Significance
Identifying factors that regulate the development of cytokine-producing immunoregulatory invariant natural killer T (iNKT) cells is critical for understanding how to modulate these cells to promote cell-mediated immunity to cancer and infectious organisms or suppress excessive inflammation in autoimmune disease. Here, we identified an essential role for the metabolic regulator Folliculin-interacting protein 1 (Fnip1) in iNKT cell development and survival. Fnip1 physically interacts with AMPK, an energy-sensing enzyme that stimulates mitochondria and ATP production in response to energy deficit, while inhibiting mammalian target of rapamycin (mTOR)-mediated cell growth. Fnip1-deficient iNKT cells contain reduced mitochondrial number and hyperactive mTOR, which resulted in decreased ATP levels and increased sensitivity to apoptosis. Our findings indicate that Fnip1 is vital for maintaining metabolic balance during iNKT cell development.
Keywords: metabolism, thymus, Birt-Hogg-Dubé syndrome
Abstract
Folliculin-interacting protein 1 (Fnip1) is an adaptor protein that physically interacts with AMPK, an energy-sensing kinase that stimulates mitochondrial biogenesis and autophagy in response to low ATP, while turning off energy consumption mediated by mammalian target of rapamycin. Previous studies with Fnip1-null mice revealed that Fnip1 is essential for pre–B-cell development. Here we report a critical role of Fnip1 in invariant natural killer T (iNKT) cell development. Thymic iNKT development in Fnip1−/− mice was arrested at stage 2 (NK1.1−CD44+) but development of CD4, CD8, γδ T-cell, and NK cell lineages proceeded normally. Enforced expression of a Vα14Jα18 iNKT TCR transgene or loss of the proapoptotic protein Bim did not rescue iNKT cell maturation in Fnip1−/− mice. Whereas most known essential transcription factors for iNKT cell development were represented normally, Fnip1−/− iNKT cells failed to down-regulate Promyelocytic leukemia zinc finger compared with their WT counterparts. Moreover, Fnip1−/− iNKT cells contained hyperactive mTOR and reduced mitochondrial number despite lower ATP levels, resulting in increased sensitivity to apoptosis. These results indicate that Fnip1 is vital for iNKT cell development by maintaining metabolic homeostasis in response to metabolic stress.
Invariant natural killer T cells (iNKT) are a unique subset of immunoregulatory T-cell receptor (TCR)-αβ T cells that express a semi-invariant T-cell antigen receptor (Vα14Jα18 in mice) combined with a limited TCR-β–chain repertoire. iNKT cells recognize mostly self- and microorganism-derived glycolipid antigens presented by the nonpolymorphic MHC class I-like molecule CD1d. Upon activation, iNKT cells participate in the early phases of the immune response to tumors and infectious organisms by producing numerous cytokines. In some instances, such as allergy and atherosclerosis, iNKT cell activity is deleterious to the host, reinforcing the importance of identifying factors that regulate iNKT cell development (1–3).
Similar to conventional T cells, iNKT cells develop in the thymus according to a carefully orchestrated series of checkpoints, which ensure completion of appropriate TCR rearrangement, proliferation, and maturation (4, 5). At least four distinct stages of iNKT development have been defined through differences in expression of CD24, CD44, and NK1.1 on TCR-αβ+ T cells that bind CD1d-α-galactosylceramide (αGalCer) tetramers. The earliest committed iNKT cells (stage 0) express CD4 and CD24, and are derived from the thymic double-positive [CD4+CD8+ (DP)] cells following successful gene rearrangement of the TCR Vα14Jα18 segments. In conjunction with the signaling lymphocyte activation molecule (SLAM), stage 0 iNKT cells then become highly proliferative as the pool of iNKT cells is expanded in stage 1. The transition from stage 1 to stage 2 is accompanied by CD44 up-regulation and continued Myc-dependent expansion (6–8). Further maturation to stage 3 involves surface expression of NK1.1 and NKG2D effector molecules, and can occur either in the thymus or following migration of stage 2 cells to the periphery (9). Their immunological features and functions may be reprogrammed in secondary lymphoid tissues (10–12).
The particular signaling proteins and transcription factors that control iNKT cell lineage commitment and development are beginning to be realized. For example, SLAM-SAP-Fyn signaling and Runt-related transcription factor (Runx)1 protein are important for commitment of DP thymocytes to stage 0 of the iNKT lineage (13). The type I basic helix–loop–helix family member, HEB, is essential for the maturation of stage 0 to stage 1, in part by increasing expression of the survival factors Rorγt and Bcl-xL (13–15). The Calcineurin/NFAT/early growth response protein 2 (Egr2) signaling pathway is important for generation of stage 1 and stage 2 iNKT cells (16), and the transcriptional regulator promyelocytic leukemia zinc finger (PLZF) have been identified as a critical regulator of iNKT cell development (17, 18). Specific deletion of c-Myc in DP thymocytes leads to a block in iNKT cell expansion at stages 1 and 2 (6, 7). The T-box transcription factor, Tbx21 (T-bet), is also essential for iNKT cell maturation at the transition from stage 2 to stage 3 (19). After migrating to peripheral lymphoid tissues, stage 2 iNKT cells mature further under control of Id2 (11) and GATA-3 (20, 21). These checkpoint molecules together help define iNKT maturation and homeostasis.
We previously reported that disruption of Folliculin-interacting protein 1 (Fnip1) arrests B-cell development at the large pre–B-cell stage because of defective cell survival in response to metabolic stress (22). Although the functions of Fnip1 are poorly defined, it physically interacts with Folliculin [encoded by the BHD (Birt-Hogg-Dubé) gene], and all three subunits of AMPK, a serine-threonine kinase that stimulates energy production and turns off energy consumption in response to low ATP/AMP balance (23, 24). AMPK decreases energy (ATP) consumption by inhibiting mammalian target of rapamycin (mTOR)-driven cell growth, by phosphorylating and activating TSC2 (25), and by phosphorylating and inactivating Raptor (26). In a recent study, the Folliculin–Fnip1/2 complex was also found to stimulate embryonic stem cell differentiation by limiting access of Tfe3 into the nucleus, thereby allowing efficient exit from naive pluripotency (27). These studies suggest that Folliculin, Fnip1, and Fnip2 may act to modulate AMPK and mTOR activities during cellular differentiation.
