Significance
The relationship between body fat and male reproduction is clearly seen when excess fat compromises fertility; however, potential consequences of adipose tissue paucity on fertility are unclear. We report that lack of seipin, a transmembrane protein localizing to the endoplasmic reticulum, causes both paucity of adipose tissue and male sterility. Human patients and mouse models lacking seipin in germ cells produce severely abnormal sperm because of impaired lipid distribution during sperm maturation. These defects are testis-intrinsic; scarcity of adipose tissue per se does not affect male fertility. Our work shows that two distinct organs—adipose tissue and the male germ line—depend on seipin function and that appropriate lipid distribution is essential for production of mature, functional sperm.
Abstract
Obesity impairs male fertility, providing evidence for a link between adipose tissue and reproductive function; however, potential consequences of adipose tissue paucity on fertility remain unknown. Lack of s.c. fat is a hallmark of Berardinelli–Seip congenital lipodystrophy type 2 (BSCL2), which is caused by mutations in BSCL2-encoding seipin. Mice with a targeted deletion of murine seipin model BSCL2 with severe lipodystrophy, insulin resistance, and fatty liver but also exhibit male sterility. Here, we report teratozoospermia syndrome in a lipodystrophic patient with compound BSCL2 mutations, with sperm defects resembling the defects of infertile seipin null mutant mice. Analysis of conditional mouse mutants revealed that adipocyte-specific loss of seipin causes progressive lipodystrophy without affecting fertility, whereas loss of seipin in germ cells results in complete male infertility and teratozoospermia. Spermatids of the human patient and mice devoid of seipin in germ cells are morphologically abnormal with large ectopic lipid droplets and aggregate in dysfunctional clusters. Elevated levels of phosphatidic acid accompanied with an altered ratio of polyunsaturated to monounsaturated and saturated fatty acids in mutant mouse testes indicate impaired phospholipid homeostasis during spermiogenesis. We conclude that testicular but not adipose tissue-derived seipin is essential for male fertility by modulating testicular phospholipid homeostasis.
Spermatogenesis encompasses many complex processes, such as the proliferation of spermatogonia, meiosis of spermatocytes, and morphological changes associated with spermatid formation. These processes are modulated by factors that are both intrinsic and extrinsic to the testis. Intrinsic factors derive from genes predominantly expressed in testis, including DEAD (Asp-Glu-Ala-Asp) box polypeptide 4 (DDX4), synaptonemal complex protein 3, and deleted in azoospermia 1 among others, whereas extrinsic factors originate from the hypothalamus, pituitary gland, and other organs. Recent studies have shown that the secretion of adipokines, such as leptin from adipose tissue, regulates reproductive function by affecting the hypothalamus–pituitary–gonadal (HPG) axis (1, 2). Evidence for a link between adipose tissue function and male fertility has also emerged from several studies showing a negative impact of obesity on male reproduction (3, 4). Whether the opposing syndrome, loss of adipose tissue, or lipodystrophy impacts on male infertility remains largely unexplored. A single metaanalysis evaluating body mass index and male semen quality reported that underweight was associated with an increased but nonsignificant risk of abnormal sperm count (5). A study on several types of lipoatrophies mentioned that men with congenital generalized lipodystrophy (CGL) were fertile, but it did not present specific data (6). CGL, also known as Berardinelli–Seip congenital lipodystrophy (BSCL), is a rare autosomal recessive disease characterized by near-complete atrophy of general adipose tissue and hypertriglyceridemia after birth as well as hyperinsulinemia and hepatomegaly because of hepatic steatosis. Mutations in two genes, 1-acylglycerol-3-phosphate O-acyltransferase 2 and BSCL2, are causative in CGL types 1 and 2, respectively; 1-acylglycerol-3-phosphate O-acyltransferase 2, localized on human chromosome 9q34, encodes an enzyme participating in biosynthesis of triglyceride and glycerophospholipids (7, 8), whereas BSCL2 on chromosome 11q13 encodes seipin, a transmembrane protein of unknown function that localizes to the endoplasmic reticulum of adipocytes and other cells (9, 10). Seipin is highly expressed in human brain, testis, and adipose tissue (9). Lack of seipin in the brain has been linked to motor neuropathy and Silver syndrome (11–13), and loss in adipose tissue has been linked with severe lipodystrophy (14); its role in testis remains unknown.
In our previous study, we made the surprising observation that seipin-deficient mice not only model BSCL2 with marked lipodystrophy (15) but also, exhibit complete male infertility. To evaluate a link between lipodystrophy and male fertility and assess the particular role of seipin, we examined reproductive function and sperm quality in male CGL type 2/BSCL2 patients and characterized mice with adipose- and germ cell-specific lack of seipin. We report that seipin deficiency causes teratozoospermia in humans and mice. Specifically, testicular but not adipose tissue-derived seipin is required for male fertility, playing a central role in modulating the testicular phospholipid (PL) homeostasis required for normal spermiogenesis.
Results
Severe Teratozoospermia Syndrome in a Male Patient with Compound Seipin Mutations.
