Significance
Inducing the growth of new blood vessels by specific factors is an attractive strategy to restore blood flow in ischemic tissues. Vascular endothelial growth factor (VEGF) is the master regulator of angiogenesis, yet clinical trials of VEGF gene delivery failed. Major challenges include the need to control the tissue distribution of factor dose and the duration of expression. Here, we developed a highly tunable fibrin-based platform to precisely control the dose and duration of VEGF protein delivery in tissues. Optimized delivery of fibrin-bound VEGF ensured normal, stable, and functional angiogenesis and improved perfusion of ischemic tissues, without genetic modification and with limited duration of VEGF delivery. These findings suggest a strategy to improve both safety and efficacy of therapeutic angiogenesis.
Abstract
Clinical trials of therapeutic angiogenesis by vascular endothelial growth factor (VEGF) gene delivery failed to show efficacy. Major challenges include the need to precisely control in vivo distribution of growth factor dose and duration of expression. Recombinant VEGF protein delivery could overcome these issues, but rapid in vivo clearance prevents the stabilization of induced angiogenesis. Here, we developed an optimized fibrin platform for controlled delivery of recombinant VEGF, to robustly induce normal, stable, and functional angiogenesis. Murine VEGF164 was fused to a sequence derived from α2-plasmin inhibitor (α2-PI1–8) that is a substrate for the coagulation factor fXIIIa, to allow its covalent cross-linking into fibrin hydrogels and release only by enzymatic cleavage. An α2-PI1–8–fused variant of the fibrinolysis inhibitor aprotinin was used to control the hydrogel degradation rate, which determines both the duration and effective dose of factor release. An optimized aprotinin-α2-PI1–8 concentration ensured ideal degradation over 4 wk. Under these conditions, fibrin-α2-PI1–8-VEGF164 allowed exquisitely dose-dependent angiogenesis: concentrations ≥25 μg/mL caused widespread aberrant vascular structures, but a 500-fold concentration range (0.01–5.0 μg/mL) induced exclusively normal, mature, nonleaky, and perfused capillaries, which were stable after 3 mo. Optimized delivery of fibrin-α2-PI1–8-VEGF164 was therapeutically effective both in ischemic hind limb and wound-healing models, significantly improving angiogenesis, tissue perfusion, and healing rate. In conclusion, this optimized platform ensured (i) controlled and highly tunable delivery of VEGF protein in ischemic tissue and (ii) stable and functional angiogenesis without introducing genetic material and with a limited and controllable duration of treatment. These findings suggest a strategy to improve safety and efficacy of therapeutic angiogenesis.
Therapeutic angiogenesis is an attractive strategy for treating ischemic conditions, such as peripheral and coronary artery diseases or chronic wounds, in which the intrinsic capacity for spontaneous vascular repair and tissue regeneration is either compromised or insufficient to restore physiological blood flow. In fact, sufficient expansion of microvascular networks is capable of increasing flow in upstream collateral arteries through retrograde signals (1, 2), thereby providing effective bypass of the obstructed feeding vessels. Vascular endothelial growth factor (VEGF) is the master regulator of both developmental and reparative vascular growth (3). However, initial clinical trials of VEGF gene delivery failed to establish clinical benefit (4). Retrospective analyses identified several issues that undermined the efficacy of those trials, particularly the difficulty to deliver a sufficient VEGF dose into the target tissue at safe vector doses (5, 6). By cell-based gene delivery, we previously found that, to effectively exploit VEGF’s therapeutic potential and robustly induce only functional and safe angiogenesis, it is key to control its microenvironmental concentration around each producing cell in vivo rather than its total dose (7) as VEGF binds tightly to the extracellular matrix (ECM) (8). However, it is challenging to achieve homogeneous expression levels in vivo with gene-therapy vectors. Further, newly induced vessels require sustained VEGF stimulation for at least 4 wk to stabilize and persist indefinitely, but unlimited duration of expression raises safety concerns (7, 9, 10). Controlled release of recombinant VEGF protein from biodegradable matrices is an attractive approach for clinical translation of these biological concepts due to the lack of genetic modification, the ease of achieving a homogenous dose distribution, and the limited duration of treatment (11).