In this study, we identified an essential role for Fnip1 in iNKT cell development and survival. This block is correlated with increased PLZF expression in stage 2 cells, and an inability to maintain metabolic homeostasis associated with iNKT precursor cell proliferation.
Results
Fnip1 Deficiency Impairs iNKT Cell Development, Whereas CD4, CD8, and TCR-γδ T Cells Develop Normally.
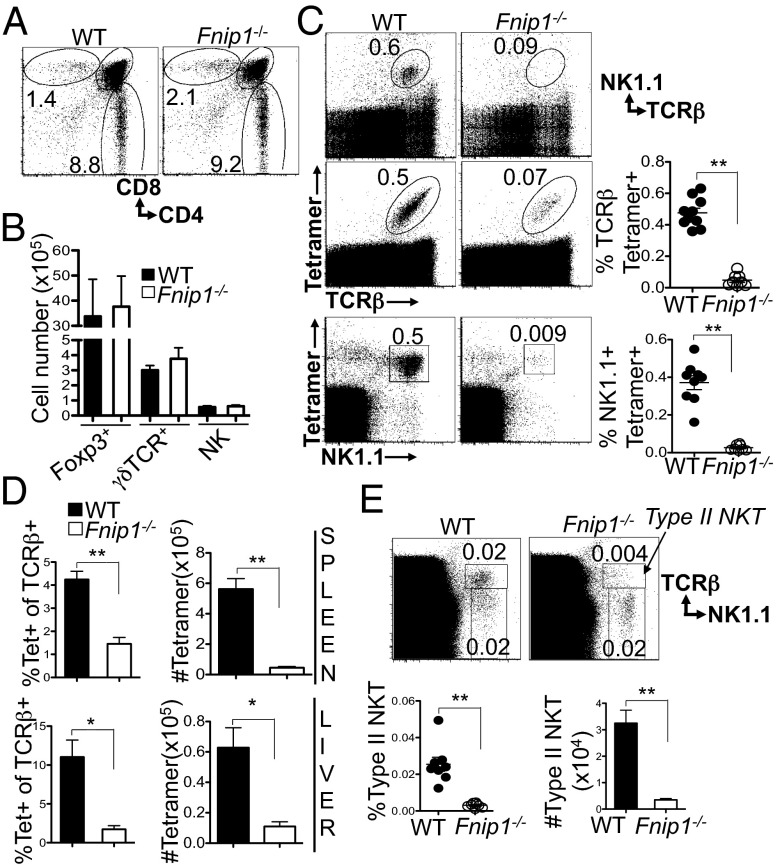
Previous studies indicated that Fnip1 deficiency completely blocks B-cell development at the large pre–B-cell stage, whereas conventional αβ T cells develop normally (22, 28). In this study, we extended our analyses of Fnip1−/− mice by examining if Fnip1 is important for the development and function of γδ, NK, and iNKT cells and dendritic cells. This analysis revealed normal representation (Fig. 1A) and total number of thymic CD4 and CD8 subsets (Fig. S1), and thymic Foxp3+ T-regulatory cells (Treg), TCR-γδ T cells, and NK cells (Fig. 1B), as well as normal representation of CD4, CD8, Treg, NK, and dendritic cells in the spleen of Fnip1−/− relative to WT mice (Fig. S1). In contrast, Fnip1 deficiency nearly completely blocked the development of thymic TCRβ+ NK1.1+ cells (iNKT cells) (Fig. 1C). To assess the development of iNKT cells in mice lacking Fnip1, thymocytes were stained with CD1d dimer or tetramer loaded with the prototypic iNKT cell antigen αGalCer, which allows for the specific detection of their TCR. Fnip1 deficiency resulted in significant reduction in the percentage and total number of CD1d tetramer-binding, TCR-β+ iNKT cells and tetramer-binding NK1.1+ iNKT cells in the thymus (Fig. 1C). There was a concomitant deficiency of CD1d tetramer-binding iNKT cells in the spleen and liver (Fig. 1D). Similar to iNKT cell development, type II NKT cells, which express TCR-β and NK1.1 but do not bind the CD1d tetramer, were also developmentally impaired in Fnip1−/− mice (Fig. 1E).
Fig. 1.
Fnip1-null mice display normal T and NK cell development but disrupted iNKT development. (A and B) Thymocytes were stained for CD4, CD8, Foxp3, NK1.1, TCR-γδ, and TCR-β. Shown are the percentages of cells within specified gates: (A) a representative from n = 8 mice per group; (B) T-cell subsets, n = 4 mice per group for Foxp3 and 6 mice per group for γδ TCR), NK cell number (n = 15 mice/group). Shown are the mean ± SEM. (C) The percentage of CD1d tetramer-positive or NK1.1+ cells within TCR-β+ thymocytes is greatly decreased in Fnip1−/− thymocytes. Shown are the means ± SEM of eight to nine mice per group. (D) Peripheral iNKT cell numbers are decreased in Fnip1−/− mice. Cells isolated from spleen or liver were stained for TCR-β and CD1d tetramer. Shown are the mean ± SEM of six to nine mice per group. (E) The percentage of type II NKT cells within TCR-β+ thymocytes is greatly decreased in Fnip1−/− thymocytes. Cells stained with TCR-β, CD1d tetramer, and NK1.1 type II NKT cells are shown among CD1d tetramer-negative cells. Shown are the mean ± SEM of eight mice per group. *P < 0.05 (unpaired t test), **P < 0.0001 (unpaired t test).