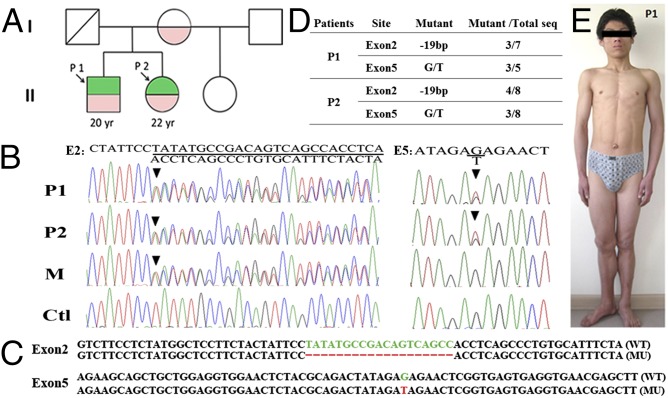
To investigate the relationship between lipodystrophy and male fertility, we studied a male patient from a family affected by lipodystrophy. The patient and his older sister exhibited lipodystrophy; mother, stepfather, and stepsister were not affected. Sequencing of all exons and exon/intron boundaries of BSCL2 revealed compound heterozygous mutations in the affected patients (Fig. 1 A and B), a 19-bp deletion from nucleotide 358 to 376 in exon 2, and a G to T mutation at nucleotide 757 (codon 253) in exon 5; these results were further confirmed by sequencing of subcloned PCR products (Fig. 1 C and D). The 19-bp deletion, inherited from the heterozygous mother, caused a frameshift (S119fsX155) and thus, premature termination of seipin, and the paternally inherited mutation of codon 253 replaced glutamic acid with a stop codon (E253X) (Fig. 1 B and C).
Fig. 1.
BSCL2 mutations in a family with congenital lipodystrophy. (A) Family tree of male patient 1 (P1) and five family members. P1 and his sister [patient 2 (P2)] exhibited lipoatrophy; his mother, stepfather, and stepsister were not affected. P1 and P2 were compound heterozygotes for a G to T mutation in exon 5 (green) and a 19-nt deletion in exon 2 (red) of the BSCL2 gene. The mother was heterozygous for this 19-nt deletion. (B) Detection of mutations by sequence analysis of the BSCL2 gene. A heterozygous 19-bp deletion from nucleotides 358 to 376 in exon 2 in both the subject and her brother causes a frameshift (S119fsX155). The G to T mutation in exon 5 converts codon 253 (glutamic acid) to a stop codon (E253X), resulting in premature termination. Ctl, control sample from an unrelated healthy man; M, mother. Arrowheads indicate the first base of the respective mutation. (C and D) The proportion of mutant to normal sequence in patient samples indicates heterozygosity for each mutation. PCR fragments amplified from patient DNA were subcloned followed by sequencing of plasmids isolated in randomly picked colonies. (E) Physical appearance of P1 with marked absence of s.c. fat and muscular appearance.
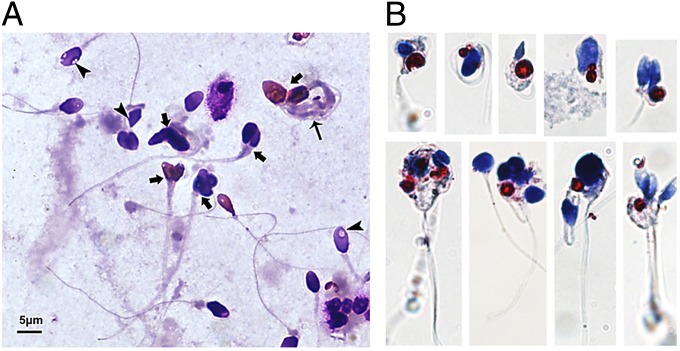
On admission, the patient presented with an extremely muscular and acromegaloid appearance; umbilical hernia and acanthosis nigricans over the axillae were also noted (Fig. 1E). A physical examination revealed enlarged penis and a testicular volume of 32 mL bilaterally. The patient had high fasting blood glucose levels (21 mM/L). Sex hormones were in the normal range, albeit at the lower end for FSH and luteinizing hormone (LH). The patient was diagnosed with teratozoospermia syndrome by two separate reproductive centers according to World Health Organization reference values for human semen characteristics (16) (Table 1). Sperm defects included abnormal head morphology and the presence of bundled sperm with two or more sperm connected to each other (Fig. 2A). Oil Red O staining of sperm samples revealed that heads of bundled sperm contained large ectopic lipid droplets (LDs), which are not present in normal sperm (Fig. 2B).
Table 1.
Hormone levels and sperm abnormalities in a male lipodystrophic patient with BSCL2 mutations
| Hormone | Concentration (reference value*) | Sperm morphology (200 sperm) | Center I (%) | Center II (%) |
| LH | 2.92 (1.24–8.62) IU/L | Normal (%) | 2.50 | 2.0 |
| FSH | 2.96 (1.27–19.46) IU/L | Head defect (%) | 95.50 | 95.0 |
| Estradiol | 15.00 (0–50) pg/mL | Neck defect (%) | 53.00 | 43.0 |
| Testosterone | 4.94 (1.75–7.81) ng/mL | Tail defect (%) | 22.50 | 15.0 |
Reference values used by the laboratory of the Second Affiliated Hospital of Wenzhou Medical University.
