Significance
Accurate gene expression requires that transcription of DNA into RNA terminate in the correct location. In bacteria, many transcription termination events rely on the termination factor Rho. Rho-dependent terminators perform critical housekeeping functions in the cell and also regulate expression of specific genes. However, the DNA and RNA sequences that promote Rho-dependent termination are poorly understood, particularly for those terminators with regulatory functions. We now define the sequence elements required for transcription termination at a regulated Rho-dependent terminator. These sequences cause RNA polymerase to pause for an unusually long time during transcription. The remarkable longevity of this pause is required for a regulatory signal to stimulate Rho-dependent termination and thereby govern expression of the gene downstream of the pause site.
Keywords: magnesium, RNA polymerase
Abstract
Up to half of all transcription termination events in bacteria rely on the RNA-dependent helicase Rho. However, the nucleic acid sequences that promote Rho-dependent termination remain poorly characterized. Defining the molecular determinants that confer Rho-dependent termination is especially important for understanding how such terminators can be regulated in response to specific signals. Here, we identify an extraordinarily long-lived pause at the site where Rho terminates transcription in the 5′-leader region of the Mg2+ transporter gene mgtA in Salmonella enterica. We dissect the sequence elements required for prolonged pausing in the mgtA leader and establish that the remarkable longevity of this pause is required for a riboswitch to stimulate Rho-dependent termination in the mgtA leader region in response to Mg2+ availability. Unlike Rho-dependent terminators described previously, where termination occurs at multiple pause sites, there is a single site of transcription termination directed by Rho in the mgtA leader. Our data suggest that Rho-dependent termination events that are subject to regulation may require elements distinct from those operating at constitutive Rho-dependent terminators.
Transcription termination is critical for accurate expression and regulation of genes. Bacteria use two types of transcription terminators: intrinsic terminators, which dissociate transcription complexes in the absence of auxiliary proteins, and factor-dependent terminators, which require the action of the RNA-dependent helicase Rho (1). Rho is responsible for 20–50% of termination events in Escherichia coli (2, 3) and performs an array of important functions in bacteria. In addition to terminating transcription at the ends of operons (4), Rho directs transcription termination within coding regions when translation slows or stalls thereby establishing transcriptional polarity (5), silences horizontally acquired DNA (3, 6), protects the chromosome from R-loops and double-strand breaks (7–9), and suppresses pervasive antisense transcription (10).
Aside from these genome-wide “housekeeping” functions, Rho has also been recruited to regulate the expression of specific genes in response to signals (11). Long-established examples include a Rho-dependent terminator that controls expression of the E. coli tnaA gene in response to tryptophan availability and terminators that regulate rho mRNA levels in response to the concentration of Rho protein (12–15). The recent identification of two riboswitches and a small regulatory RNA (sRNA) that control gene expression by modulating Rho-dependent termination (11, 16, 17) has now refocused attention on Rho as a regulatory factor. However, it is unclear whether regulated Rho-dependent terminators differ from those performing housekeeping functions.
Rho-dependent terminators consist of two elements: a Rho utilization (rut) site in the nascent RNA and a downstream sequence that induces pausing of RNA polymerase (RNAP). The ATPase activity of Rho is stimulated upon binding to a rut site, which drives 5′-to-3′ translocation of Rho along the RNA, and thereby permits Rho to establish interactions with RNAP that result in termination of transcription. The RNAP pause site provides sufficient time for Rho to “catch up” with RNAP and terminate transcription in the correct location (1, 18). The sequence features of both the rut sites, which tend to be C-rich but lack a clear consensus sequence, and the pause sites associated with Rho-dependent terminators remain poorly defined. Understanding of regulated Rho-dependent terminators is especially limited because the few Rho-dependent terminators that have been investigated in some detail are constitutive housekeeping terminators or strong terminators from bacteriophage (19–24). In addition, most work has focused on the rut site, with less attention paid to the molecular determinants of RNAP pausing in the context of Rho-dependent termination. Here, we dissect the sequence components that promote an unusual pause required for a regulated Rho-dependent termination event.
Expression of the Mg2+ transporter gene mgtA from Salmonella enterica serovar Typhimurium is governed by a Mg2+-sensing riboswitch that functions by modulating the activity of Rho (17, 25). The 5′-leader region of the mgtA mRNA responds to intracellular levels of Mg2+ by adopting one of two mutually exclusive conformations (25) (Fig. 1A). High Mg2+ favors an RNA conformation (i.e., stem-loops A + B) that permits Rho to interact with the nascent RNA and terminate transcription within the mgtA leader. By contrast, in the conformer fostered under low Mg2+ conditions (i.e., stem-loop C), sequences required for Rho binding are sequestered, which prevents Rho from terminating transcription and enables transcription to continue into the mgtA coding region (17). Therefore, the mgtA riboswitch differs from previously described transcriptionally acting riboswitches, which typically respond to ligand binding by forming an intrinsic transcription terminator (26).
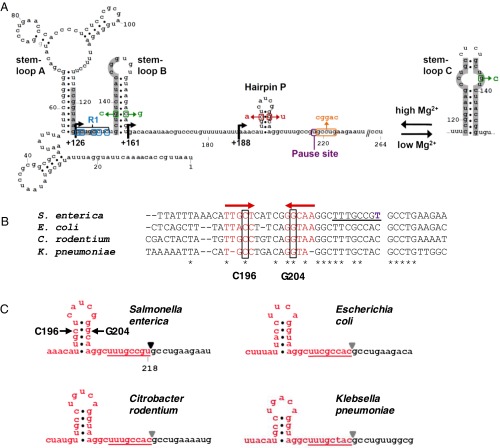
Fig. 1.
Sequence and structure of the mgtA leader mRNA. (A) Schematic illustrates the two mutually exclusive RNA conformations that can be adopted by the Salmonella mgtA leader. Stem-loops A and B are promoted at high Mg2+ whereas low Mg2+ favors the stem-loop C conformation. Sequences involved in alternate base pairing in response to Mg2+ are shaded gray. Bent arrows indicate the locations of deletions from the 5′ end of the leader that were used in this study. The position of the long, Mg2+-sensitive pause is indicated in purple. The locations of the G138C and C157G mutations are shown in green, and those of the C196A and G204T mutations are shown in red. The mutated nucleotides downstream of the pause site are shown in orange, and those in the R1 region are shown in blue. (B) Alignment of the mgtA leader sequence around the T218 pause from Salmonella with the corresponding mgtA leader sequences from E. coli strain MG1655, Citrobacter rodentium strain ICC168, and Klebsiella pneumoniae strain 342. T218 is shown in purple in the Salmonella sequence, and the locations of C196 and G204 are boxed. Red arrows indicate sequences that are predicted to form a hairpin upstream of the pause site, and the sequence predicted to form the 8-bp RNA–DNA hybrid in a transcription elongation complex paused at T218 is underlined. (C) Predicted secondary structure of the mRNA upstream of the T218 pause in the Salmonella mgtA leader and homologous sequences in the mgtA leaders from E. coli, C. rodentium, and K. pneumoniae. Secondary structure predictions were generated by entering the Salmonella mgtA leader sequence from +1 to +218 (or corresponding sequences from the other bacteria) into mfold (http://mfold.rna.albany.edu/?q=mfold). Triangles (▼) indicate the location of the RNA 3′ end in a transcription elongation complex paused at the position corresponding to T218 in the Salmonella mgtA leader, and the sequence predicted to form the RNA–DNA hybrid in this paused complex is underlined.
In common with other Rho-dependent terminators, Rho promotes transcription termination at an RNAP pause site in the mgtA leader (17). We now report that this pause differs from any pause described to date in that (i) it is remarkably long-lived both in vitro and in vivo, and (ii) its duration is longer in high Mg2+ conditions than in low Mg2+ conditions in vitro. We define sequence elements that are required for prolonged Mg2+-sensitive pausing and show that an extended pause is required for high Mg2+ to promote Rho-dependent transcription termination in the mgtA leader region in vivo. Our results establish previously unidentified molecular features that play a central role in enabling gene regulation via Rho-dependent termination.
Results and Discussion
RNAP Pauses for an Unusually Long Time in the mgtA Leader in Vivo.
Rho-dependent transcription termination takes place at a site in the mgtA leader where RNAP pauses in the absence of Rho (17). In vitro studies revealed that this pause is located ∼220 nt downstream from the transcription start site and that it is atypically long-lived (17). To determine whether RNAP pauses at this location in vivo, we carried out KMnO4 footprinting experiments in WT Salmonella harboring a plasmid-borne fusion of the mgtA leader to the lacZ coding region, driven by the constitutive plac1–6 promoter (27). We chose this construct because it has been characterized extensively in vivo (17, 25) and because Mg2+ does not regulate the plac1–6 promoter.