Three subsets of iNKT cells, referred to as iNKT1, iNKT2, and iNKT17, have recently been identified according to differential expression of PLZF, T-bet, IL-17RB, and Rorγt, and distinct cytokine profiles (10, 12). Analysis of spleen and lymph node cells revealed that the number of all three iNKT subsets was reduced in Fnip1−/− mice (Fig. S2). These results indicate that Fnip1 is selectively required for the development of NKT cells, whereas other T-lineage cells develop normally.
Fnip1−/− iNKT Cell Development Is Arrested at an Immature Stage.
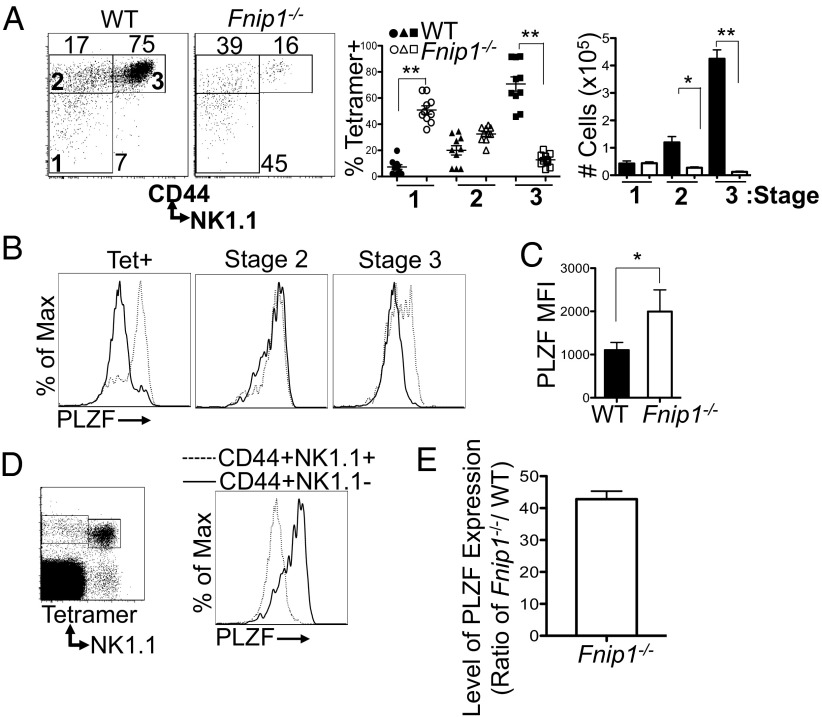
To more specifically define the stage at which loss of Fnip1 inhibits the development of iNKT cells, thymocytes from WT and Fnip1−/− mice were stained with CD1d tetramer and antibodies against iNKT cell surface markers. Analysis of CD44 and NK1.1 profiles revealed that CD1d tetramer binding cells in knockout mice were largely arrested at stages 1 and 2, with far fewer mature iNKT cells bearing NK1.1, CD44, NKG2D, CD94, CD69, CD122, CD244.2, and Ly49G2 markers (Fig. 2A and Fig. S3). Expression of CD1d, and CD150 (SLAM) on thymocytes was normal (Fig. S4), suggesting Fnip1−/− Vα14Jα18 iNKT cells interact normally with CD1d autoantigen complex in the Fnip1−/− thymi. Next we examined the expression of Fnip1 in the different stages of iNKT development in control thymocytes by real-time PCR. Total iNKT cells from WT thymocytes were enriched by tetramer selection using magnetic beads and iNKT developmental subsets were FACS-sorted based on CD44 and NK1.1 markers. Fnip1 expression was robust in stage 1 and 2 iNKT cells, but was minimally expressed in mature stage 3 iNKT cells (CD44+NK1.1+) (Fig. S5). To examine whether loss of Fnip1 affected expression of transcription factors known to be important for iNKT cell development, CD1d tetramer-binding WT and Fnip1−/− iNKT cells were stained with antibodies specific for Egr2, Runx1, Rorγt, PLZF, and T-bet proteins in conjunction with CD24, CD44, and NK1.1 markers. Interestingly, we found that PLZF expression was increased in iNKT cells from Fnip1−/− versus WT mice (Fig. 2 B and C). In contrast, T-bet, Rorγt, Egr2, and Runx1 were present at similar levels throughout development of Fnip1−/− and WT iNKT cells (Fig. S6). Previous studies have suggested that PLZF expression is tightly regulated during iNKT cell development, and is down-regulated following maturation of iNKT cells to stage 3 (17, 29, 30). In line with this, we found that PLZF expression in WT mice was down-regulated during the transition from stage 2 to stage 3 (Fig. 2D). By contrast, analysis of Fnip1−/− iNKT cells revealed PLZF protein was only partially down-regulated in phenotypically mature stage 3 cells (Fig. 2 B and C). Both mRNA and protein levels of PLZF were elevated in Fnip1−/− NK1.1+CD44+ stage 3 iNKT cells compared with their WT counterparts (Fig. 2 C and E).
Fig. 2.
Loss of Fnip1 results in a block in iNKT cell development at Stage 2. (A) Thymocytes from WT and Fnip1−/− mice were stained for CD44, NK1.1, TCR-β, and CD1d tetramer. CD44 and NK1.1 expression profiles within tetramer-positive cells were analyzed and the percentage and cell number of stage 1 (CD44−NK1.1−), stage 2 (CD44+NK1.1−), and stage 3 (CD44+NK1.1+) iNKT cells are shown. Mean ± SEM of 10 mice per group is shown. (B and C) Thymic Fnip1−/− tetramer-positive iNKT cells (dashed line) express high levels of PLZF relative to WT iNKT cells (solid line). Mean fluorescence intensity (MFI) is shown on NK1.1+ cells within tetramer-positive cells (C). Mean ± SEM is shown from n = 7 mice per group. (D) PLZF expression is down-regulated at the transition from stage 2 to stage 3. WT thymocytes were stained with CD1d tetramer and antibodies specific for NK1.1 and CD44. After fixation and permeabilization, cells were stained with antibody specific for PLZF (representative of n = 7 mice per group). (E) PLZF transcript is highly elevated in stage 3 Fnip1−/− iNKT cells compared with WT. WT and Fnip1−/− NK1.1+CD44+ cells were FACS-sorted and PLZF transcript was analyzed by real-time PCR. Shown is the mean PLZF expression (± SEM) of three mice per group. *P < 0.05 (unpaired t test), **P < 0.0001 (unpaired t test).
