silencing through RISC recruitment of eIF6. Nature. 2007;447(7146):823–828. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- 18.Haase AD, et al. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6(10):961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landthaler M, et al. Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. RNA. 2008;14(12):2580–2596. doi: 10.1261/rna.1351608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Wang L, Berman M, Kong YY, Dorf ME. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity. 2011;35(3):426–440. doi: 10.1016/j.immuni.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18(5):504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höck J, et al. Proteomic and functional analysis of Argonaute-containing mRNA-protein complexes in human cells. EMBO Rep. 2007;8(11):1052–1060. doi: 10.1038/sj.embor.7401088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eystathioy T, et al. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol Biol Cell. 2002;13(4):1338–1351. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11(9):1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 26.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 27.Talotta F, et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. 2009;28(1):73–84. doi: 10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
- 28.Boyerinas B, et al. Identification of let-7-regulated oncofetal genes. Cancer Res. 2008;68(8):2587–2591. doi: 10.1158/0008-5472.CAN-08-0264. [DOI] [PubMed] [Google Scholar]
- 29.Frohn A, et al. Dicer-dependent and -independent Argonaute2 protein interaction networks in mammalian cells. Mol Cell Proteomics. 2012;11(11):1442–1456. doi: 10.1074/mcp.M112.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janas MM, et al. Reduced expression of ribosomal proteins relieves microRNA-mediated repression. Mol Cell. 2012;46(2):171–186. doi: 10.1016/j.molcel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Johnston M, Geoffroy MC, Sobala A, Hay R, Hutvagner G. HSP90 protein stabilizes unloaded argonaute complexes and microscopic P-bodies in human cells. Mol Biol Cell. 2010;21(9):1462–1469. doi: 10.1091/mbc.E09-10-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eulalio A, Tritschler F, Izaurralde E. The GW182 protein family in animal cells: New insights into domains required for miRNA-mediated gene silencing. RNA. 2009;15(8):1433–1442. doi: 10.1261/rna.1703809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyoshi K, Okada TN, Siomi H, Siomi MC. Characterization of the miRNA-RISC loading complex and miRNA-RISC formed in the Drosophila miRNA pathway. RNA. 2009;15(7):1282–1291. doi: 10.1261/rna.1541209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu CY, Rana TM. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 2006;4(7):e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zipprich JT, Bhattacharyya S, Mathys H, Filipowicz W. Importance of the C-terminal domain of the human GW182 protein TNRC6C for translational repression. RNA. 2009;15(5):781–793. doi: 10.1261/rna.1448009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fornage M, et al. Genome-wide association studies of cerebral white matter lesion burden: The CHARGE consortium. Ann Neurol. 2011;69(6):928–939. doi: 10.1002/ana.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freudenberger P, Schmidt R, Schmidt H. Genetics of age-related white matter lesions from linkage to genome wide association studies. J Neurol Sci. 2012;322(1-2):82–86. doi: 10.1016/j.jns.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albor A, et al. The interaction of Piasy with Trim32, an E3-ubiquitin ligase mutated in limb-girdle muscular dystrophy type 2H, promotes Piasy degradation and regulates UVB-induced keratinocyte apoptosis through NFkappaB. J Biol Chem. 2006;281(35):25850–25866. doi: 10.1074/jbc.M601655200. [DOI] [PubMed] [Google Scholar]
- 39.Schwamborn JC, Berezikov E, Knoblich JA. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell. 2009;136(5):913–925. doi: 10.1016/j.cell.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammell CM, Lubin I, Boag PR, Blackwell TK, Ambros V. nhl-2 Modulates microRNA activity in Caenorhabditis elegans. Cell. 2009;136(5):926–938. doi: 10.1016/j.cell.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rybak A, et al. The let-7 target gene mouse lin-41 is a stem cell specific E3 ubiquitin ligase for the miRNA pathway protein Ago2. Nat Cell Biol. 2009;11(12):1411–1420. doi: 10.1038/ncb1987. [DOI] [PubMed] [Google Scholar]
- 42.Chang HM, et al. Trim71 cooperates with microRNAs to repress Cdkn1a expression and promote embryonic stem cell proliferation. Nat Commun. 2012;3:923. doi: 10.1038/ncomms1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Lai F, Niswander L. The ubiquitin ligase mLin41 temporally promotes neural progenitor cell maintenance through FGF signaling. Genes Dev. 2012;26(8):803–815. doi: 10.1101/gad.187641.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loedige I, Gaidatzis D, Sack R, Meister G, Filipowicz W. The mammalian TRIM-NHL protein TRIM71/LIN-41 is a repressor of mRNA function. Nucleic Acids Res. 2013;41(1):518–532. doi: 10.1093/nar/gks1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumüller RA, et al. Mei-P26 regulates microRNAs and cell growth in the Drosophila ovarian stem cell lineage. Nature. 2008;454(7201):241–245. doi: 10.1038/nature07014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.