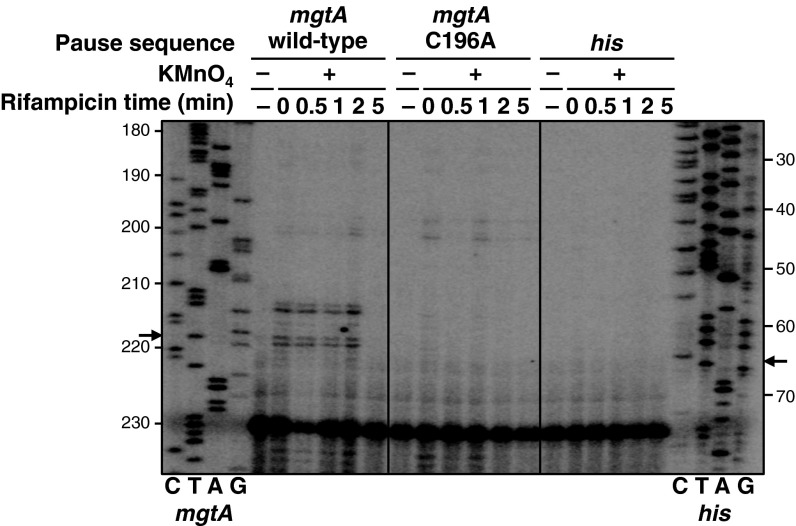
KMnO4 enables detection of DNA melted inside a transcription bubble because it preferentially modifies nucleotides (particularly T residues) in single-stranded regions of DNA. Such modifications are more readily observed when RNAP is paused, providing additional time for the melted DNA to react with KMnO4 (28). We detected modification of T212, T213, T218, and G219 in the nontemplate strand of the mgtA leader DNA after treatment with KMnO4 in vivo (Fig. 2). These results are in agreement with the pause location determined in vitro (17, 25). A strong pause signal was still detected 2 min after treatment with rifampicin, an antibiotic that inhibits reinitiation of transcription. By contrast, no KMnO4 reactivity was observed around the well-characterized his pause site (29) in bacteria harboring a plasmid with a plac1–6-driven fusion of the his pause sequence to a promoterless lacZ gene (Fig. 2). This lack of KMnO4 reactivity is likely because the his pause is too short-lived to be detected by this in vivo assay. Our data indicate that the duration of the mgtA pause is much longer than that of the his pause and, to our knowledge, of other natural bacterial pauses that have been detected in vivo (30–33).
Fig. 2.
The mgtA leader contains an unusually long-lived pause that can be detected in vivo. A primer extension analysis of plasmid DNA extracted from KMnO4-treated WT Salmonella 14028s harboring a plasmid with a plac1–6-driven fusion of either the WT mgtA leader (pYS1010), the C196A mutant mgtA leader (pYS10120), or the his pause sequence (pYS10138) to a promoterless lacZ gene was performed. Bacteria were treated with KMnO4 at the indicated times after addition of rifampicin to inhibit reinitiation of transcription. “CTAG” lanes represent DNA sequencing reactions generated using the same primer and plasmid pYS1010 (mgtA) or pYS10138 (his) as a template. Sequence positions are numbered with respect to the first nucleotide transcribed from plac1–6 (designated +1). Arrows indicate the location of the mgtA and his pause sites detected in vitro (Fig. 3 B and C).
High Mg2+ Extends the Duration of the Long-Lived Pause in the mgtA Leader.
To quantify the kinetic parameters of the mgtA pause, we carried out single-round in vitro transcription assays using a DNA template corresponding to the mgtA leader cloned downstream of the constitutive λ PR promoter and a C-less initial transcribed sequence (ITS) (Fig. 3A). This strategy, which has been used extensively to study transcriptional pauses in vitro (29), enabled us to synchronize transcription through the mgtA leader.
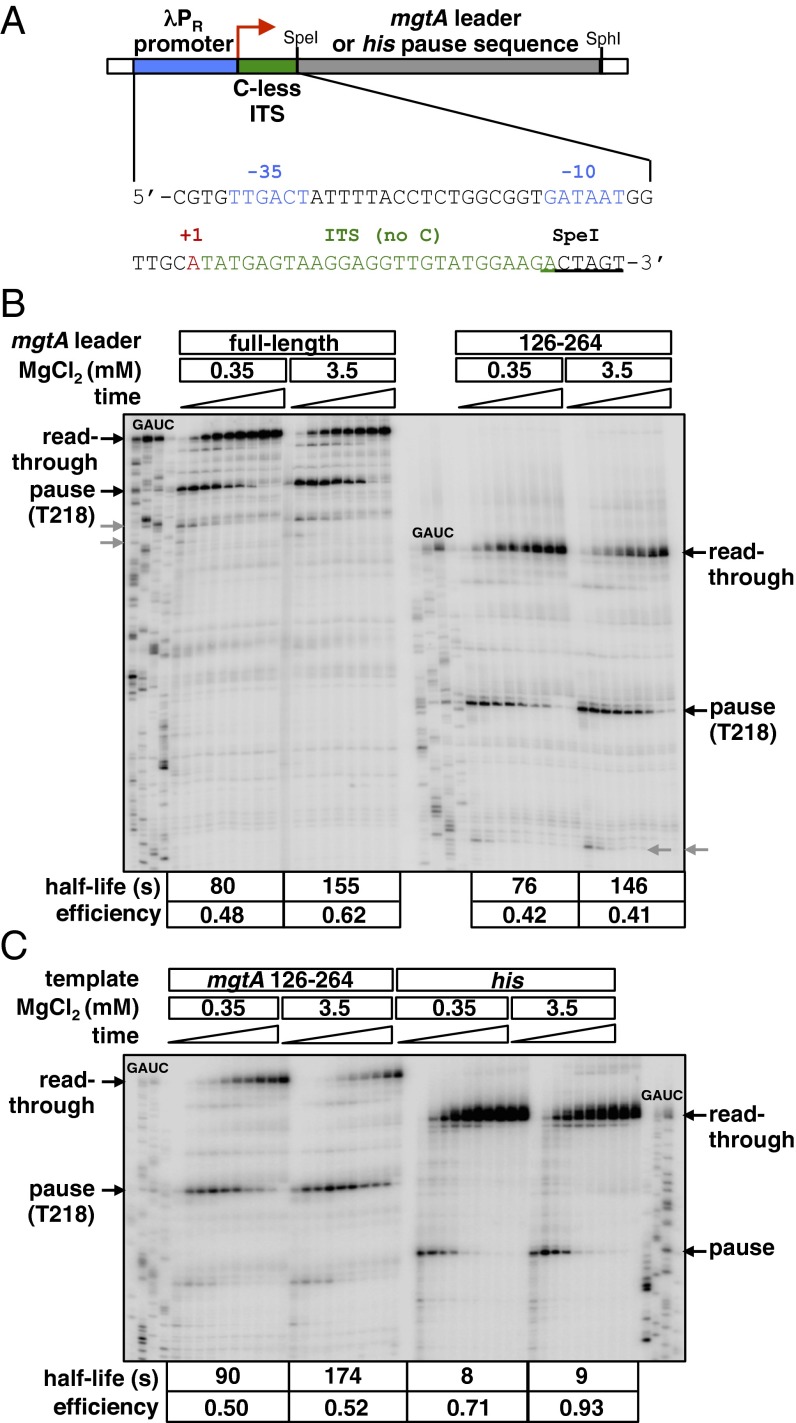
Fig. 3.