Impaired iNKT Cell Development in Fnip1-Deficient Mice Is Cell Autonomous.
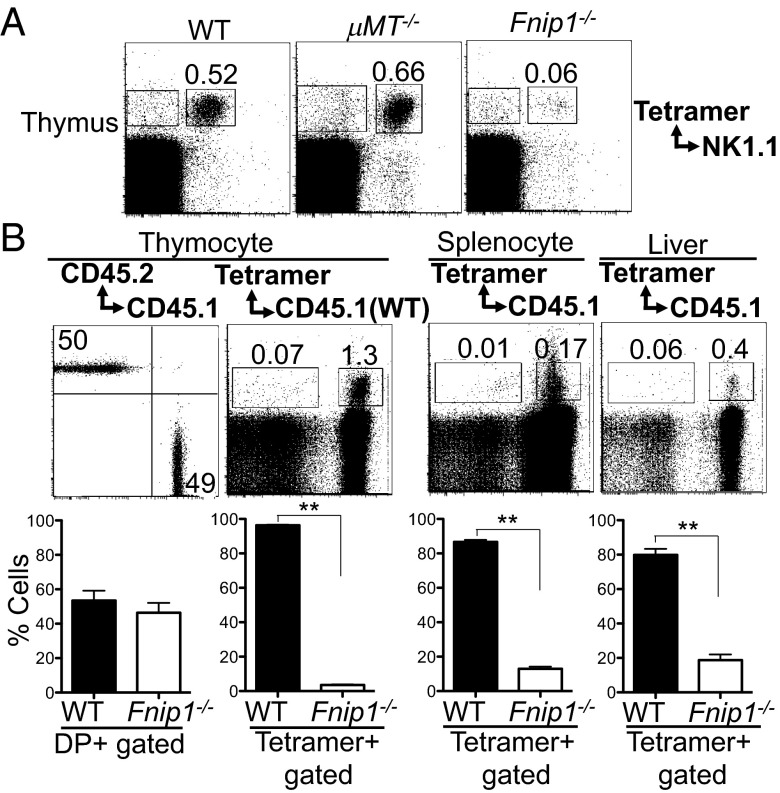
To examine whether defective iNKT cell development in Fnip1−/− mice is secondary to the B-cell deficiency, we assessed iNKT cell development in Fnip1-null mice compared with B-cell–deficient μMT−/− mice. Thymocytes from μMT−/− mice contained normal representation and number of NK1.1+CD1d tetramer-positive iNKT cells, whereas Fnip1−/− mice lacked mature iNKT cells (Fig. 3A). To determine whether the defects in iNKT cell development were cell autonomous, we transferred a 1:1 mixture of bone marrow cells from WT (CD45.1+) and Fnip1−/− (CD45.2+) mice into irradiated Rag2−/−γc−/− recipients and analyzed hematopoietic cell development 10 wk later. There was an equal contribution of donor-derived cells to the development of CD4 and CD8 thymocytes (Fig. 3B), whereas B cells were exclusively derived from WT donor cells (22). Analysis of cells isolated from thymus, spleen, and liver indicated that Fnip1−/− iNKT cells failed to develop, whereas WT iNKT cells in the same animal developed normally (Fig. 3B). These results indicate that the defect in the development of Fnip1−/− iNKT cells is cell autonomous and is not because of a defective environment.
Fig. 3.
Defective iNKT cell development in Fnip1−/− mice is cell-autonomous. (A) Defective iNKT cell development in Fnip1−/− mice does not result from peripheral B-cell deficiency. Thymocytes from WT, μMT−/−, and Fnip1−/− mice were stained with antibodies specific for NK1.1 and CD1d tetramer. Shown are representative flow cytometric plots from four mice per group. (B) Bone marrow cells from Fnip1−/− (CD45.2) and WT (CD45.1) mice were mixed 1:1 and injected into irradiated Rag2−/−γc−/− recipient mice. Thymus, spleen, and liver were harvested from the recipient mice 10 wk after transplantation analyzed by flow cytometry. Shown are representative histograms (Upper) and mean ± SEM (Lower) of the percentages of cells within each gate determined from six mice per group. **P < 0.0001 (unpaired t test).
Expression of Vα14Jα18 Transgene Does Not Rescue iNKT Cell Differentiation in Fnip1-Deficient Mice.
We next tested whether the transgenic provision of rearranged Vα14Jα18 in Fnip1−/− mice could overcome the arrest in iNKT cell development. Fnip1−/− and WT mice were bred with Vα14Jα18 transgenic mice (31). Expression of the Vα14Jα18 transgene did not drive iNKT cell maturation in Fnip1−/− mice, as shown by a failure to increase stage 3 numbers and to up-regulate NK1.1 and NKG2D (Fig. 4A). As expected, Vα14Jα18 transgene expression in Fnip1−/− mice increased the representation of immature tetramer-positive cells (Fig. 4A, column 3). Further analysis of CD44 and NK1.1 within CD1d tetramer-positive cells indicated that considerable numbers of stage 1 iNKT cells accumulated in Vα14Jα18Tg x Fnip1−/− mice compared with Vα14-Jα18Tg × WT mice (Fig. 4A) and Fnip1−/− mice (Fig. 2A), suggesting that other intrinsic cellular programs of iNKT cells are required to proceed beyond stage 1 (31–33).
Fig. 4.