Fig. 2.
Teratozoospermia phenotype of P1. (A) Abnormal sperm heads (arrowheads), bundled sperms (arrows), and bundled tails (long arrow). (B) Ectopic accumulation of LDs in sperm of P1. Blue, nuclei; red, LDs.
Seipin Antibody Generation and Stage-Specific Expression of Seipin in the Testis.
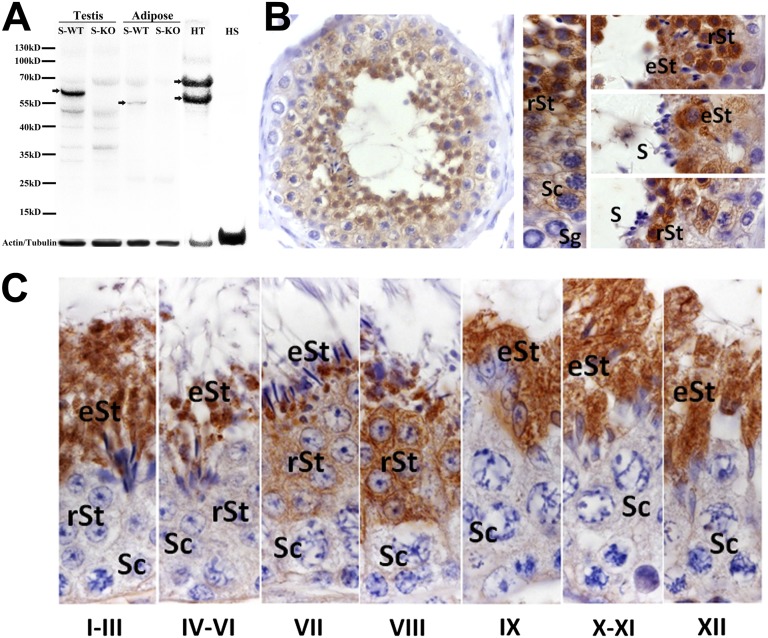
To characterize the distribution of seipin in testicular tissue, we used a rabbit polyclonal antibody generated against the C-terminal domain of seipin. The specificity of this antibody was confirmed by Western blot analysis detecting specific bands in extracts from testis and adipose tissue from WT littermate mice (S-WT) but not mice with ubiquitous deletion of seipin (S-KO) (Fig. 3A). In extracts from human testis tissue, the antibody detected two bands at 55 and 70 kDa in accordance with previous analyses of the two known human isoforms that result from both alternative splicing and alternative translation initiation start site use on human transcripts (17) but absence of reactivity in isolates from mature sperm (Fig. 3A). In the mouse, a long (NM_001136064.2) and a short (NM_008144.4) transcript of seipin have been identified; they result from differential splicing of a 5′ UTR exon. By RT-PCR analysis, we detected higher expression levels and marked predominance of the long transcript in murine testis tissue, whereas murine adipose tissue contained low levels of both transcripts (Fig. S1). Because both transcripts are predicted to encode the same protein (443 aa), the observed size difference of seipin protein from murine testis (between 55 and 70 kDa) and adipose tissue (55 kDa) (Fig. 3A) may be because of different posttranslational modifications in different tissues.
Fig. 3.
Stage-specific expression of seipin in human and murine testis. (A) Western blot analysis using a rabbit polyclonal antibody raised against the C-terminal end of seipin; this antibody detects a specific seipin band in testis (between 55 and 70 kDa) and adipose tissue (at 55 kDa) from S-WT (arrows) but not from S-KO mice. In human testis tissue, the antibody detects two bands at ∼55 and 70 kDa, consistent with the two predicted human isoforms, whereas mature human sperm lacks seipin reactivity. The loading control for murine samples is β-actin, and for human samples, it is β-tubulin. HS, human sperm; HT, human testis. (B) In human testis, seipin is present in later spermatocytes (Sc) and round (rSt) and elongating (eSt) spermatid stages but not spermatogonia (Sg) or mature sperm (S). (C) During murine spermatogenesis, seipin is detectable in steps 7–16 spermatids during spermiogenesis.
Immunohistochemical analysis of human and murine testicular tissue confirmed stage-specific expression of seipin during spermatogenesis. In humans, seipin was present in the cytoplasm of late spermatocytes to spermatids but absent from mature sperm (Fig. 3B), whereas murine seipin appeared at later phases of spermatogenesis and was detectable in steps 7–16 spermatids before incorporation into the residual body (Fig. 3C). Human and mouse seipin transcript and protein were also detected in Leydig cells at a lower level compared with germ cells (Fig. S2).
Loss of Testicular but Not Adipose Seipin Causes Male Infertility.