High Mg2+ extends the duration of the long-lived pause in the mgtA leader. (A) Schematic of the DNA templates used to synchronize transcription elongation complexes (ECs) for in vitro pause assays. The mgtA leader or his pause sequence was cloned downstream of the constitutive λ PR promoter (blue) and a 26-nt ITS that lacks C residues (green). Initiation of transcription in the absence of CTP generates ECs stalled after the ITS (EC26), and subsequent addition of all four NTPs permits transcription to continue into the downstream sequence. The transcription start site (+1) is indicated by the red arrow. The sequences of the λ PR promoter (with −10 and −35 promoter elements highlighted in blue) and C-less ITS are shown. (B) In vitro transcription of derivatives of the DNA template shown in A containing either the full-length mgtA leader or nucleotides 126–264 of the mgtA leader. Transcripts were analyzed 0.5, 1, 2, 3, 5, 7, 10, 30, and 60 min after addition of all four NTPs to EC26. Gels were calibrated using RNA sequencing reactions (GAUC). Black arrows indicate the positions of the Mg2+-sensitive long pause at T218 and of the read-through transcript. Gray arrows indicate shorter Mg2+-insensitive pauses upstream of the T218 pause. The half-life and efficiency of the pause at position T218 are shown for each condition. (C) In vitro transcription of derivatives of the DNA template shown in A containing either the mgtA leader sequence from positions +126 to +264 or the his pause sequence. Transcripts were analyzed 0.25, 0.5, 0.75, 1, 2, 3, 5, 7, 10, and 45 min after addition of all four NTPs to EC26. The half-life and efficiency of the pause at position T218 are shown for each condition.
We observed a long-lived pause at position T218 in the mgtA leader (Figs. 1A and 3B), which corresponds to the site where transcription terminates in the presence of Rho (17). This finding is consistent with our previous estimate of RNAP pausing near position 220 (17, 25). That the pause confers a single site of transcription termination (17) is a unique feature of Rho-dependent termination in the mgtA leader because Rho typically terminates transcription at clusters of tandem pause sites to generate RNA products with heterogeneous 3′ ends (34, 35).
Remarkably, high Mg2+ extended the half-life of the pause at T218: It was twice as long in reactions carried out at 3.5 mM Mg2+ as in those performed at 0.35 mM Mg2+ (Fig. 3B). This lengthening of the pause is particularly striking given that Mg2+ is required for RNAP to catalyze nucleotide addition. Therefore, one would expect high Mg2+ to have the opposite effect, that is, to favor a more rapid escape from the paused state rather than actually increasing the longevity of the pause. The effect of Mg2+ on RNAP pausing is distinct from its impact on alternate folding of the mgtA leader RNA because Mg2+-regulated pausing was still observed on a template in which nucleotides 1–125 of the mgtA leader were deleted (Fig. 3B). This truncated derivative of the mgtA leader is unable to switch between the stem-loop A + B and stem-loop C conformers (25). This conformational switch is required for Mg2+ to regulate Rho loading onto the RNA (17) (Fig. 1A).
The long duration and Mg2+ sensitivity are distinct characteristics of the mgtA pause at position T218 because the half-life of the well-characterized his pause was 11- and 19-fold shorter than the mgtA pause in low and high Mg2+, respectively, and because the his pause did not respond to Mg2+ under the same assay conditions (Fig. 3C). In addition, Mg2+ did not affect the duration of a number of shorter pauses observed in the mgtA leader (Fig. 3B). In contrast to its effect on pause duration, Mg2+ had only a minor effect on the efficiency of the T218 pause (i.e., the fraction of RNAP molecules that enter the paused state), which was similar to the effect of Mg2+ on the efficiency of the his pause (Fig. 3 B and C).
Taken together, these data indicate that the mgtA leader contains a pause that is distinctive because (i) it is unusually long-lived, (ii) its duration is increased in high Mg2+, and (iii) it confers a single site of transcription termination in the presence of Rho.
RNA Sequences >50 nt Upstream of T218 Are Required for High Mg2+ to Prolong Pausing in the mgtA Leader.
Pausing occurs when the transcription elongation complex undergoes conformational changes that disrupt the normal nucleotide addition cycle. Pausing can be induced by sequence elements in the DNA template, the nascent RNA transcript, and/or by regulatory proteins that contact the elongation complex (36). Because the long, Mg2+-sensitive pause in the mgtA leader can be detected in the absence of auxiliary proteins in vitro (Fig. 3B), it must be promoted by DNA or RNA sequences in the leader itself. Thus, we sought to identify sequence determinants responsible for this unusual pause.
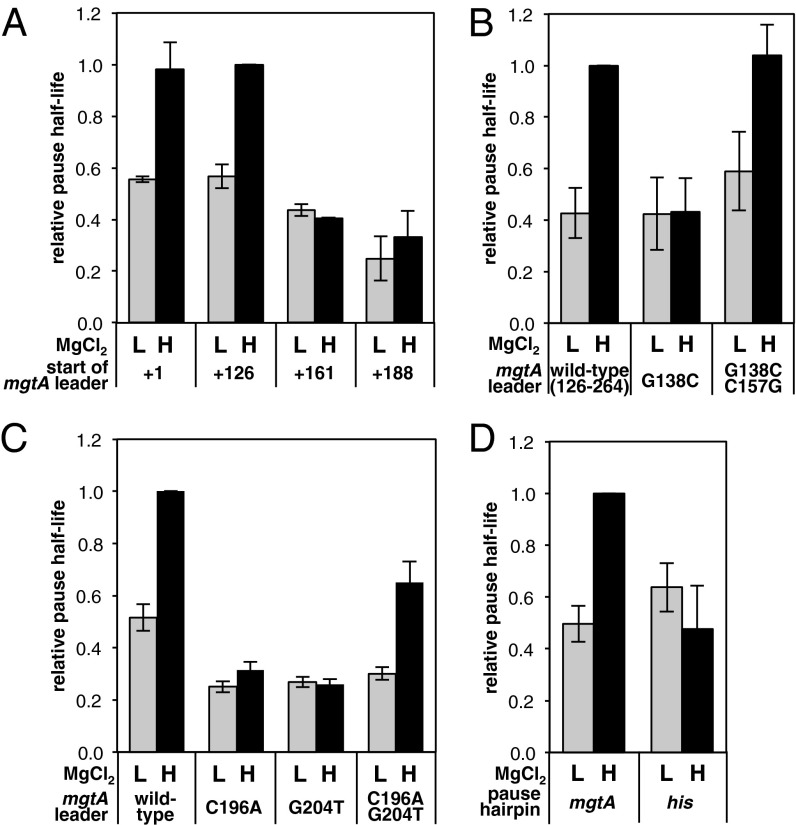
We began by making a series of deletions from the start of the mgtA leader to investigate whether upstream sequences have an impact on the pause (Fig. 1A). Pausing in vitro was unaffected by deletion of nucleotides 1–125 (Figs. 3B and 4A). However, deletion of nucleotides 1–160 reduced the half-life of the pause 2.5-fold in high Mg2+ but had little effect in low Mg2+, resulting in a similar half-life under both low and high Mg2+ conditions (Fig. 4A and Fig. S1A). Deletion of nucleotides 1–187 resulted in a small additional decrease in pause duration (Fig. 4A and Fig. S1A). These results suggest that some sequence between positions 126 and 160 is required for Mg2+-sensitive pausing in the mgtA leader. This region harbors a sequence previously shown to adopt an RNA stem-loop (stem-loop B) in high Mg2+ conditions (25) (Fig. 1A). We determined that stem-loop B is required for high Mg2+ to promote extended pausing in the mgtA leader because a G138C substitution predicted to disrupt stem-loop B halved the pause half-life in high Mg2+ conditions but had no effect in low Mg2+ conditions (Fig. 4B and Fig. S1B). Furthermore, incorporation of a compensatory C157G mutation that restores the ability to form stem-loop B reestablished the long-lived pause in high Mg2+ conditions (Fig. 4B and Fig. S1B). The mutations in stem-loop B affected the T218 pause specifically because they had little impact on the shorter Mg2+-insensitive pauses earlier in the mgtA leader (Fig. S1B).
Fig. 4.
RNA sequences in the mgtA leader are required for long, Mg2+-sensitive pausing. (A) Relative half-life of the T218 pause measured after in vitro transcription of DNA templates containing nucleotides 1–264, 126–264, 161–264, or 188–264 of the mgtA leader at low (L, 0.35 mM) or high (H, 3.5 mM) MgCl2. The half-lives were normalized to the half-life measured using the nucleotide 126–264 template at high Mg2+. Data are averages from at least three independent experiments, and error bars show the SD. A representative gel from these experiments is shown in Fig. S1A. (B) Relative half-life of the T218 pause measured after in vitro transcription of DNA templates containing nucleotides 126–264 of either the WT mgtA leader or the mgtA leader harboring the indicated substitutions in stem-loop B at low (0.35 mM) or high (3.5 mM) MgCl2. Pause half-lives were normalized to the half-life measured using the WT template at high Mg2+. Data are averages from at least three independent experiments, and error bars show the SD. A representative gel from these experiments is shown in Fig. S1B. (C) Relative half-life of the T218 pause measured after in vitro transcription of DNA templates containing either the full-length WT mgtA leader or derivatives harboring the indicated substitutions in hairpin P, at low (0.35 mM) or high (3.5 mM) MgCl2. Pause half-lives were normalized to the half-life measured using the WT template at high Mg2+. Data are averages from three independent experiments, and error bars show the SD. A representative gel from these experiments is shown in Fig. S1C. (D) Relative half-life of the T218 pause measured after in vitro transcription of DNA templates containing nucleotides 126–264 of either the WT mgtA leader or a derivative in which hairpin P was replaced with the corresponding hairpin from the his pause. Pause half-lives were normalized to the half-life measured using the WT template at high Mg2+. Data are averages from three independent experiments, and error bars show the SD. A representative gel from these experiments is shown in Fig. S1D.