Expression of a Vα14Jα18 TCR-α transgene fails to drive iNKT cell maturation in Fnip1−/− mice. (A) Thymocytes from control and Fnip1−/− Vα14Jα18 transgenic, mice were analyzed by flow cytometry with the indicated antibodies. Representative FACS dot-plots are shown from three mice per group. *P < 0.05 (unpaired t test). (B) Thymocytes from WT and Fnip1−/− mice were stained with antibodies specific for CD44, CD24, NK1.1, TCR-β, and the CD1d tetramer. The mean ± SEM percentage and cell number of Stage 0 CD44−CD24+NK1.1− tetramer-positive cells is graphed (n = 13 mice per group). (C) Real-time PCR analysis of TCR-α rearrangement (Vα14 or Vα3 to Jα56, Jα18, or Jα9) in FACS-sorted DP thymocytes from Fnip1−/− and WT mice. Shown is the mean ± SEM from three mice per group. mRNA expression standardized to Cα loading is shown.
iNKT cells express a TCR-α chain derived from rearranged Vα14 and Jα18 TCR gene segments (15, 34). Because the Jα18 gene segment is located at the distal end of the Jα locus, its use requires secondary rearrangements and is dependent on an extended lifespan of DP thymocytes (1). We investigated whether impaired iNKT cell generation is caused by fewer distal Jα gene segment rearrangements in Fnip1−/− DP cells, which would skew the TCR-α repertoires. Analysis of CD24, CD44, and NK1.1 surface expression within CD1d tetramer-positive cells showed that early committed stage 0 iNKT precursors were represented normally in Fnip1−/− mice relative to WT mice (Fig. 4B). Total DP thymocytes from WT and Fnip1−/− mice were FACS-sorted, and quantitative real-time PCR analysis was used to detect Vα14 and Vα3 rearrangements to proximal Jα56 and distal Jα18 and Jα9 gene segments. We found normal use of distal Jα segments relative to WT mice, even though there was a profound defect of mature tetramer-positive cells in Fnip1−/− thymocytes (Fig. 4C).
Immature Fnip1−/− iNKT Cells Proliferate Normally but Are More Sensitive to Apoptosis.
We next examined the ability of Fnip1−/− iNKT thymic precursors to expand and survive in the presence of growth factors. The addition of IL-7 and IL-15 to cultures resulted in significant expansion of WT iNKT cells, as measured by an increase in the percentage of tetramer-positive cells, whereas Fnip1−/− cells failed to respond (Fig. S7). Fnip1−/− CD1d tetramer-positive cells also expressed lower levels of the IL-7 receptor α-chain (IL-7Rα) compared with controls. However, transgenic expression of the IL-7Rα in Fnip1−/− mice did not rescue iNKT cell maturation (Fig. S8). WT iNKT cell maturation and cellularity were not affected by overexpression of IL-7Rα.
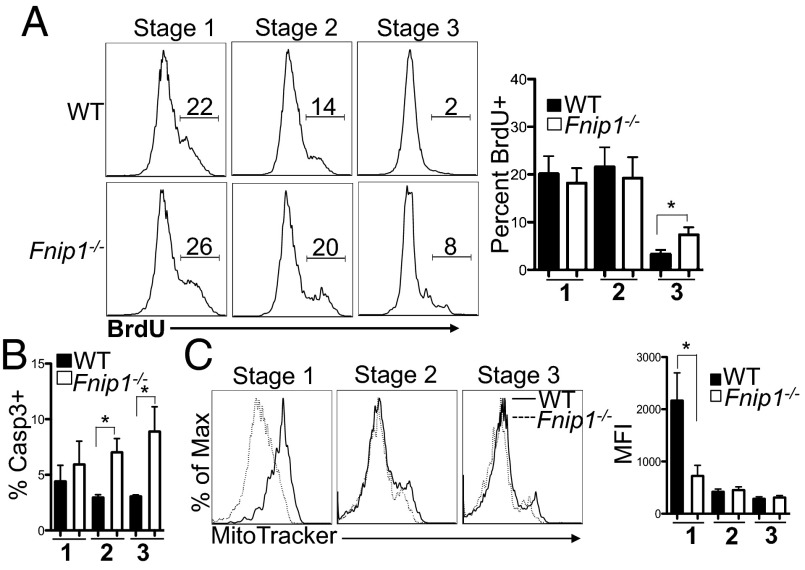
We next examined whether defective maturation of Fnip1−/− iNKT cells was a result of defective cell proliferation or impaired cell survival. To assess cell division, mice were pulsed with BrdU and assayed 24 h later. Relative to WT iNKT cells, Fnip1−/− iNKT cells divided equivalently during stages 1 and 2, but exhibited increased division during stage 3 based on increased BrdU incorporation (Fig. 5A). In contrast, Fnip1 deficiency decreased cell viability during iNKT cell development based on an increased percentage of Caspase 3-positive cells in stage 2 and stage 3 (Fig. 5B). To examine whether increased apoptosis of iNKT cells in Fnip1−/− mice was associated with the Bim pathway, Fnip1−/− mice were bred to Bim1−/− mice and iNKT cell development was assessed. Bim, a proapoptotic member of the Bcl-2 family, induces cell death in response to cytokine withdrawal (35). Maturation and cellularity of Fnip1−/− iNKT cells was not restored by the removal of Bim (Fig. S8). These results suggest that Fnip1−/− iNKT cells are more sensitive to apoptosis, whereas proliferative signals are transmitted relatively normally.
Fig. 5.
Thymic iNKT cells from Fnip1−/− mice proliferate normally but are less viable. (A) WT (Upper) and Fnip1−/− (Lower) mice were injected with BrdU and 24 h later thymocytes were harvested and tetramer-enriched for iNKT cells. Purified cells were stained with antibodies against CD44, NK1.1, and BrdU. Shown are representative flow cytometric histograms of the percentage of BrdU+ cells at each stage of iNKT cell development and the mean ± SEM from five mice per group. *P < 0.05 (unpaired t test). (B) Fnip1−/− iNKT cells have decreased viability. Thymocytes isolated from WT and Fnip1−/− mice were stained for CD44, NK1.1, CD1d tetramer, and Caspase 3. The mean ± SEM of the percentage of Caspase 3-positive stage 1, 2, and 3 iNKT cells is shown from n = 6 mice per group. *P < 0.05 (unpaired t test). (C) Fnip1−/− iNKT cells have reduced numbers of mitochondria. Thymic iNKT cells were analyzed with CD44, NK1.1, CD1d tetramer, MitoTracker green (25 nM). Mean ± SEM is shown from four mice per group. *P < 0.05 (unpaired t test).