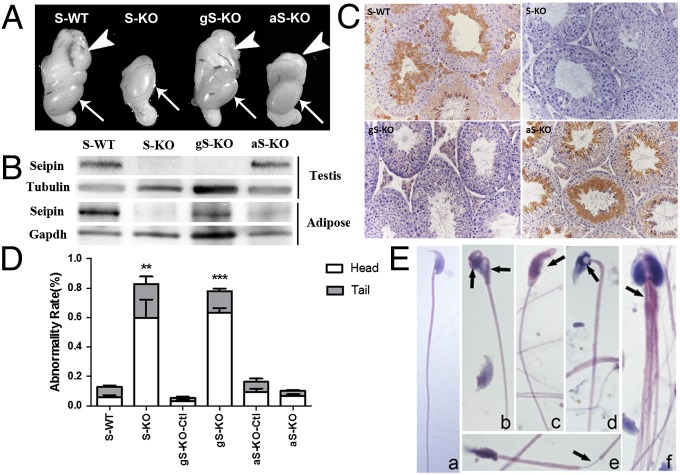
To investigate the consequences of tissue-specific loss of seipin on male reproduction, we crossed mice with a floxed seipin allele (exon 3;E3fl/fl) (15) with mice expressing Cre recombinase in germ cells (DDX4-Cre mice) and adipocytes (adipocyte protein 2-Cre mice), generating mice with germ cell-specific (gS-KO) and adipocyte-specific (aS-KO) deletion of seipin, respectively. S-KO mice were obtained by intercrossing seipin+/− mice (15). S-KO males displayed severe lipodystrophy and absence of seipin protein in both somatic and germ cells of the testis, whereas gS-KO males had normal levels of adipose tissue and lacked detectable seipin in germ cells but not Leydig cells of the testis (Fig. 4 A–C). The aS-KO males showed progressive lipodystrophy (14) but normal levels of seipin in testis (Fig. 4 A–C).
Fig. 4.
Consequences of tissue-specific lack of seipin in mouse male reproductive organs. (A) Distribution of adipose tissue in mouse models with ubiquitous-, germ cell-, or adipocyte-specific loss of seipin. Absence of the epididymal fat pad (arrowheads) near the testes (arrows) in S-KO and aS-KO but not gS-KO mice. (B and C) S-WT and aS-KO males express comparable levels of seipin in testis, whereas S-KO testis and seminiferous tubules of gS-KO males lack detectable seipin. (D) Percentage of sperm with abnormal morphology in controls and mice with tissue-specific deletion of seipin. **P ≤ 0.01; ***P ≤ 0.001. (E) Representative examples of abnormal morphology of sperm from S-KO and gS-KO males. (a) Normal sperm. (b–f) Head defects include lack of the (b) usual hook, (c) banana shape, or (d) apparent cavities. Tail defects include (b) folded sperm and (e) sheath defects (e). (f) Bundle-like masses of connected sperm. Arrows indicate morphological abnormalities.
Males with the three different seipin mutations (S-KO, gS-KO, and aS-KO) exhibited normal mating behavior, evident from copulatory plugs in WT females after overnight mating. However, during a 3-month fertility test, no pregnancies or offspring were observed in breeding pairs of WT females and S-KO or gS-KO males, whereas aS-KO males sired offspring at approximately the same frequency as WT controls (Table S1).
Serum levels of sex hormones, including FSH, LH, testosterone, and estradiol, were normal in S-KO, gS-KO, and aS-KO males (Fig. S3). However, both S-KO and gS-KO mice had significantly lower sperm counts and percentages of motile sperm compared with littermate controls (Table S2). Sperm from S-KO and gS-KO males was morphologically highly abnormal, with head and tail abnormalities in 60% ± 15% and 18% ± 6% of sperm, respectively, whereas head or tail defects were found in only 6% ± 4% of sperm from respective littermate control mice (Fig. 4D). Defects in sperm heads included lack of the usual hook and banana-like or amorphous forms (Fig. 4 E, b and c). Tail defects manifested with folded sperm or as sheath abnormalities (Fig. 4 E, b and e). Some sperm exhibited cavities in the sperm head (Fig. 4 E, d). Similar to the teratozoospermia syndrome of patient 1, testes from S-KO and gS-KO males contained prominent bundles of two or more interconnected sperm (Fig. 4 E, f). In contrast, aS-KO males had normal sperm, similar to normal littermate controls (Fig. 4D and Table S2). Normal male reproductive function in aS-KO males but infertility of S-KO and gS-KO males indicates that loss of seipin function in germ cells causes male infertility, specifically by causing oligoasthenoteratozoospermia. To verify that these observations were not related to the metabolic status of the males, we assessed fasting plasma levels of serum cholesterol (total cholesterol), triglycerides, and glucose and serum levels of insulin at fed state. None of these metabolic markers differed significantly between gS-KO and control mice (Fig. S4). S-KO and aS-KO mice had higher total cholesterol levels and significantly elevated fed plasma insulin levels compared with controls, whereas fasting triglycerides and glucose levels of all mutants were similar to controls (Fig. S4). The absence of detectable metabolic abnormalities in gS-KO mice further confirms that the infertility of seipin-deficient mice is not caused by metabolic changes but because of the absence of seipin protein in germ cells.
Lack of Seipin in Germ Cells Causes Structural Defects in Late Spermiogenesis.