Physiological angiogenesis crucially depends on a spatially restricted organization of growth factors through their binding to the ECM (12). Fibrin, a natural product of blood coagulation, provides unique features for physiological presentation of angiogenic signals: (i) It is injectable as a liquid and solidifies in situ without cytotoxicity; (ii) it is remodeled by cell-associated enzymes like metalloproteinases and plasmin; and (iii) it is a natural cell-infiltration matrix (13). Therefore, we previously developed an approach to enzymatically link growth factors into fibrin hydrogels: the α2-plasmin inhibitor-derived octapeptide NQEQVSPL (α2-PI1–8), which is a substrate for the transglutaminase coagulation factor fXIIIa and has no plasmin inhibitory function itself, fused onto a factor N terminus, ensures its covalent binding to fibrin during the fibrinogen cross-linking reaction and subsequent release only through matrix degradation by local cell-associated proteases (14, 15). Matrix-bound presentation of diverse so-engineered growth factors, such as α2-PI1–8–fused variants of VEGF-A121, BMP-2, and IGF1, considerably accentuated their biological effects compared with the wild-type factors (16–19). However, the brief persistence of fibrin hydrogels in vivo (16) is insufficient to ensure stabilization of newly induced vessels and is a major obstacle to its exploitation for therapeutic angiogenesis (20). To gain control over fibrin-remodeling rates and significantly prolong gel persistence in vivo, we have engineered an α2-PI1–8–fused variant of the fibrinolysis inhibitor aprotinin (21). Here, we developed a fibrin platform to ensure both controlled and sustained delivery of α2-PI1–8-VEGF-A164 and achieve robust induction of normal, stable, and functional angiogenesis in therapeutically relevant target tissues.
Results
Generation of a Recombinant Murine VEGF164 Variant, α2PI1–8-VEGF164.
The α2-PI1–8 peptide was fused at the N terminus of murine VEGF164 by a previously developed method of protein engineering (15). The coupling efficiency of α2-PI1–8-VEGF164 in fibrin gels was determined measuring the factor release into buffer every 24 h over 7 d: 5.1 ± 1.2% of the incorporated α2-PI1–8-VEGF164 was released in the first day without significant increases by 7 d (6.9 ± 1.2%) whereas the native VEGF164 was almost completely released already after 1 d (88.2 ± 2.4%) (Fig. S1A). The bioactivity of α2-PI1–8-VEGF164 was equivalent to native VEGF164, as determined by their ability to induce VEGFR-2 phosphorylation on endothelial cells (Fig. S1B).
In Vivo Fibrin-Gel Degradation as a Function of Composition.
Fibrinogen content proportionally determines the maximum amount of α2-PI1–8-VEGF incorporated into the gel. Therefore, the highest concentration compatible with in vivo injection in liquid form (polymerization time >10 s) was determined to be 25 mg/mL and selected for subsequent experiments. Small-strain oscillatory shear rheometry showed that gel stiffness varied between 2.9 ± 0.4 and 6.9 ± 1.2 kPa, depending on the concentration of cross-linking enzymes. To determine the effect of gel composition on in vivo degradation rate after intramuscular injection, the amount of remaining gel was assessed by histological analysis after 4 d (Fig. S2A) and by noninvasive multispectral imaging of gels labeled with fluorescent fibrinogen (Fig. S2B). All compositions showed similar gel persistence and degradation rates, being essentially consumed by 5 d (Fig. S2). Therefore, the composition with the lowest stiffness (2.9 kPa: fibrinogen 25 mg/mL, FXIII 2 U/mL, thrombin 2 U/mL) was selected to investigate growth-factor delivery in vivo, to maximize compliance and minimize tissue invasiveness.