These data indicate that stem-loop B, and possibly other sequences >57 nt upstream of the pause site, promote extended pausing in the mgtA leader in high Mg2+. To our knowledge, this is the first time that RNA this far upstream has been found to stimulate pausing. RNA elements in the putL transcript from bacteriophage HK022 have been shown to bind RNAP and to influence its elongation properties. However, in this case, the nascent RNA suppresses, rather than promotes, pausing at a downstream site (37, 38). It is notable that in the case of putL, formation of a stem-loop is also required for the nascent RNA to affect pausing.
Sequences Downstream of the mgtA Pause Are Necessary for Entry into the Paused State.
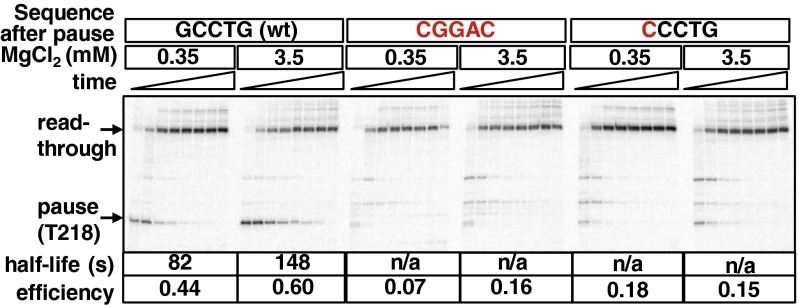
The first step in pausing involves rearrangement of the RNAP active site into a conformation termed the “elemental pause state” in which incorporation of the incoming nucleotide into the 3′ end of the transcript is hindered (36, 39–41). Because sequences downstream of the pause site can have an impact on entry into the elemental pause state (42–44), we analyzed the effect of mutations downstream of T218 on pausing in the mgtA leader in vitro. Consistent with this region promoting entry into the paused state, mutation of the GCCTG sequence from positions 219–223, which is strikingly conserved among the mgtA leader regions from different enterobacteria (Fig. 1B), virtually eliminated pausing at T218 (Fig. 5). The effect of these mutations appears to be due primarily to the G-to-C substitution at position 219, because a G219C substitution alone was sufficient to reduce the pause efficiency drastically in vitro (Fig. 5). This result is in agreement with previous findings at other pause sites where the identity of the incoming nucleotide is an important determinant of entry into the elemental pause state (40, 45).
Fig. 5.
Sequences downstream of the pause are required for entry into the paused state. In vitro transcription of derivatives of DNA templates containing either the full-length WT mgtA leader or derivatives with the GCCTG sequence from +219 to +223 mutated to CGGAC, or with a single G-to-C substitution at position 219. Transcripts were analyzed 0.5, 1, 2, 3, 5, 7, 10, and 45 min after addition of all four NTPs to EC26. Arrows indicate positions of the Mg2+-sensitive pause at T218 and of the read-through transcript. The efficiency of the pause at position T218 is shown for each condition, together with the pause half-life for the WT template. n/a, not applicable.
An RNA Hairpin Is Required to Stabilize Paused RNAP in both Low and High Mg2+.
Once RNAP enters the elemental pause state, the transcription elongation complex may undergo additional reorganization to generate pauses that are more stable (36). One way in which this pause stabilization can be achieved is by formation of a hairpin in the nascent RNA (45–49). We noticed that the mgtA leader RNA has the potential to form a hairpin with a 5-bp stem located 11 nt upstream of the T218 pause site (Fig. 1 B and C). Moreover, the ability to form a hairpin in this region of the mgtA leader RNA is conserved among other enterobacteria (Fig. 1 B and C). We termed this hairpin “hairpin P.”
To examine the role of hairpin P, we monitored the effect of nucleotide substitutions anticipated to disrupt its formation. Nucleotide C196 is predicted to base-pair with G204 in the stem of hairpin P (Fig. 1A). A C196A substitution disrupts pausing at T218 in vivo because KMnO4 reactivity at positions T212, T213, T218, and G219 was lost in the C196A mutant (Fig. 2). In vitro, the C196A substitution reduced the half-life of the T218 pause twofold in low Mg2+and threefold in high Mg2+, whereas the G204T mutation decreased the half-life two- and fourfold in low and high Mg2+, respectively (Fig. 4C and Fig. S1C). In both the C196A and G204T mutants, high Mg2+ was unable to extend the duration of the pause (Fig. 4C and Fig. S1C). Mg2+-sensitive pausing was restored in a C196A G204T double mutant that regained the capacity to form hairpin P (Fig. 4C and Fig. S1C). The C196A and G204T mutations (individually and in combination) interfered with pausing at T218 specifically because they did not have an impact on a short pause earlier in the mgtA leader (Fig. S1C). These results indicate that formation of hairpin P is required for stabilization of the pause in both low and high Mg2+, and also for high Mg2+ to extend the pause duration.
The configuration of the mgtA pause sequence resembles that of other well-characterized hairpin-stabilized pauses (45–49). In particular, it is strikingly similar to that of the his pause, which also consists of a 5-nt hairpin located 11 nt upstream of the pause site (46–48). Given this likeness, we investigated whether the unusual properties of the mgtA pause might depend on particular features of hairpin P or whether hairpin P could be substituted with an alternative pause hairpin. Hence, we monitored transcription using DNA templates in which the nucleotides specifying hairpin P were replaced with those encoding the his pause hairpin. Replacement of hairpin P with the his pause hairpin reduced the half-life of the pause under high Mg2+ conditions but had no effect in low Mg2+ (Fig. 4D and Fig. S1D). This result indicates that some distinctive feature of hairpin P in particular, and not just any pause-stabilizing hairpin, is required for high Mg2+ to prolong pausing in the mgtA leader.
A Model for Prolonged, Mg2+-Sensitive Pausing in the mgtA Leader.
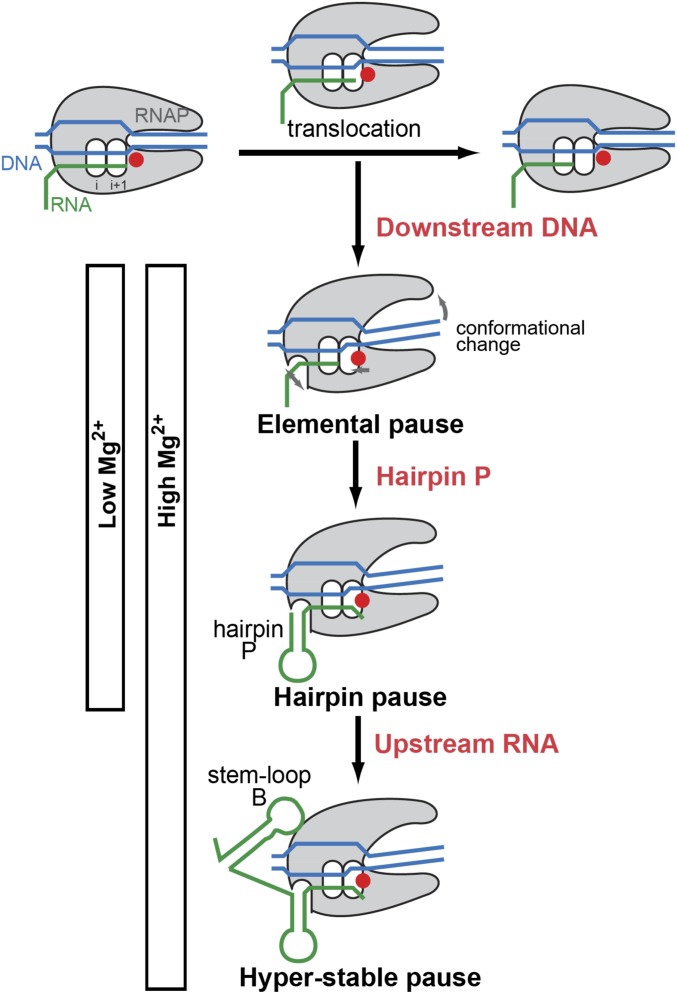
We suggest that transcription elongation through the mgtA leader region is disrupted by entry of the transcription elongation complex into an elemental pause state, which is then stabilized by formation of hairpin P in the nascent RNA (Fig. 6). These events likely occur in low and high Mg2+ because the pause takes place and is as stable as other hairpin-stabilized pauses in both conditions (Fig. 3C). In this regard, the initial stages of pausing in the mgtA leader are similar to those described for other hairpin-stabilized pauses (36). However, the mgtA pause appears to be able to achieve an even longer-lived state that we term a “hyperstable” pause (Fig. 6). This state does not represent an “arrested” transcription complex because RNAP can eventually escape the paused state and resume transcript elongation in the absence of other protein factors (Fig. 3B). The hyperstable paused state appears to be attained more readily when Mg2+ is high, resulting in a remarkably long pause (Fig. 6).