In the three seipin-deficient mouse models, spermatogonia and spermatocytes did not exhibit obvious morphological differences. However, in S-KO and gS-KO males, we observed abnormal clustering of late spermatids of seminiferous tubules at stages VII and VIII of the seminiferous epithelial cycle (Fig. 5A). The heads of these spermatids were randomly oriented, whereas the heads of the spermatids from the WT controls were aligned perpendicular to the seminiferous tubule basement membrane. S-KO and gS-KO tubule sections near completion of spermiogenesis contained massive accumulations of spermatids in bundle-like structures, suggestive of defects during spermatid individualization, which defines the final stage of normal spermiogenesis. The numbers of spermatocytes in stages VII and VIII seminiferous tubules from S-KO, gS-KO, and aS-KO mice were similar to the numbers in S-WT mice, but tubules from S-KO and gS-KO mice contained significantly fewer round spermatids (Fig. S5A). We speculate that the loss of round spermatids was caused by disruption of the release of step 16 spermatids that are normally not bundled and become released into the lumen of the seminiferous tubule. Because of the bundle-like aggregation of most of these spermatids in the mutant, disengagement would likely be aberrant and may cause defects in the architecture of the seminiferous tubule wall. Cauda epididymides from S-KO and gS-KO mice were almost entirely devoid of sperm but contained numerous spherical cells, whereas aS-KO males and WT controls had similar numbers of epidydimal sperm (Fig. 5A). The morphological abnormalities associated with lack of seipin in germ cells, therefore, originate from disturbances in morphological changes associated with spermiogenesis.
Fig. 5.
Defective spermiogenesis in adult S-KO and gS-KO mice. (A) Tubules from S-KO and gS-KO males at stages VI and VII of the seminiferous epithelial cycle contain clusters of late spermatids (short arrows) and bundle-like structures (circles) without significant residual bodies. In S-WT and aS-KO tubules, spermatid heads are oriented perpendicular to the lining membrane of the tubules (arrowheads) and contain many small residual bodies (long arrows). Cauda epididymides from S-KO and gS-KO lack sperm but contain numerous spherical cells and vacuoles (arrows), whereas S-WT and aS-KO are filled with sperm (arrowheads). Sections were stained with H&E. (B) Oil Red O staining of S-KO and gS-KO testes reveals multiple large ectopic LDs near the nuclei of spermatids. In contrast, S-WT and aS-KO contain small LDs in residual bodies of the seminiferous epithelium. Arrows indicate LDs. Blue, nucleus; red, LDs.
Lipid Accumulation in Sperm Caused by Altered PL Metabolism in Seipin-Deficient Testis.
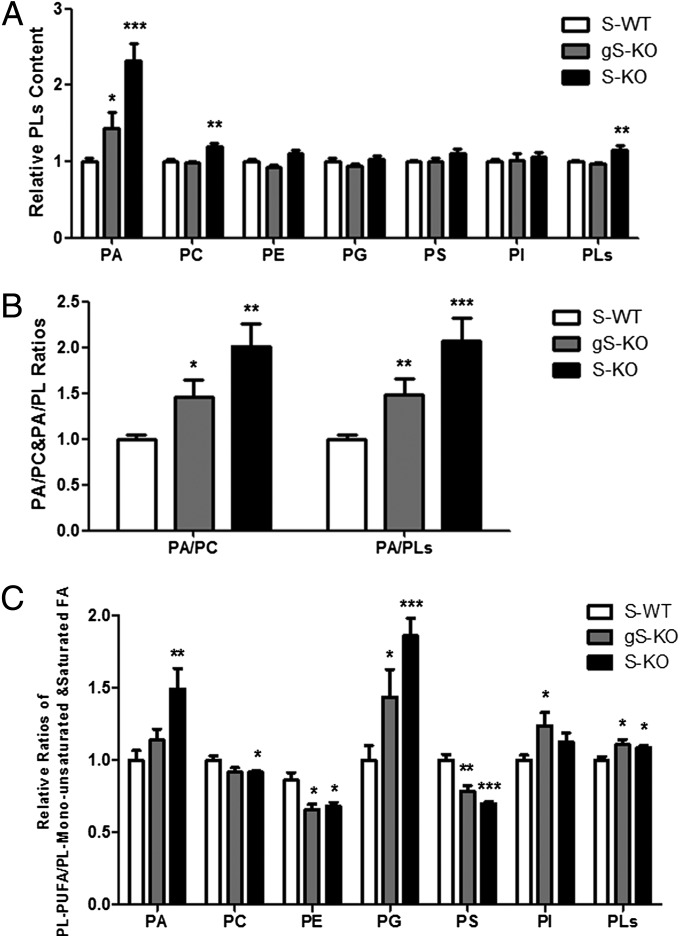
Similar to the ectopic lipid spheres observed in sperm from patient 1, testicular tissue from S-KO and gS-KO mice contained large ectopic LDs in the perinuclear region of round to elongated spermatids. These LDs were present in every tubule of each section assessed and predominated in elongating spermatids, which exhibited the highest expression levels of seipin during spermatogenesis (Fig. 5B and Fig. S5B). In contrast, only small LDs colocalized with residual bodies of elongated sperm in WT and aS-KO testes (Fig. 5B). Electron microscopic analyses of S-KO testes confirmed the presence of large ectopic LDs in round, elongating, and elongated spermatids (Fig. 6 A and B). A similar phenotype was observed in gS-KO mice (Fig. S5C), whereas in WT and aS-KO testes, only LDs of small size were present, located within residual bodies. Spermatids from S-KO and gS-KO mice exhibited defects in chromatin condensation (Fig. 6B), and S-KO and gS-KO testes contained many degenerating masses composed of fragmented spermatids and large LDs (Fig. 6C). Clusters of spermatid tails were present and appeared as bundles in longitudinal sections (Fig. 6C). Purification of haploid cells with ectopic large LDs for additional analysis was not feasible, and Oil Red O staining of LDs in Leydig cells did not reveal any apparent differences between testis tissue from S-WT, S-KO, gS-KO, and aS-KO mice (Fig. S6). We, therefore, performed lipidomic analysis of PLs by liquid chromatography–tandem MS of whole testes from S-KO, gS-KO, and S-WT males. Analyses of fatty acid compositions and their relative levels in testes tissue were assessed for glycero-PLs (GPL), including phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, phosphatidylglycerol, and total PLs. The relative content of PA was significantly increased in the testes of both S-KO and gS-KO males compared with WT (133% and 