Aprotinin-α2-PI1–8 Concentration Determines both Rate and Duration of VEGF Release.
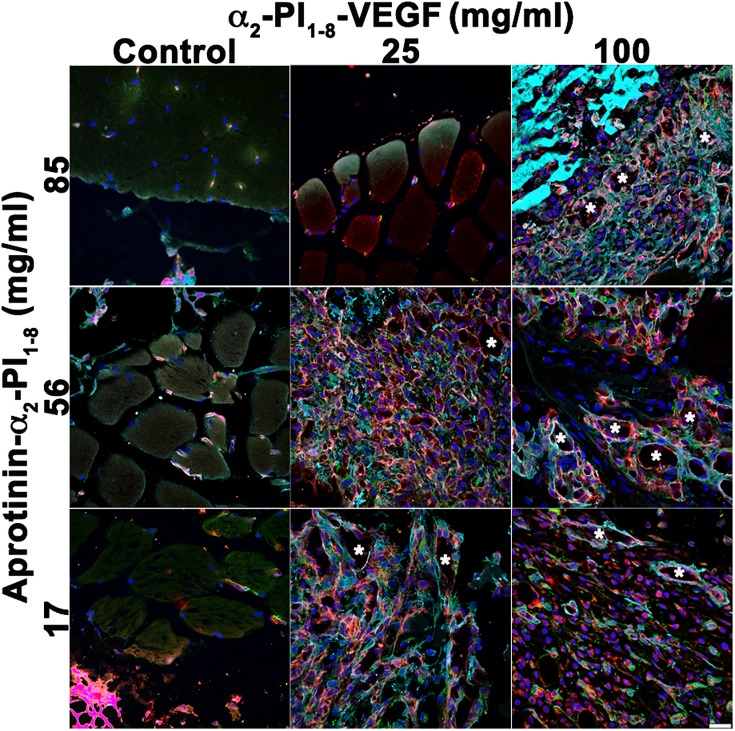
Aprotinin inhibits fibrin degradation by proteases, and the engineered variant aprotinin-α2-PI1–8 (21) was covalently cross-linked in the gels to prolong in vivo persistence and the duration of VEGF release, required for vascular stabilization. Gel degradation rate affects both key parameters controlling angiogenesis (i.e., the rate and duration of VEGF release), but in opposite directions, as a reduction in degradation rate would increase the duration of VEGF release but also reduce its rate (i.e., the effective delivered dose). Therefore, we first sought to determine the optimal aprotinin-α2-PI1–8 concentration that would ensure both sufficient in vivo persistence and an adequate VEGF release rate. Gels were prepared with the maximum α2-PI1–8-VEGF164 concentration that would not affect their mechanical properties (100 μg/mL) and a fourfold lower one (25 μg/mL), in combination with three different aprotinin-α2-PI1–8 concentrations (17 μg/mL, 56 μg/mL, and 85 μg/mL), to analyze the effect of aprotinin-α2-PI1–8 dose on the effective VEGF release rate, based on induced vascular morphology. As shown in Fig. 1, 9 d after intramuscular injection, the negative control gels, containing only aprotinin-α2-PI1–8, did not induce any angiogenesis whereas 100 μg/mL α2-PI1–8-VEGF164 induced aberrant vessels independently of aprotinin concentration. Such vessels displayed irregularly dilated diameters and multiple lumens, were devoid of pericytes, and were covered with a thick layer of smooth muscle cells, similar to previously described angioma-like structures induced by excessive VEGF doses (7). However, the effects of the fourfold lower α2-PI1–8-VEGF164 concentration of 25 μg/mL were clearly dependent on aprotinin-α2-PI1–8 amount: no angiogenesis was detectable with 85 μg/mL, aberrant angioma-like structures were induced with 17 μg/mL, and an abundant network of morphologically normal, pericyte-covered capillaries was generated with the intermediate 56 μg/mL concentration, even if rare enlarged vessels were still detectable. Thus, an aprotinin-α2-PI1–8 concentration of 56 μg/mL, which allowed a VEGF dose-dependent transition between normal and aberrant angiogenesis, was used in subsequent experiments.