Apoptosis is often associated with impaired mitochondrial function and increased mitochondrial stress, resulting in production of reactive oxygen species (36). To examine mitochondria functions in Fnip1−/− iNKT cells, thymic iNKT cells from WT and Fnip1−/− mice were stained with MitoTracker and MitoSox, which detect mitochondrial mass and superoxide accumulation respectively. We found that Fnip1−/− iNKT mitochondria mass was significantly decreased during stage 1 but was normal during stages 2 and 3 compared with WT iNKT cells (Fig. 5C). In contrast, superoxides were increased in Fnip1−/− compared with WT iNKT cells during stages 2 and 3 (Fig. S9). These data suggest disruption of Fnip1 results in altered mitochondrial homeostasis and potentially impaired mitochondrial function.
Fnip1 Deficiency Results in Increased mTOR Activation and Cell Growth.
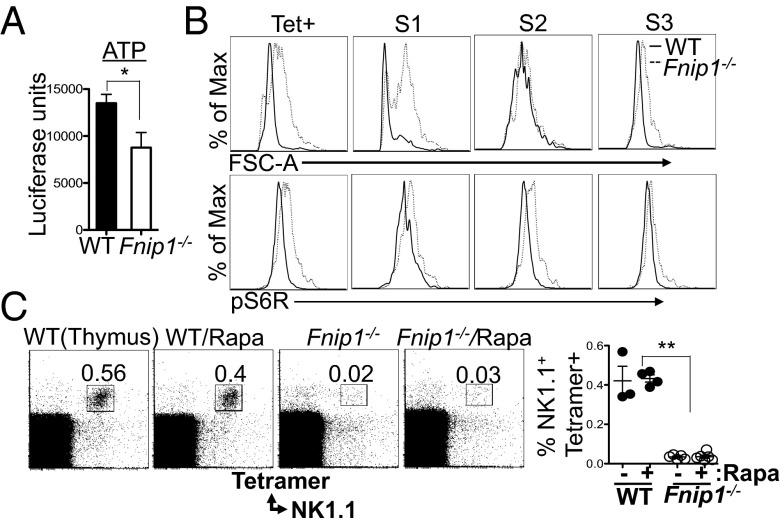
Fnip1 and Fnip2 physically interact with the energy sensor AMPK, and thus may modulate AMPK/mTOR signaling and cellular metabolism (23, 37, 38). Because Fnip1−/− iNKT cells are more sensitive to apoptosis, we examined whether Fnip1−/− iNKT cells are in energy deficit. Endogenous ATP levels were measured on sorted stage 1 NKT cells from WT and Fnip1−/− thymocytes, revealing that stage 1 Fnip1−/− iNKT cells have decreased basal ATP levels compared with their WT counterparts (Fig. 6A). Moreover, relative to WT iNKT cells, Fnip1−/− iNKT cells are larger in size based on increased forward scatter characteristics, and contain increased intracellular phospho-S6R, a downstream effector of mTOR (Fig. 6B). These results suggest that even with low ATP levels, Fnip1−/− iNKT cells fail to properly inhibit mTOR signaling, which further contributes to energy imbalance.
Fig. 6.
iNKT cells from Fnip1−/− mice have reduced ATP levels, are larger in size, and exhibit hyperactive mTOR signaling. (A) ATP levels were determined on sorted stage 1 iNKT cells (NK1.1−CD44−) from WT and Fnip1−/− mice using a luciferase assay. Shown is the mean ± SEM of five mice per group. *P < 0.05 (paired t test). (B) Fnip1−/− iNKT cells are larger and contain increased phospho-S6 ribosomal protein (pS6R). Shown are representative flow cytometric histograms of six mice per group. (C) WT and Fnip1−/− mice were fed a rapamycin-supplemented diet for 50 d and thymocytes were stained with the indicated reagents. The percentage of NK1.1+ CD1d tetramer-positive cells is graphed. Shown is the mean ± SEM of three to six mice per group. **P < 0.0001 (unpaired t test).
To determine whether excessive mTOR signaling is the sole cause of impaired iNKT cell development in Fnip1−/− mice, we treated Fnip1−/− and control mice with the mTOR inhibitor rapamycin for up to 7 wk, beginning in utero and extending to 6 wk of age. We found that long-term in vivo rapamycin treatment did not rescue iNKT cell development in Fnip1−/− mice (Fig. 6C), despite efficient inhibition of mTOR.
Discussion
Developing lymphocytes undergo progressive periods of differentiation and proliferation that require distinct changes in cell metabolism to support cell growth and division (39). The coordination of energy availability and molecular synthesis, with cellular expansion and maturation, is crucial for maintaining metabolic balance and ensuring cell survival. In this study, we found that the AMPK-interacting protein Fnip1 is essential for iNKT cell development, and we propose that disruption of Fnip1 results in metabolic imbalance, which triggers apoptosis in response to metabolic stress during iNKT cell proliferation. Regulation of the AMPK/mTOR axis by Fnip1 may ensure that iNKT progenitors have adequate metabolic capacity to fuel clonal expansion during development and to fully mature.
Our analysis of Fnip1−/− mice revealed a very specific effect of Fnip1 loss on iNKT cell and type II NKT cell development, whereas the development of NK cells, γδ T cells, and conventional TCR-αβ T cells was unperturbed. Mixed bone-marrow chimera experiments showed that the defect in Fnip1 null iNKT cells is cell-intrinsic. Analysis of CD24, CD44, NK1.1 expression within CD1d tetramer-positive cells showed that Fnip1−/− mice have normal numbers of committed stage 0 iNKT cell precursors (CD24+CD44−NK1.1−) relative to WT counterparts. Moreover, analysis of TCR-α repertoire using quantitative real-time PCR indicated that use of proximal versus distal Jα gene segments is not biased in Fnip1−/− mice. Overexpression of rearranged Vα14Jα18 TCR-α chain failed to stimulate iNKT cell differentiation in Fnip1−/− mice. Our results suggest that Fnip1 is essential for the development of iNKT cells in a cell-autonomous manner after TCR rearrangement and expression.