46% higher, respectively; P < 0.01 and P < 0.05, respectively). PC and PLs levels were elevated in S-KO testis compared with control (19% and 16% higher, respectively; P < 0.01 compared with WT) but not significantly different between gS-KO and WT (Fig. 7A). The ratios of PA to PC and PLs in both S-KO and gS-KO testes were higher than in WT, confirming the elevated PA in S-KO and gS-KO testes (Fig. 7B). In addition, the ratio of polyunsaturated fatty acids to monounsaturated and saturated fatty acids in different PL fractions was increased in phosphatidylglycerol and PL in both S-KO and gS-KO testes. For phosphatidylethanolamine and phosphatidylserine, this ratio was reduced in both S-KO and gS-KO testes. S-KO but not gS-KO testes also exhibited an increased PA and decreased PC ratio, and lastly, gS-KO but not S-KO had elevated phosphatidylinositol levels (Fig. 7C). These results show elevated levels of PA and altered PL homeostasis in S-KO and gS-KO testes, concomitant with the appearance of large ectopic LDs in sperm.
Fig. 6.
Lipid abnormalities and chromatin defects in seipin-deficient male germ cells. (A) Electron microscopic analysis reveals large LDs in spermatids from the seminiferous epithelium of S-KO males and much smaller LDs in residual bodies in tubules from S-WT males. Black dotted line outlines the contour of LDs; arrows show residual bodies. (B) In contrast to spermatids from control littermates, round, elongating, and elongated spermatids from S-KO males contain large ectopic LDs and exhibit defective compaction and condensation of nuclear chromatin and abnormal morphology. (C) The seminiferous epithelium of S-KO males contains degenerating clusters of several spermatids and large LDs. Clusters of spermatid tails are visualized as bundles in longitudinal sections. Arrows, LDs; arrowheads, nuclei.
Fig. 7.
Altered PL homeostasis in S-KO and gS-KO testes. (A) Relative PLs level in gS-KO and S-KO testes compared with S-WT. PA was significantly increased in both S-KO and gS-KO; PC and PLs in S-KO were also increased slightly. (B) The ratios of PA to PC and PLs were elevated in S-KO and gS-KO compared with S-WT. (C) S-KO and gS-KO testes exhibit altered ratios of polyunsaturated fatty acid (PUFA) to monounsaturated and saturated fatty acids in different PL fractions. PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In the present study, we report severe teratozoospermia syndrome in a patient with complex BSCL2 mutations predicted to cause a lack of seipin. We find that mice with ubiquitous or germ cell-specific lack of seipin exhibit completely male infertility accompanied by the same morphological abnormalities observed in the patient’s sperm, particularly defective sperm head, ectopic accumulation of large LDs, and aggregation in dysfunctional bundles. These data indicate that seipin plays an important role for spermatogenesis and may be critical for appropriate lipid distribution during the morphological changes associated with spermiogenesis and release of mature individual sperm.
Normal spermatogenesis and fertility of aS-KO males reveal that loss of adipose tissue because of the lack of seipin does not affect male reproduction. Furthermore, the phenotypic differences of aS-KO and gS-KO mice show that seipin plays separate and distinct roles in adipose tissue homeostasis and during spermatogenesis.
Previous studies have shown that adipose tissue can affect male fertility by secretion of leptin, which regulates various compounds of the HPG axis. Enhanced leptin secretion associated with obesity presumably disturbs the HPG axis, negatively affecting male reproductive capacity. We find that adipocyte-specific seipin deficiency and the ensuing paucity of adipose tissue do not affect male fertility. Consistent with this observation, male fertility is preserved in several other mouse models of systemic lipodystrophy, including mice with overexpression of SREBP-1c in adipose tissue that have markedly reduced fat depots (18), transgenic A-ZIP/F-1 mice lacking white fat tissue (19), or PPAR-hypomorphic mice with severe lipodystrophy and hyperlipidemia (20). Therefore, paucity of adipose tissue, despite being associated with low leptin levels, does not necessarily impair normal spermatogenesis and male fertility. Male mice with the fatty liver dystrophy gene mutation represent an exception to this observation, because they exhibit both lipodystrophy and infertility (21). However, lipin, the gene product of fatty liver dystrophy, is highly expressed in testis; therefore, male infertility may be caused by the loss of lipin function in testis rather than adipose tissue dysfunction resulting in lipodystrophy (22).