Fig. 1.
Aprotinin-α2-PI1–8 concentration determines the effective released dose of α2-PI1–8-VEGF164 and the angiogenic outcome. Fibrin gels were injected into the gastrocnemius muscles of SCID mice, and tissues were analyzed 9 d later. Two different α2-PI1–8-VEGF164 concentrations (25 μg/mL and 100 μg/mL) were tested in combination with three aprotinin-α2-PI1–8 concentrations (17 μg/mL, 56 μg/mL, and 81 μg/mL). Negative control conditions contained only aprotinin-α2-PI1–8. Frozen sections were immunostained for endothelial cells (CD31, in red), pericytes (NG2, in green), smooth-muscle cells (α-SMA, in cyan) and nuclei (DAPI, in blue). Asterisks, enlarged aberrant vascular structures. n = 3. (Scale bar: 20 μm.)
α2-PI1–8-VEGF164 Bioactivity After Gel Incorporation and in Vivo Implantation.
To determine whether α2-PI1–8-VEGF164 could retain its bioactivity while incorporated into the fibrin gels in vivo, before being released, gels were preformed at 37 °C with 100 μg/mL α2-PI1–8-VEGF164 and 56 μg/mL aprotinin-α2-PI1–8 and implanted s.c. in nude mice. After 2 wk, gels still contained ∼30% of the α2-PI1–8-VEGF164 amount incorporated at day 0 (Fig. S3A). Furthermore, α2-PI1–8-VEGF164 extracted from gels after 2 wk of in vivo incubation was capable of inducing endothelial proliferation as efficiently as the nonimplanted factor (Fig. S3B), indicating that gel incorporation effectively protects VEGF bioactivity despite prolonged in vivo exposure. The extracted factor showed a slightly reduced activity compared with fresh recombinant VEGF (∼80% relative efficacy) as a consequence of the prolonged manipulations necessary for the gel-extraction procedure.
Dose-Dependent Angiogenesis by Fibrin-Bound α2-PI1–8-VEGF164.
Since decreasing α2-PI1–8-VEGF164 from 100 μg/mL to 25 μg/mL caused a shift from completely aberrant to mostly normal angiogenesis, we investigated the effects of lower α2-PI1–8-VEGF164 concentrations. Nine days after intramuscular injection, 5 μg/mL, 1 μg/mL, and 0.1 μg/mL α2-PI1–8-VEGF164 all induced exclusively normal and mature capillaries, associated with nerve/glia antigen 2 (NG2)-positive and α-smooth muscle actin (α-SMA)-negative pericytes (Fig. S4).