Fig. 6.
Model for the mechanism of prolonged, Mg2+-sensitive pausing in the mgtA leader. During transcription elongation, incorporation of a nucleotide into the 3′ end of the nascent RNA is followed by downstream translocation of RNAP to free up the active center for addition of the next nucleotide. Pausing occurs when the transcription elongation complex (TEC) undergoes conformational changes that disrupt this normal nucleotide addition cycle. In the mgtA leader, downstream DNA sequences induce conformational changes in the TEC that inhibit nucleotide addition, causing RNAP to enter an “elemental pause” state at position T218. This elemental pause state is then stabilized by formation of hairpin P in the nascent RNA. Formation of the elemental and hairpin-stabilized pause states appears to occur in both low and high Mg2+ conditions, and is similar to other hairpin-stabilized pauses. However, in high Mg2+ conditions, upstream RNA sequences (including, but possibly not limited to, stem-loop B) promote a unique “hyperstable” pause in the mgtA leader that is substantially longer-lived than any pause described previously. Here, stem-loop B is shown interacting directly with RNAP, but it might also stabilize the pause by interacting with other RNA sequences involved in pausing. Modified from Weixlbaumer et al. (41).
Our data suggest that distinct regions of the mgtA leader sequence enforce different stages of this pausing pathway (Fig. 6). In common with other pause sites (40, 45), the sequence immediately downstream of T218 (particularly the G at position 219) appears to promote entry of RNAP into the elemental paused state because mutations that alter this sequence essentially abolish pausing at T218 (Fig. 5). Formation of hairpin P in the nascent RNA furthers initial stabilization of the pause because mutations that disrupt hairpin P reduced the overall pause duration in both low and high Mg2+ conditions (Fig. 4C and Fig. S1C). This finding also echoes the role of RNA hairpins at other hairpin-stabilized pauses, such as those in the leader regions of the Salmonella his, E. coli trp, and Bacillus subtilis trp operons, where mutations that disrupt hairpin formation also reduce pause longevity (45, 48, 49). However, in contrast to these other pauses, RNA sequences upstream of hairpin P (particularly stem-loop B) appear to drive hyperstablization of the mgtA pause in high Mg2+ conditions because mutations that disrupt stem-loop B prevented high Mg2+ from extending the pause duration but had no effect in low Mg2+ (Fig. 4B and Fig. S1B). Hyperstabilization also appears to require particular sequences in hairpin P because replacement of this hairpin with that from the his pause specifically reduced pausing in high Mg2+ (Fig. 4D and Fig. S1D).
Stem-loop B and hairpin P may bring about hyperstablization of the pause in high Mg2+ in a variety of nonmutually exclusive ways. For instance, Mg2+ may have an impact on the ability of these RNA elements to interact with RNAP in ways that stabilize the pause. Alternatively or in addition, Mg2+ may control the capacity of these RNA sequences to interact with each other and/or with other pause-inducing signals in the RNA. In either of these scenarios, Mg2+ could act either by stabilizing RNA–protein or RNA–RNA interactions directly, or by altering the conformational state of the RNA to favor or hinder such contacts. These conformational changes would differ from the stem-loop A + B vs. stem-loop C switch previously implicated in controlling Rho loading (17) because the pause remains Mg2+-sensitive even in templates where this transition cannot take place (Fig. 3B).
One way in which stem-loop B and hairpin P could hyperstabilize the pause is via a “kissing” interaction between their respective loops. Indeed, examination of the Salmonella mgtA leader sequence suggests potential base-pairing interactions that could occur between the loops of stem-loop B and hairpin P (Fig. S2A). However, such an interaction is unlikely to explain prolonged, Mg2+-sensitive pausing in the mgtA leader because the putative interaction is not conserved in other enterobacteria (25) (Fig. 1B) and also because mutations in the loops of stem-loop B or hairpin P predicted to disrupt such an interaction had little effect on transcription (Fig. S2 B and C). Thus, alternative RNA–protein or RNA–RNA interactions are likely required to achieve hyperstable pausing in the mgtA leader.
Prolonged Pausing Is Required for High Mg2+ to Promote Rho-Dependent Termination in the mgtA Leader Region in Vivo.
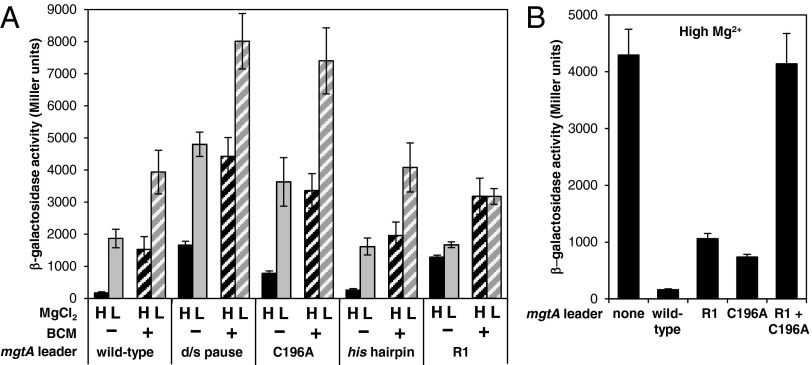
Because the T218 pause corresponds exactly to the site where transcription terminates in the presence of Rho, and because Rho-dependent termination at this site is controlled by a Mg2+-sensing riboswitch (17), we hypothesized that the unusual features of this pause might contribute to the ability of the mgtA riboswitch to control transcription elongation into the associated coding region. To test this notion, we examined the effect of mutations that disrupt pausing on the β-gal activity expressed by WT Salmonella harboring a plasmid-borne transcriptional fusion between the full-length mgtA leader and lacZ (driven by the plac1–6 promoter) after growth at high or low Mg2+ in the absence or presence of the Rho-specific inhibitor bicyclomycin (BCM) (50).
First, mutations downstream of the pause site that prevent entry into the paused state (Fig. 5) enhanced β-gal activity 10-fold in high Mg2+, a condition that represses transcription elongation through the WT mgtA leader (Fig. 7A). The effect of these mutations is most pronounced in cells grown in high Mg2+ because they exerted a smaller (less than threefold) derepression effect in low Mg2+ conditions (Fig. 7A). Moreover, it is dependent on a functional Rho protein because the mutations resulted in only approximately twofold derepression when bacteria were grown in the presence of BCM (Fig. 7A). [Note that it was necessary to use a BCM concentration that allowed some limited Rho-dependent transcription termination because complete inhibition of Rho is lethal (51).] These data indicate that pausing per se is required for high Mg2+ to promote Rho-dependent termination in the mgtA leader.
Fig. 7.
A long-lived pause is required for high Mg2+ to promote Rho-dependent termination in the mgtA leader in vivo. (A) β-Gal activity expressed by WT Salmonella 14028s harboring a plasmid with a plac1–6-driven fusion of the mgtA leader to a promoterless lacZ gene (pYS1010) or derivatives of pYS1010 with the GCCTG sequence from +219 to +223 mutated to CGGAC (“d/s pause”; pYS10150), the C196A substitution (pYS10120), the mgtA pause hairpin substituted for that from the his pause (“his hairpin”; pYS10176), or mutations in the “R1” region (pYS10116). Bacteria were grown in N-minimal medium with 10 μM (L) or 10 mM (H) MgCl2 in the absence or presence of BCM. Data shown are averages from at least three independent experiments, and error bars represent the SD. (B) β-Gal activity expressed by WT Salmonella 14028s harboring a plasmid with a plac1–6-driven fusion of the mgtA leader to lacZ (pYS1010) or derivatives of pYS1010 containing mutations in the R1 region (pYS10116), the C196A substitution (pYS10120), or both (pYS10128). The term “none” corresponds to data for bacteria harboring the control plasmid pYS1000, which contains the plac1–6-driven lacZ fusion without the mgtA leader. Bacteria were grown in N-minimal medium with 10 mM MgCl2. Data shown are averages from four independent experiments, and error bars represent the SD.