The hormone-sensitive lipase (HSL) gene encodes a protein essential for male fertility; male mice lacking HSL are infertile, with testicular abnormalities resembling the abnormalities of seipin-deficient mice, including multinuclear masses and ectopic LDs (23, 24). Defects associated with the loss of seipin or HSL emphasize a central role of appropriate lipid metabolism and distribution during spermiogenesis. Membrane-structured organelles contribute to the morphological changes occurring during spermiogenesis. The endoplasmic reticulum and Golgi apparatus facilitate acrosome formation, and nuclear condensation and elongation of the flagellum require changes in membrane structure. With completion of spermiogenesis, redundant organelles and materials are packed into residual bodies, a process involving the formation of small LDs inside residual bodies and associated with a reduction of the volume of spermatids to ∼25% before spermiation (25). Residual bodies become phagocytosed by Sertoli cells to undergo eventual recycling, and mature spermatids separate and become released into the lumen of seminiferous tubule. The coordinated execution of such specialized events requires fine regulation of germ cell lipid metabolism, especially membrane PLs, during spermiogenesis. Oresti et al. (26) showed, that with progressing differentiation, the amount of polyunsaturated fatty acid-rich GPL per spermatid decreased with the reduction of cell size, whereas the ratio of fatty acids (22:5/20:4) in GPL was increased, but the precise requirements for particular changes in PL composition during spermiogenesis are not yet well-understood. The severe morphological defects of seipin-deficient sperm reported here likely result from disturbances in lipid metabolism during spermiogenesis. Morphological abnormalities of spermatids with ectopic large LDs may be caused solely by the space-occupying effect of such large LDs during condensation. The disturbance of PL homeostasis in spermatids could affect the stability of intercellular bridges and hence, impair the separation of matured spermatids, leading to massive accumulation of S-KO and gS-KO spermatids in bundle-like structures. Previous studies have shown that elevated PA enhances the formation of very large LDs (27). PA is a cone-shaped lipid that alters the membrane curvature and can promote membrane fusion events. In our lipidomics analysis, we found higher total PA and higher ratios of PA to PC and PA to PL in S-KO and gS-KO testes compared with controls. Higher PA levels in S-KO compared with gS-KO testes may be associated with a diluting effect of normal lipids present in the Leydig cells of gS-KO mice, which express low levels of seipin. Elevated PA levels have also been reported from seipin-deleted yeast and seipin-KO Drosophila (27–30). We speculate that seipin may participate in the regulation of PA metabolism in testis, thereby controlling LD morphology and formation. In summary, we find that testicular but not adipose tissue-derived seipin is essential for male fertility by modulating testicular PL homeostasis.
Materials and Methods
This study was approved by the Institutional Ethical Committee of Nanjing Medical University (2013-26); written informed consent was obtained from all human subjects. Exons and exon/intron boundaries of seipin (cDNA NM_001122955) were amplified by PCR (Table S3 shows oligonucleotide sequences) and analyzed by direct sequencing and sequencing of fragments subcloned into T vector (code no. 3271; Takara)
All procedures involving mice were approved by the Animal Care Committee, Peking University Health Science Center (LA2010-059). The gS-KO and aS-KO mouse strains were obtained by crossing seipin-LoxP/LoxP (15) with DDX4-Cre (006954; Jax) and adipocyte protein 2-Cre mice (14); genotyping was performed by PCR using the oligonucleotide primers listed in Table S3. The murine plasma levels of LH, FSH, estradiol, and testosterone were assayed at the Beijing Sino–United Kingdom Institute of Biological Technology. Neutral lipid dye Oil Red O staining, histological analysis, and computer-assisted semen analysis were performed as described previously (31, 32) with minor modifications (SI Materials and Methods). Western blot and immunohistochemical analyses were performed using a rabbit polyclonal antibody raised against the C terminus of murine seipin (Vazyme Biotech) (SI Materials and Methods). Lipid extracts from mouse testis were prepared and analyzed using a TSQ Vantage triple-quadrupole mass spectrometer (Thermo Fisher Scientific) equipped with Xcalibur system software as described previously (33, 34). All data are presented as means ± SEMs. Statistical comparison between the two groups was performed using Student t test or one-way ANOVA. Values of P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Huiyong Yin and Tao Meng for lipidomics technical assistance and Dr. Sigrid Eckardt for revising the manuscript. This work was funded by National Key Basic Research Program Grant 2011CB944304, Major National Basic Research Program of the People’s Republic of China Grants 2011CB503900 and 2012CB517505, and National Natural Science Foundation of the People’s Republic of China Grants 81222006, 30930037, and 81121061.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324025111/-/DCSupplemental.