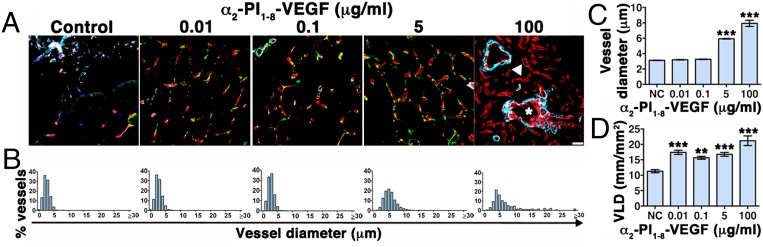
Sustained VEGF stimulation for at least 4 wk is necessary for newly induced vessels to stabilize and become VEGF-independent (7, 9, 10). Four weeks after intramuscular implantation of gels carrying four different α2-PI1–8-VEGF164 concentrations (0.01 μg/mL, 0.1 μg/mL, 5 μg/mL, and 100 μg/mL), small amounts of nondegraded gel were still detectable (Fig. S5), and robust angiogenesis was present in all conditions (Fig. 2 A and D). Similarly to the 9-d results, 100 μg/mL α2-PI1–8-VEGF164 generated aberrant angioma-like structures mixed with more regular capillaries, which were, however, completely devoid of mural cells whereas both 0.1 μg/mL and 5 μg/mL induced exclusively normal networks of mature capillaries. Remarkably, the further 10-fold lower VEGF concentration of 0.01 μg/mL also induced efficient capillary growth, suggesting that normal angiogenesis can be effectively generated by a very wide range of α2-PI1–8-VEGF164 doses. All vessels induced by α2-PI1–8-VEGF164 concentrations up to 5 μg/mL displayed the morphology of normal skeletal-muscle capillaries as they were covered by NG2+/SMA− pericytes, but no SMA+ smooth muscle (22) and had homogeneous diameter distributions in a narrow range within 10 µm (90th percentile, 0.01 μg/mL = 4.62 µm; 0.1 μg/mL = 4.53 µm; 5 μg/mL = 8.47 µm), similar to normal capillaries in control tissues (90th percentile, 4.48 µm). In contrast, aberrant structures induced by 100 μg/mL displayed very heterogeneous and enlarged sizes (90th percentile, 16.05 µm) and were covered by a thick smooth-muscle coat (Fig. 2 A and B). Interestingly, concentrations ≤0.1 μg/mL induced vessels with the same average diameter as control tissue whereas the capillaries induced by 5 μg/mL were significantly larger (Fig. 2C), although homogeneous in size and smaller than 10 µm and normal in morphology. In contrast to vessel diameters, the amount of angiogenesis, quantified as vessel length density (VLD), did not depend on the α2-PI1–8-VEGF164 dose as all concentrations between 0.01 µg/mL and 5 µg/mL similarly increased VLD by 50–60% compared with controls (Fig. 2D).
Fig. 2.
Dose-dependent angiogenesis 4 wk after optimized delivery of fibrin-bound α2-PI1–8-VEGF164. Fibrin gels containing 56 μg/mL aprotinin-α2-PI1–8 and 0 μg/mL (negative control), 0.01 μg/mL, 0.1 μg/mL, 5 μg/mL, or 100 μg/mL α2-PI1–8-VEGF164 were injected into gastrocnemius muscles of SCID mice. Tissues were analyzed 4 wk later. (A) Frozen sections were immunostained to detect endothelial cells (CD31, in red), pericytes (NG2, in green), and smooth-muscle cells (α-SMA, in cyan). n = 3. (Scale bar: 20 μm.) Asterisks, enlarged aberrant vascular structures; arrowhead, regular capillaries devoid of mural cells. (B and C) Quantification of vessel diameters (n > 500 per group), shown as their distribution in 1-μm intervals (B) and mean ± SEM (C). (D) The amount of angiogenesis was quantified as vessel length density (VLD): i.e., the total vessel length in the area of each measured field (n = 5–10 fields per group); ***P < 0.001, **P < 0.01 vs. negative control (NC).
Sustained vascular leakage is a side effect of VEGF delivery that can lead to harmful tissue edema (23). Therefore, plasma leakage was quantified 4 wk after implantation of gels containing 5 µg/mL α2-PI1–8-VEGF164: i.e., the highest concentration inducing morphologically normal angiogenesis at the latest time when gel-bound factor was still present (Fig. S5). Consistent with previous results (7), newly induced normal vessels were not leaky after 4 wk of VEGF delivery, with similar levels of Evans blue extravasation as the background measured in control tissues implanted with empty gels containing no VEGF (controls, 6.49 ± 1.54 ng Evans blue/mg tissue vs. VEGF, 5 μg/mL, 6.98 ± 1.83 ng Evans blue/mg tissue; P = n.s.), suggesting they had acquired normal functionality. Moreover, no macroscopic edema was observed in the implanted limbs at any time up to 3 mo.