PLZF expression was persistently elevated in Fnip1−/− iNKT cells. Targeted disruption of PLZF inhibits iNKT cell development at stage 1 and immature iNKT cells accumulate in lymph nodes instead of liver. Conversely, ectopic overexpression of PLZF under control of the CD4 promoter inhibits expression of late iNKT cell markers, such as CD122, NK1.1, and NKG2D (17, 29, 30). These studies suggest that iNKT cell development is exquisitely sensitive to levels of PLZF. Recent findings indicate that iNKT cells consist of at least three subsets (iNKT1, iNKT2, iNKT17), whereby lineage determination is guided by distinct transcription factors, including T-bet (iNKT1 cells), Rorγt (iNKT17 cells), and PLZF (iNKT2 cells) (10, 12). Our studies show that development of three iNKT subsets is impaired in Fnip1−/− mice (Fig. S2).
Fnip1, Fnip2, and Folliculin physically interact as a complex with HSP-90 and all three subunits of AMPK (40). In response to low energy, AMPK stimulates ATP and nutrient production by stimulating autophagy (23, 41) and by increasing mitochondrial biogenesis. AMPK also turns off energy and nutrient consumption by inhibiting mTOR (25, 26). In this study, we found that basal ATP levels are decreased in stage 1 Fnip1−/− iNKT cells relative to WT iNKT cells, perhaps because of decreased mitochondria mass and function, or increased mTOR-mediated energy consumption. Fnip1−/− iNKT cell progenitors are also larger in size and contain increased levels of phospho-S6R, indicative of increased mTOR activation. These results suggest that Fnip1 is required for inhibition of mTOR in response to metabolic stress, resulting in excessive nutrient/energy consumption and increased sensitivity to apoptosis. Consistent with this notion, conditional disruption of the mTOR inhibitor TSC1 using CD4-Cre also results in excessive mTOR activation and inhibition of iNKT cell development because of increased apoptosis (42). However, whereas rapamycin rescues cell size and survival in TSC1-null mice, rapamycin failed to rescue iNKT cell development in Fnip1−/− mice. These results suggest that Fnip1 controls iNKT cell development in both mTOR-dependent and -independent manners.
Recent studies suggest that Fnip-family proteins contain DENN (differentially expressed in normal and neoplastic cells) domains, known to be important for activating vesicle fusion and induction of autophagy in other systems (43). In addition, Fnip1 and Folliculin have been implicated in autophagy induction in other cell types (44). Whereas the mechanism of how loss of Fnip1 inhibits autophagy is unclear, AMPK activates the proautophagy PI3 kinase vacuolar sorting protein 34 (Vps34) complex (45, 46), and conditional disruption of Vps34 also inhibits iNKT cell development at stage 1 (47). These reports suggest that impaired autophagy combined with dysregulated mTOR may contribute to impaired iNKT cell development in Fnip1−/− mice. Disruption of Fnip1 inhibits B-cell development and iNKT cell development at transitions where differentiation is coincident with massive expansion and metabolic stress associated with expression of c-Myc (22). Conditional disruption of c-Myc mirrors loss of Fnip1, including inhibition of B-cell development at the pre–B-cell stage (48) and impaired iNKT development at stages 1–2 (6, 7). However, whereas Myc-dependent pre-B and iNKT cell division proceeds normally in the absence of Fnip1, cell survival is significantly impaired. Recent studies indicate that Myc stimulates cell division and growth in part by modulating cell metabolism to support cell division (see ref. 49 for review). For example, Myc increases protein synthesis (50) and stimulates a shift toward glycolysis at the expense of oxidative phosphorylation. We speculate that Fnip1 is important for ensuring that energy and nutrient supply exceed consumption during Myc-dependent cell growth. “Resting” Fnip1-null pro-B cells and stage 0 iNKT cells are able to maintain metabolic homeostasis, but are unable to do so following Myc-dependent expansion resulting in apoptosis. Consistent with this notion is the finding that loss of Fnip1 blocks B-cell transformation by the Eμ-Myc transgene (22).
It remains unclear why B- and iNKT cell development are exclusively disrupted by the loss of Fnip1, whereas conventional TCR-αβ T-cell development proceeds unperturbed. Because Fnip2 is also expressed in developing T-lineage cells (22), targeted disruption of the Fnip2 gene should shed light on understanding differential functions of Fnip family members in immune cell function and metabolism.
Materials and Methods
Mice.
Fnip1−/− were generated as previously described (22). Rag2−/−γc−/− (Taconic), μMT−/− (Jackson Laboratories), Vα14-Jα18 transgenic (Jackson Laboratories), and B6.SJL-Ptprca Pepcb/BoyJ (Jackson Laboratories) mice were purchased. CD127 transgenic or Bim−/− were provided by Keith B. Elkon and Pamela J. Fink, respectively (University of Washington, Seattle, WA). All mice were on C57BL/6 genetic background. Fnip1−/− mice were backcrossed to C57BL/6 mice for 14 generations. Experimental mice used were littermates or age-/sex-matched C57BL/6 (Jackson Laboratories) as WT mice at 6–12 wk old. Mice were housed under specific pathogen-free conditions and all animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee (Seattle, WA).
Enrichment of iNKT Cells.
Total thymocytes were incubated with Fc receptor block, washed and stained with biotinylated CD1d/αGalCer complex (BD DimerX). iNKT cells were then purified with a MACS cell sorting system (Miltenyl Biotec) after streptavidin microbead labeling. For convenience, these cell preparations are referred to as “CD1d tetramer-enriched” in the text.
Statistical Analysis.
The statistical significance of differences between WT and KO groups were analyzed using the Student’s two-tailed t test. P < 0.05 values were considered as significant. P values were calculated by using Prism software (GraphPad).