References
- 1.Landry D, Cloutier F, Martin LJ. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod Biol. 2013;13(1):1–14. doi: 10.1016/j.repbio.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Hamm ML, Bhat GK, Thompson WE, Mann DR. Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol Reprod. 2004;71(1):66–72. doi: 10.1095/biolreprod.104.027292. [DOI] [PubMed] [Google Scholar]
- 3.Teerds KJ, de Rooij DG, Keijer J. Functional relationship between obesity and male reproduction: From humans to animal models. Hum Reprod Update. 2011;17(5):667–683. doi: 10.1093/humupd/dmr017. [DOI] [PubMed] [Google Scholar]
- 4.Sermondade N, Faure C, Fezeu L, Lévy R, Czernichow S. Obesity-Fertility Collaborative Group Obesity and increased risk for oligozoospermia and azoospermia. Arch Intern Med. 2012;172(5):440–442. doi: 10.1001/archinternmed.2011.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sermondade N, et al. BMI in relation to sperm count: An updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19(3):221–231. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal AK, et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31(1):21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal AK, et al. Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: Biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice. J Biol Chem. 2011;286(43):37676–37691. doi: 10.1074/jbc.M111.250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magré J, et al. BSCL Working Group Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28(4):365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 10.Ito D, Suzuki N. Seipinopathy: A novel endoplasmic reticulum stress-associated disease. Brain. 2009;132(Pt 1):8–15. doi: 10.1093/brain/awn216. [DOI] [PubMed] [Google Scholar]
- 11.Windpassinger C, et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet. 2004;36(3):271–276. doi: 10.1038/ng1313. [DOI] [PubMed] [Google Scholar]
- 12.Ito D, Fujisawa T, Iida H, Suzuki N. Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17. Neurobiol Dis. 2008;31(2):266–277. doi: 10.1016/j.nbd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, et al. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology. 2009;150(10):4552–4561. doi: 10.1210/en.2009-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, et al. Adipose-specific knockout of Seipin/Bscl2 results in progressive lipodystrophy. Diabetes. 2014 doi: 10.2337/db13-0729. [DOI] [PubMed] [Google Scholar]
- 15.Cui X, et al. Seipin ablation in mice results in severe generalized lipodystrophy. Hum Mol Genet. 2011;20(15):3022–3030. doi: 10.1093/hmg/ddr205. [DOI] [PubMed] [Google Scholar]
- 16.Cooper TG, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 17.Lundin C, et al. Membrane topology of the human seipin protein. FEBS Lett. 2006;580(9):2281–2284. doi: 10.1016/j.febslet.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 18.Shimomura I, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: Model for congenital generalized lipodystrophy. Genes Dev. 1998;12(20):3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moitra J, et al. Life without white fat: A transgenic mouse. Genes Dev. 1998;12(20):3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koutnikova H, et al. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc Natl Acad Sci USA. 2003;100(24):14457–14462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reue K, Xu P, Wang XP, Slavin BG. Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J Lipid Res. 2000;41(7):1067–1076. [PubMed] [Google Scholar]
- 22.Péterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27(1):121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 23.Vallet-Erdtmann V, et al. The testicular form of hormone-sensitive lipase HSLtes confers rescue of male infertility in HSL-deficient mice. J Biol Chem. 2004;279(41):42875–42880. doi: 10.1074/jbc.M403495200. [DOI] [PubMed] [Google Scholar]
- 24.Wang SP, et al. Expression of human hormone-sensitive lipase (HSL) in postmeiotic germ cells confers normal fertility to HSL-deficient mice. Endocrinology. 2004;145(12):5688–5693. doi: 10.1210/en.2004-0919. [DOI] [PubMed] [Google Scholar]
- 25.Sprando RL, Russell LD. Comparative study of cytoplasmic elimination in spermatids of selected mammalian species. Am J Anat. 1987;178(1):72–80. doi: 10.1002/aja.1001780109. [DOI] [PubMed] [Google Scholar]
- 26.Oresti GM, et al. Differentiation-related changes in lipid classes with long-chain and very long-chain polyenoic fatty acids in rat spermatogenic cells. J Lipid Res. 2010;51(10):2909–2921. doi: 10.1194/jlr.M006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fei W, et al. A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet. 2011;7(7):e1002201. doi: 10.1371/journal.pgen.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolinski H, Kolb D, Hermann S, Koning RI, Kohlwein SD. A role for seipin in lipid droplet dynamics and inheritance in yeast. J Cell Sci. 2011;124(Pt 22):3894–3904. doi: 10.1242/jcs.091454. [DOI] [PubMed] [Google Scholar]
- 29.Tian Y, et al. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet. 2011;7(4):e1001364. doi: 10.1371/journal.pgen.1001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fei W, Du X, Yang H. Seipin, adipogenesis and lipid droplets. Trends Endocrinol Metab. 2011;22(6):204–210. doi: 10.1016/j.tem.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, et al. Transient scrotal hyperthermia induces lipid droplet accumulation and reveals a different ADFP expression pattern between the testes and liver in mice. PLoS ONE. 2012;7(10):e45694. doi: 10.1371/journal.pone.0045694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction. 2009;138(4):655–666. doi: 10.1530/REP-09-0101. [DOI] [PubMed] [Google Scholar]
- 33.Han X, et al. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: A shotgun lipidomics study. Biochemistry. 2007;46(21):6417–6428. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang K, Cheng H, Gross RW, Han X. Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal Chem. 2009;81(11):4356–4368. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.