It is noteworthy that the derepression effect caused by mutations that prevent entry into the pause is at least as great as that exerted by mutations in the “R1 region” (Figs. 1A and 7A), which reduce the ability of the RNA to interact with Rho but do not have an impact on pausing (17). Thus, pausing appears to be just as critical as Rho loading for Rho to terminate transcription in the mgtA leader.
Second, the C196A mutation, which reduces the pause duration in both low and high Mg2+ (Fig. 4C), increased β-gal activity 4.5-fold relative to the WT mgtA leader when bacteria were grown in high Mg2+ conditions but exerted a smaller (approximately twofold) effect in low Mg2+ and/or when Rho was inhibited by treatment with BCM (Fig. 7A). These results indicate that C196A specifically prevents high Mg2+ from promoting Rho-dependent termination in the mgtA leader. Conceivably, the effect of the C196A substitution could be ascribed to an effect on the ability of the RNA to interact with Rho, but this is not the case because an mgtA leader RNA with the C196A substitution retained a WT ability to stimulate Rho’s ATPase activity in vitro (Fig. S3). This notion is further supported by the finding that the C196A substitution acts synergistically with mutations in the R1 region [which hinder Rho loading but do not have an impact on pausing (16)], thereby abolishing termination in the mgtA leader in high Mg2+ (Fig. 7B).
Finally, replacement of hairpin P with the hairpin from the his pause, which prevents hyperstabilization of the pause in high Mg2+ in vitro (Fig. 4D), did not have an impact on transcription through the mgtA leader in vivo under the tested conditions (Fig. 7A). This result suggests that although a long pause is required for high Mg2+ to promote Rho-dependent termination in the mgtA leader, additional hyperstabilization of the pause by high Mg2+ is not. It remains possible, however, that Mg2+ sensing by the pause affects gene expression under conditions other than those tested here.
Concluding Remarks.
Most Rho-dependent terminators characterized to date either terminate transcription at the ends of operons and therefore are constitutively active (e.g., the trp t′ terminator downstream of the E. coli trp operon), or participate in bacteriophage development and thus act in an “all or none” manner (e.g., the tR1 terminator from phage lambda) (19–24). By contrast, for Rho-dependent terminators to regulate gene expression in response to signals, their activities must be “fine-tuned” in response to fluctuating signal levels. Thus, they likely rely on molecular determinants distinct from those described for constitutive Rho-dependent terminators. We have conducted what is, to our knowledge, the first detailed molecular dissection of a pause site that participates in a regulated Rho-dependent termination event. We have established that this pause is remarkably long-lived and that the extraordinary longevity of the pause is required for high Mg2+ to promote Rho-dependent termination in the mgtA leader region.
Why should regulation of Rho-dependent termination in the mgtA leader rely on such a long-lived pause? It does not appear to be a requirement imposed by regulation of Rho-dependent termination by a riboswitch per se because there is no evidence of such a long pause in the 5′-leader region of the E. coli ribB transcript, which contains an flavin-mononucleotide–sensing riboswitch that also acts on Rho-dependent termination (17). Instead, we envision several nonmutually exclusive explanations. First, prolonged pausing may grant additional time for sensing of Mg2+ by the mgtA riboswitch aptamer. Thus, the mgtA riboswitch could potentially be driven thermodynamically (i.e., by the affinity of the riboswitch RNA for Mg2+). This potential for thermodynamic regulation is in contrast to most transcriptionally acting riboswitches, which tend to be driven by the kinetic competition between the rates of ligand binding and transcription elongation (52, 53). Second, the long pause may provide additional time for Rho to load onto the mgtA leader RNA. This RNA is likely a suboptimal substrate for Rho because it is highly structured, whereas Rho typically binds to RNAs that are fairly single-stranded (1, 18). Thus, by providing a longer time window over which the RNA can sense Mg2+ and Rho loading onto the RNA can be modulated, the long pause may act together with slow Rho loading to exert precise regulation over Rho-dependent termination.
Third, the long pause may provide time for sensing of additional signals by the mgtA leader RNA. For instance, the mgtA leader contains a short, proline codon-rich ORF, termed mgtL, the translation of which results in transcription termination in the mgtA leader by favoring the formation of stem-loop B (and thus, presumably promoting Rho loading) (54, 55). Proline limitation is thought to induce ribosome stalling at the proline codons in mgtL, which favors stem-loop C and permits transcription elongation into the mgtA coding region. The Mg2+ and proline signals act synergistically on the mgtA leader RNA (54) and appear to influence the same Rho-dependent termination event even though translation of mgtL would occlude sequences required for Mg2+ sensing by the riboswitch (25). Prolonged pausing may allow time for mgtL translation to complete, thereby freeing up the riboswitch RNA to detect intracellular Mg2+ levels before the transcription termination decision must be made.
The extraordinary longevity of the mgtA pause may also be responsible for Rho directing transcription termination at a single site. This unorthodox behavior is in contrast to Rho-dependent terminators described previously, where termination typically takes place at a series of weaker pause sites spread over a region of up to ∼100 nt (34, 35). A possible explanation for the single Rho-dependent termination site in the mgtA leader is evoked by the finding that a truncated RNA generated by riboswitch-directed transcription termination may function in trans as an sRNA (56). Thus, if the product of transcription termination in the mgtA leader were to act as an sRNA (52), it might require that termination generate a single, defined RNA product rather than a collection of RNA molecules with heterogeneous 3′ ends.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
Bacterial strains and plasmids used in this study are listed in Table S1, and oligonucleotide primers are listed in Table S2. Details of plasmid constructions are described in SI Materials and Methods. Bacteria were grown in either N-minimal medium (pH 7.4) (57) supplemented with 0.1% casamino acids, 38 mM glycerol, and the indicated concentration of MgCl2, or in M9 medium (58) containing the indicated concentration of MgSO4. Chloramphenicol (20 μg/mL) was added for growth of strains harboring derivatives of plasmid pYS1000.
In Vivo KMnO4 Footprinting.
In vivo KMnO4 footprinting was carried out by following a published protocol (28) with some modifications. Bacteria were grown overnight in M9 medium containing 1 mM MgSO4, washed twice in M9 medium lacking MgSO4, and inoculated 1:100 into fresh M9 medium containing 10 μM MgSO4. (Note that bacteria were grown in M9 medium instead of N-minimal medium because N-minimal medium appeared to quench permanganate.) Cultures were incubated for 3 h at 37 °C (OD600 ∼ 0.4), and 12-mL samples were then treated with 10 mM KMnO4 for 2 min at 37 °C. To monitor the pause duration, cultures were incubated with rifampicin at 0.2 mg/mL for the indicated time (0.5–5 min) before addition of KMnO4. After treatment with KMnO4, cultures were immediately transferred to prechilled centrifuge tubes and placed on ice. Cells were harvested by centrifugation, washed in 0.9% NaCl, and pelleted again. Plasmid DNA was isolated from cell pellets using the boiling method (59). After precipitation with isopropanol, DNA pellets were resuspended in 600 μL of Tris⋅EDTA (TE) buffer and treated with 1 μL of 20 mg/mL RNaseA (Life Technologies) for 20 min at 37 °C. Plasmid DNA was then extracted once with phenol, three to four times with phenol/chloroform/isoamyl alcohol [25:24:1 (vol/vol/vol)], and once with chloroform/isoamyl alcohol [24:1 (vol/vol)] before precipitation with ethanol and resuspension in 30 μL of TE buffer.
For detection of KMnO4 modifications, primer extension reactions were carried out using 2 μg of plasmid DNA and 0.3–0.5 × 106 cpm of 32P end-labeled primer 12901 as described (28). After precipitation with ethanol, primer extension products were resuspended in 7 μL of gel loading dye [50 mM EDTA, 80% (vol/vol) deionized formamide, 0.1% bromophenol blue, and 0.1% xylene cyanol FF]. Samples were heated for 2 min at 90 °C and then analyzed on denaturing 6% (vol/vol) polyacrylamide sequencing gels (Ureagel; National Diagnostics). Gels were calibrated using DNA sequencing reactions generated using primer 12901 and the Sequenase 2.0 DNA sequencing kit (Affymetrix) according to the manufacturer’s instructions.