Long-Term Stability and Perfusion of Vessels Induced by Optimized α2-PI1–8-VEGF164 Delivery.
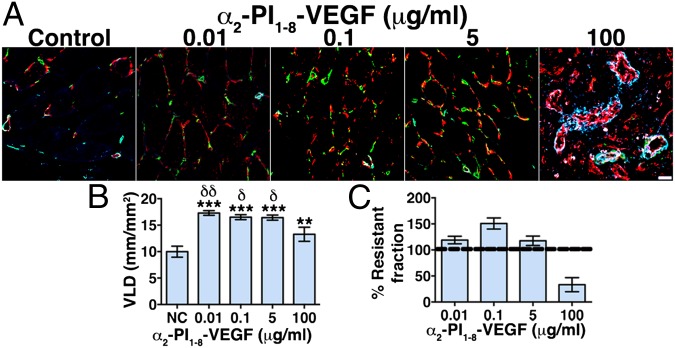
We assessed whether α2-PI1–8-VEGF164–induced vessels had stabilized and achieved complete independence from exogenous VEGF 3 mo after intramuscular gel injection. Angiogenesis was still present in all conditions, displaying similar morphologies as after 4 wk (Fig. 3A) although no trace of the injected gels could be found anymore (Fig. S5). All α2-PI1–8-VEGF164 concentrations causing normal angiogenesis (0.01–5 μg/mL) induced about a 70% increase in VLD, which was also significantly more than the amount still present after delivery of 100 μg/mL that induced aberrant angiogenesis (Fig. 3B). A comparison between VLD after 3 mo and 4 wk showed that the normal vessels generated by concentrations between 0.01 μg/mL and 5 μg/mL were completely stable and even further increased whereas greater than 60% of the aberrant vasculature induced by 100 μg/mL regressed (Fig. 3C). Further, i.v. injection of a fluorescein-labeled tomato lectin (FITC-lectin) showed that essentially all endothelial structures induced by the different α2-PI1–8-VEGF164 concentrations were reached by blood flow and thereby functionally connected to the systemic circulation both after 4 wk (Fig. S6) and 3 mo (Fig. S7), as demonstrated by colocalization of CD31 immunostaining and FITC-lectin. Therefore, optimized delivery of fibrin-bound α2-PI1–8-VEGF164 induced the growth of morphologically normal and mature capillary networks that were both stable and functional.
Fig. 3.
Long-term stability of normal, but not aberrant, angiogenesis induced by optimized delivery of fibrin-bound α2-PI1–8-VEGF164. Fibrin gels containing 56 μg/mL aprotinin-α2-PI1–8 and 0 μg/mL (negative control), 0.01 μg/mL, 0.1 μg/mL, 5 μg/mL, and 100 μg/mL α2-PI1–8-VEGF164 were injected into gastrocnemius muscles of SCID mice. Tissues were analyzed 3 mo later. (A) Frozen sections were immunostained to detect endothelial cells (CD31, in red), pericytes (NG2, in green), and smooth-muscle cells (α-SMA, in cyan). n = 3. (Scale bar: 20 μm.) (B) The amount of angiogenesis was quantified as vessel length density (mean VLD ± SEM; n = 5–10 fields per group); ***P < 0.001 or **P < 0.01 vs. negative control, δδP < 0.01, δP < 0.05 vs. the 100 μg/mL condition. (C) The stabilization rate of vessels induced in each condition was calculated as the percentage ratio between VLD 3 mo and 4 wk after gel injection (% Resistant fraction), with a value of 100% or higher indicating complete stabilization and a value lower than 100% indicating vascular regression between the two time points.
Functional Improvement in Ischemia.
Lastly, we tested the efficacy of optimized delivery of fibrin-bound α2-PI1–8-VEGF164 to cause functional improvement in two different rodent models of clinically relevant ischemic diseases: i.e., hind-limb ischemia and ischemic wound healing.