Supplementary Material
Acknowledgments
We thank Michelle Torres, XiaoCun Pan, and Jacky Chan for assistance with the mouse colony. This study was supported by National Institutes of Health Grant AI19335 and the Howard Hughes Medical Institute (to M.J.B.); and National Institutes of Health Grants K26RR024462, P30 CA015704, and R56AI092093 (to B.M.I.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406473111/-/DCSupplemental.
References
- 1.Engel I, Kronenberg M. Making memory at birth: Understanding the differentiation of natural killer T cells. Curr Opin Immunol. 2012;24(2):184–190. doi: 10.1016/j.coi.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 3.Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells as sensors and managers of inflammation. Trends Immunol. 2013;34(2):50–58. doi: 10.1016/j.it.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25(2):161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11(3):197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 6.Mycko MP, et al. Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. J Immunol. 2009;182(8):4641–4648. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- 7.Dose M, et al. Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proc Natl Acad Sci USA. 2009;106(21):8641–8646. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dose M, Gounari F. The My(c)stery of iNKT cell ontogeny. Cell Cycle. 2009;8(19):3082–3085. doi: 10.4161/cc.8.19.9615. [DOI] [PubMed] [Google Scholar]
- 9.McNab FW, et al. Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts. J Immunol. 2007;179(10):6630–6637. doi: 10.4049/jimmunol.179.10.6630. [DOI] [PubMed] [Google Scholar]
- 10.Watarai H, et al. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10(2):e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monticelli LA, et al. Transcriptional regulator Id2 controls survival of hepatic NKT cells. Proc Natl Acad Sci USA. 2009;106(46):19461–19466. doi: 10.1073/pnas.0908249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14(11):1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egawa T, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22(6):705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc Natl Acad Sci USA. 2005;102(14):5114–5119. doi: 10.1073/pnas.0408449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol. 2010;11(3):240–249. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarevic V, et al. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10(3):306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalovsky D, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9(9):1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20(4):477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 20.Kim PJ, et al. GATA-3 regulates the development and function of invariant NKT cells. J Immunol. 2006;177(10):6650–6659. doi: 10.4049/jimmunol.177.10.6650. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, et al. The sequential activity of Gata3 and Thpok is required for the differentiation of CD1d-restricted CD4+ NKT cells. Eur J Immunol. 2010;40(9):2385–2390. doi: 10.1002/eji.201040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, et al. Disruption of Fnip1 reveals a metabolic checkpoint controlling B lymphocyte development. Immunity. 2012;36(5):769–781. doi: 10.1016/j.immuni.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba M, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci USA. 2006;103(42):15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: A metabolic disease. Nat Rev Urol. 2010;7(5):277–285. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoki K, Zhu T, Guan K-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 26.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betschinger J, et al. Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell. 2013;153(2):335–347. doi: 10.1016/j.cell.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baba M, et al. The folliculin-FNIP1 pathway deleted in human Birt-Hogg-Dubé syndrome is required for murine B-cell development. Blood. 2012;120(6):1254–1261. doi: 10.1182/blood-2012-02-410407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gleimer M, von Boehmer H, Kreslavsky T. PLZF controls the expression of a limited number of genes essential for NKT cell function. Front Immunol. 2012;3:374. doi: 10.3389/fimmu.2012.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovalovsky D, et al. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. J Immunol. 2010;184(12):6746–6755. doi: 10.4049/jimmunol.1000776. [DOI] [PubMed] [Google Scholar]
- 31.Wakao H, et al. A novel mouse model for invariant NKT cell study. J Immunol. 2007;179(6):3888–3895. doi: 10.4049/jimmunol.179.6.3888. [DOI] [PubMed] [Google Scholar]
- 32.Mendiratta SK, et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6(4):469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 33.Griewank K, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27(5):751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hager E, Hawwari A, Matsuda JL, Krangel MS, Gapin L. Multiple constraints at the level of TCRalpha rearrangement impact Valpha14i NKT cell development. J Immunol. 2007;179(4):2228–2234. doi: 10.4049/jimmunol.179.4.2228. [DOI] [PubMed] [Google Scholar]
- 35.Huntington ND, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8(8):856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3(3):214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 37.Takagi Y, et al. Interaction of Folliculin (Birt-Hogg-Dubé gene product) with a novel Fnip1-like (FnipL/Fnip2) protein. Oncogene. 2008;27(40):5339–5347. doi: 10.1038/onc.2008.261. [DOI] [PubMed] [Google Scholar]
- 38.Gaur K, et al. The Birt-Hogg-Dubé tumor suppressor Folliculin negatively regulates ribosomal RNA synthesis. Hum Mol Genet. 2013;22(2):284–299. doi: 10.1093/hmg/dds428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathmell JC. B-cell homeostasis: Digital survival or analog growth? Immunol Rev. 2004;197:116–128. doi: 10.1111/j.0105-2896.2004.0096.x. [DOI] [PubMed] [Google Scholar]
- 40.Hasumi H, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415(1-2):60–67. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12(9):888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang D, Iyer LM, He F, Aravind L. Discovery of novel DENN proteins: Implications for the evolution of eukaryotic intracellular membrane structures and human disease. Front Genet. 2012;3:283. doi: 10.3389/fgene.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466(7302):68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaber N, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci USA. 2012;109(6):2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152(1-2):290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parekh VV, et al. Impaired autophagy, defective T cell homeostasis, and a wasting syndrome in mice with a T cell-specific deletion of Vps34. J Immunol. 2013;190(10):5086–5101. doi: 10.4049/jimmunol.1202071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habib T, et al. Myc stimulates B lymphocyte differentiation and amplifies calcium signaling. J Cell Biol. 2007;179(4):717–731. doi: 10.1083/jcb.200704173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013;3(8) doi: 10.1101/cshperspect.a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iritani BM, Eisenman RN. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci USA. 1999;96(23):13180–13185. doi: 10.1073/pnas.96.23.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.