In Vitro Transcription.
To examine pausing, we adapted previously described single-round in vitro transcription assays (29). Linear DNA templates harboring the λ PR promoter and a 26-nt C-less ITS cloned upstream of WT or mutant derivatives of the mgtA leader or the his pause sequence (Fig. 3A) were generated by PCR using primers W174 and W175 and plasmid pKH100 or derivatives harboring mutations in the mgtA leader region (Table S1). We used previously described methods to purify E. coli core RNAP (60) and σ70 (61). RNAP holoenzyme was reconstituted by mixing core RNAP with a twofold molar excess of σ70 and incubating for 20 min at room temperature. Transcription elongation complexes stalled at the end of the ITS (EC26) were prepared by incubating 50 nM template DNA, 60 nM RNAP holoenzyme, 5 µM ATP, 5 µM UTP, 1 µM GTP, 0.1 µM ApU RNA primer, and 0.2 μCi/μL α32P-GTP in transcription buffer [100 mM KCl and 10 mM Tris⋅HCl (pH 7.9)] containing 5 mM MgCl2 and 1 U/μL SUPERaseIn (Life Technologies) for 15 min at 37 °C. Reaction mixtures were purified using Sephadex G-50 columns preequilibrated with transcription buffer lacking MgCl2, then the MgCl2 concentration was adjusted to 0.35 or 3.5 mM. Transcription was initiated by addition of all four NTPs to a final concentration of 50 μM and rifampicin to 8 μg/mL, followed by incubation at 37 °C. Samples were taken at various time points and mixed with an equal volume of stop solution [50 mM EDTA, 80% (vol/vol) deionized formamide, 0.1% bromophenol blue, and 0.1% xylene cyanol FF]. DNA samples were analyzed on denaturing 6% (vol/vol) polyacrylamide sequencing gels (Ureagel; National Diagostics) alongside RNA sequencing reactions prepared as described (62). Gels shown are representative of at least three independent experiments. Pause half-lives and efficiencies were estimated as described (29).
β-Gal Assays.
β-gal assays were performed as described (17).
Rho ATPase Assays.
RNA corresponding to WT or mutant derivatives of the mgtA leader was synthesized with the Megascript T7 kit (Ambion) according to the manufacturer’s instructions, using T7 promoter-driven templates generated by PCR with primers 6712 and 11882 and plasmid pYS1010 (WT), pYS10120 (C196A), or pYS10116 (R1 mutant) as a template. Rho ATPase activity in the presence of different RNA substrates was measured as described (17).
Supplementary Material
Acknowledgments
We thank Barbara Stitt for supplying purified Rho, Max Gottesman for providing BCM, and David Lee and Yixin Shi for plasmid constructions. We also thank Sergey Proshkin, Alex Yakhnin, and Paul Babitzke for technical advice regarding the in vivo KMnO4 footprinting experiments, Robert Landick for useful discussions, and Albert Weixlbaumer for providing the original drawing used as the basis for Fig. 6. This work was supported, in part, by National Institutes of Health Grant AI49561 (to E.A.G., who is an investigator of the Howard Hughes Medical Institute).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319193111/-/DCSupplemental.
References
- 1.Peters JM, Vangeloff AD, Landick R. Bacterial transcription terminators: The RNA 3′-end chronicles. J Mol Biol. 2011;412(5):793–813. doi: 10.1016/j.jmb.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters JM, et al. Rho directs widespread termination of intragenic and stable RNA transcription. Proc Natl Acad Sci USA. 2009;106(36):15406–15411. doi: 10.1073/pnas.0903846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardinale CJ, et al. Termination factor Rho and its cofactors NusA and NusG silence foreign DNA in E. coli. Science. 2008;320(5878):935–938. doi: 10.1126/science.1152763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts JW. Termination factor for RNA synthesis. Nature. 1969;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- 5.Richardson JP, Grimley C, Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci USA. 1975;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menouni R, Champ S, Espinosa L, Boudvillain M, Ansaldi M. Transcription termination controls prophage maintenance in Escherichia coli genomes. Proc Natl Acad Sci USA. 2013;110(35):14414–14419. doi: 10.1073/pnas.1303400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta D, Shatalin K, Epshtein V, Gottesman ME, Nudler E. Linking RNA polymerase backtracking to genome instability in E. coli. Cell. 2011;146(4):533–543. doi: 10.1016/j.cell.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leela JK, Syeda AH, Anupama K, Gowrishankar J. Rho-dependent transcription termination is essential to prevent excessive genome-wide R-loops in Escherichia coli. Proc Natl Acad Sci USA. 2013;110(1):258–263. doi: 10.1073/pnas.1213123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Washburn RS, Gottesman ME. Transcription termination maintains chromosome integrity. Proc Natl Acad Sci USA. 2011;108(2):792–797. doi: 10.1073/pnas.1009564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters JM, et al. Rho and NusG suppress pervasive antisense transcription in Escherichia coli. Genes Dev. 2012;26(23):2621–2633. doi: 10.1101/gad.196741.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudvillain M, Figueroa-Bossi N, Bossi L. Terminator still moving forward: Expanding roles for Rho factor. Curr Opin Microbiol. 2013;16(2):118–124. doi: 10.1016/j.mib.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Konan KV, Yanofsky C. Rho-dependent transcription termination in the tna operon of Escherichia coli: Roles of the boxA sequence and the rut site. J Bacteriol. 2000;182(14):3981–3988. doi: 10.1128/jb.182.14.3981-3988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart V, Landick R, Yanofsky C. Rho-dependent transcription termination in the tryptophanase operon leader region of Escherichia coli K-12. J Bacteriol. 1986;166(1):217–223. doi: 10.1128/jb.166.1.217-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanofsky C, Konan KV, Sarsero JP. Some novel transcription attenuation mechanisms used by bacteria. Biochimie. 1996;78(11-12):1017–1024. doi: 10.1016/s0300-9084(97)86725-9. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y, Shigesada K, Hirano M, Imai M. Autogenous regulation of the gene for transcription termination factor rho in Escherichia coli: Localization and function of its attenuators. J Bacteriol. 1986;166(3):945–958. doi: 10.1128/jb.166.3.945-958.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossi L, Schwartz A, Guillemardet B, Boudvillain M, Figueroa-Bossi N. A role for Rho-dependent polarity in gene regulation by a noncoding small RNA. Genes Dev. 2012;26(16):1864–1873. doi: 10.1101/gad.195412.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollands K, et al. Riboswitch control of Rho-dependent transcription termination. Proc Natl Acad Sci USA. 2012;109(14):5376–5381. doi: 10.1073/pnas.1112211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabhi M, Rahmouni AR, Boudvillain M. Transcription termination factor Rho: A ring-shaped RNA helicase from bacteria. In: Jankowsky E, editor. RNA Helicases. Royal Society of Chemistry, Cambridge, UK; 2010. pp. 243–271. [Google Scholar]
- 19.Chen CY, Richardson JP. Sequence elements essential for rho-dependent transcription termination at lambda tR1. J Biol Chem. 1987;262(23):11292–11299. [PubMed] [Google Scholar]
- 20.Ciampi MS. Rho-dependent terminators and transcription termination. Microbiology. 2006;152(Pt 9):2515–2528. doi: 10.1099/mic.0.28982-0. [DOI] [PubMed] [Google Scholar]
- 21.Graham JE, Richardson JP. rut Sites in the nascent transcript mediate Rho-dependent transcription termination in vivo. J Biol Chem. 1998;273(33):20764–20769. doi: 10.1074/jbc.273.33.20764. [DOI] [PubMed] [Google Scholar]
- 22.Lau LF, Roberts JW, Wu R. RNA polymerase pausing and transcript release at the lambda tR1 terminator in vitro. J Biol Chem. 1983;258(15):9391–9397. [PubMed] [Google Scholar]
- 23.Zalatan F, Galloway-Salvo J, Platt T. Deletion analysis of the Escherichia coli rho-dependent transcription terminator trp t′. J Biol Chem. 1993;268(23):17051–17056. [PubMed] [Google Scholar]
- 24.Zhu AQ, von Hippel PH. Rho-dependent termination within the trp t′ terminator. I. Effects of rho loading and template sequence. Biochemistry. 1998;37(32):11202–11214. doi: 10.1021/bi9729110. [DOI] [PubMed] [Google Scholar]
- 25.Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg(2+) Cell. 2006;125(1):71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 26.Henkin TM. Riboswitch RNAs: Using RNA to sense cellular metabolism. Genes Dev. 2008;22(24):3383–3390. doi: 10.1101/gad.1747308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M, Tolstorukov M, Zhurkin V, Garges S, Adhya S. A mutant spacer sequence between -35 and -10 elements makes the Plac promoter hyperactive and cAMP receptor protein-independent. Proc Natl Acad Sci USA. 2004;101(18):6911–6916. doi: 10.1073/pnas.0401929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasse-Dwight S, Gralla JD. Footprinting protein-DNA complexes in vivo. Methods Enzymol. 1991;208:146–168. doi: 10.1016/0076-6879(91)08012-7. [DOI] [PubMed] [Google Scholar]
- 29.Landick R, Wang D, Chan CL. Quantitative analysis of transcriptional pausing by Escherichia coli RNA polymerase: his leader pause site as paradigm. Methods Enzymol. 1996;274:334–353. doi: 10.1016/s0076-6879(96)74029-6. [DOI] [PubMed] [Google Scholar]
- 30.Hatoum A, Roberts J. Prevalence of RNA polymerase stalling at Escherichia coli promoters after open complex formation. Mol Microbiol. 2008;68(1):17–28. doi: 10.1111/j.1365-2958.2008.06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yakhnin AV, Yakhnin H, Babitzke P. RNA polymerase pausing regulates translation initiation by providing additional time for TRAP-RNA interaction. Mol Cell. 2006;24(4):547–557. doi: 10.1016/j.molcel.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Yakhnin AV, Yakhnin H, Babitzke P. Function of the Bacillus subtilis transcription elongation factor NusG in hairpin-dependent RNA polymerase pausing in the trp leader. Proc Natl Acad Sci USA. 2008;105(42):16131–16136. doi: 10.1073/pnas.0808842105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kainz M, Roberts JW. Kinetics of RNA polymerase initiation and pausing at the lambda late gene promoter in vivo. J Mol Biol. 1995;254(5):808–814. doi: 10.1006/jmbi.1995.0657. [DOI] [PubMed] [Google Scholar]
- 34.Lau LF, Roberts JW, Wu R. Transcription terminates at lambda tR1 in three clusters. Proc Natl Acad Sci USA. 1982;79(20):6171–6175. doi: 10.1073/pnas.79.20.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu AM, Christie GE, Platt T. Tandem termination sites in the tryptophan operon of Escherichia coli. Proc Natl Acad Sci USA. 1981;78(5):2913–2917. doi: 10.1073/pnas.78.5.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landick R. The regulatory roles and mechanism of transcriptional pausing. Biochem Soc Trans. 2006;34(Pt 6):1062–1066. doi: 10.1042/BST0341062. [DOI] [PubMed] [Google Scholar]
- 37.King RA, Banik-Maiti S, Jin DJ, Weisberg RA. Transcripts that increase the processivity and elongation rate of RNA polymerase. Cell. 1996;87(5):893–903. doi: 10.1016/s0092-8674(00)81996-0. [DOI] [PubMed] [Google Scholar]
- 38.Komissarova N, et al. Inhibition of a transcriptional pause by RNA anchoring to RNA polymerase. Mol Cell. 2008;31(5):683–694. doi: 10.1016/j.molcel.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbert KM, et al. Sequence-resolved detection of pausing by single RNA polymerase molecules. Cell. 2006;125(6):1083–1094. doi: 10.1016/j.cell.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kireeva ML, Kashlev M. Mechanism of sequence-specific pausing of bacterial RNA polymerase. Proc Natl Acad Sci USA. 2009;106(22):8900–8905. doi: 10.1073/pnas.0900407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weixlbaumer A, Leon K, Landick R, Darst SA. Structural basis of transcriptional pausing in bacteria. Cell. 2013;152(3):431–441. doi: 10.1016/j.cell.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan CL, Landick R. The Salmonella typhimurium his operon leader region contains an RNA hairpin-dependent transcription pause site. Mechanistic implications of the effect on pausing of altered RNA hairpins. J Biol Chem. 1989;264(34):20796–20804. [PubMed] [Google Scholar]
- 43.Lee DN, Phung L, Stewart J, Landick R. Transcription pausing by Escherichia coli RNA polymerase is modulated by downstream DNA sequences. J Biol Chem. 1990;265(25):15145–15153. [PubMed] [Google Scholar]
- 44.Levin JR, Chamberlin MJ. Mapping and characterization of transcriptional pause sites in the early genetic region of bacteriophage T7. J Mol Biol. 1987;196(1):61–84. doi: 10.1016/0022-2836(87)90511-0. [DOI] [PubMed] [Google Scholar]
- 45.Yakhnin AV, Babitzke P. Mechanism of NusG-stimulated pausing, hairpin-dependent pause site selection and intrinsic termination at overlapping pause and termination sites in the Bacillus subtilis trp leader. Mol Microbiol. 2010;76(3):690–705. doi: 10.1111/j.1365-2958.2010.07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Artsimovitch I, Landick R. Interaction of a nascent RNA structure with RNA polymerase is required for hairpin-dependent transcriptional pausing but not for transcript release. Genes Dev. 1998;12(19):3110–3122. doi: 10.1101/gad.12.19.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Artsimovitch I, Landick R. Pausing by bacterial RNA polymerase is mediated by mechanistically distinct classes of signals. Proc Natl Acad Sci USA. 2000;97(13):7090–7095. doi: 10.1073/pnas.97.13.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan CL, Landick R. Dissection of the his leader pause site by base substitution reveals a multipartite signal that includes a pause RNA hairpin. J Mol Biol. 1993;233(1):25–42. doi: 10.1006/jmbi.1993.1482. [DOI] [PubMed] [Google Scholar]
- 49.Landick R, Yanofsky C. Stability of an RNA secondary structure affects in vitro transcription pausing in the trp operon leader region. J Biol Chem. 1984;259(18):11550–11555. [PubMed] [Google Scholar]
- 50.Kohn H, Widger W. The molecular basis for the mode of action of bicyclomycin. Curr Drug Targets Infect Disord. 2005;5(3):273–295. doi: 10.2174/1568005054880136. [DOI] [PubMed] [Google Scholar]
- 51.Zwiefka A, Kohn H, Widger WR. Transcription termination factor rho: The site of bicyclomycin inhibition in Escherichia coli. Biochemistry. 1993;32(14):3564–3570. doi: 10.1021/bi00065a007. [DOI] [PubMed] [Google Scholar]
- 52.Coppins RL, Hall KB, Groisman EA. The intricate world of riboswitches. Curr Opin Microbiol. 2007;10(2):176–181. doi: 10.1016/j.mib.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wickiser JK, Winkler WC, Breaker RR, Crothers DM. The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol Cell. 2005;18(1):49–60. doi: 10.1016/j.molcel.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 54.Park SY, Cromie MJ, Lee EJ, Groisman EA. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell. 2010;142(5):737–748. doi: 10.1016/j.cell.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao G, Kong W, Weatherspoon-Griffin N, Clark-Curtiss J, Shi Y. Mg2+ facilitates leader peptide translation to induce riboswitch-mediated transcription termination. EMBO J. 2011;30(8):1485–1496. doi: 10.1038/emboj.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loh E, et al. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell. 2009;139(4):770–779. doi: 10.1016/j.cell.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 57.Snavely MD, Miller CG, Maguire ME. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991;266(2):815–823. [PubMed] [Google Scholar]
- 58.Elbing K, Brent R. Media preparation and bacteriological tools. Curr Protoc Mol Biol. 2002 doi: 10.1002/0471142727.mb0101s59. Chapter 1:Unit 1.1. [DOI] [PubMed] [Google Scholar]
- 59.Holmes DS, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 60.Belogurov GA, et al. Structural basis for converting a general transcription factor into an operon-specific virulence regulator. Mol Cell. 2007;26(1):117–129. doi: 10.1016/j.molcel.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grainger DC, Goldberg MD, Lee DJ, Busby SJ. Selective repression by Fis and H-NS at the Escherichia coli dps promoter. Mol Microbiol. 2008;68(6):1366–1377. doi: 10.1111/j.1365-2958.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- 62.Artsimovitch I, Henkin TM. In vitro approaches to analysis of transcription termination. Methods. 2009;47(1):37–43. doi: 10.1016/j.ymeth.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.