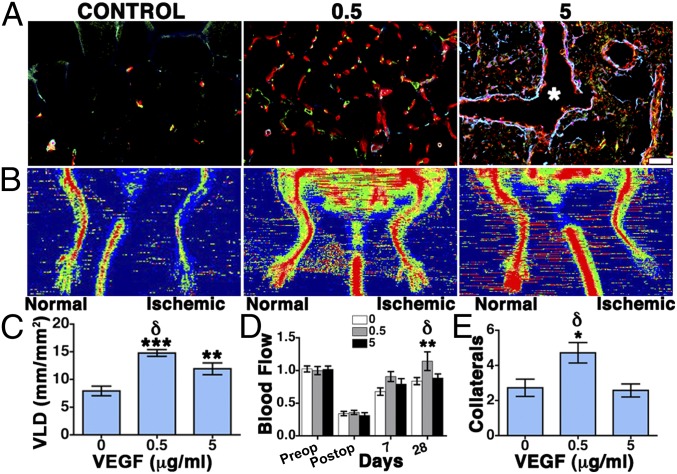
Based on the results described in Figs. 2 and 3 for normal skeletal muscle, two α2-PI1–8-VEGF concentrations in a 10-fold range (0.5 μg/mL and 5 μg/mL) were delivered through the optimized gel composition in a murine model of hind-limb ischemia. An empty gel of the same composition was used as a negative control. Histological analysis (Fig. 4A) and VLD quantification (Fig. 4C) showed that, after 4 wk, 0.5 μg/mL α2-PI1–8-VEGF164 promoted the growth of an abundant network of morphologically normal and mature capillaries, associated with NG2+/SMA− pericytes, compared with negative control. However, contrary to the results in normal muscle, 5 μg/mL α2-PI1–8-VEGF led to a lower increase in VLD (Fig. 4C) and actually caused the growth of aberrant angioma-like structures, devoid of pericytes, and encompassed by smooth-muscle cells (asterisk in Fig. 4A). Blood flow was recorded by laser Doppler imaging both in the ischemic and nonoperated contralateral leg preoperatively, immediately after surgery and 7 d and 28 d later. To account for variables, including ambient light and temperature, calculated perfusion was expressed as a ratio of that in the ischemic to normal limb, as previously described (24). Blood flow in ischemic muscles was unaffected by 5 μg/mL α2-PI1–8-VEGF164 compared with control (Fig. 4 B and D) but was significantly improved by 0.5 μg/mL α2-PI1–8-VEGF164 after 4 wk compared with both control and 5 μg/mL VEGF (Fig. 4 B and D). Consistent with the improvement in blood flow, after 4 wk, the 0.5 μg/mL α2-PI1–8-VEGF164 condition also significantly increased the number of histologically visible collateral arteries in the cranial part of the adductor thigh muscles compared with both other treatments (Fig. 4E).
Fig. 4.
Functional improvement of hind-limb ischemia by α2-PI1–8-VEGF164. Fibrin gels containing 56 μg/mL aprotinin-α2-PI1–8 and 0 μg/mL (negative control), 0.5 μg/mL, and 5 μg/mL α2-PI1–8-VEGF164 were injected into the lower thigh muscles. Tissues were analyzed 4 wk later. (A) Frozen sections were immunostained to detect endothelial cells (CD31, in red), pericytes (NG2, in green), and smooth-muscle cells (α-SMA, in cyan). n = 3. (Scale bar: 20 μm.) (B) Representative laser Doppler images of nonischemic and ischemic limbs (left and right side of each image, respectively) 28 d after treatment with control gels (control) or 0.5 μg/mL and 5 μg/mL α2-PI1–8-VEGF164. (C) The amount of angiogenesis was quantified as vessel length density (mean VLD ± SEM; n = 5–10 fields per group); ***P < 0.001 and **P < 0.01 vs. negative control, δP < 0.05 vs. the 0.5 μg/mL condition. (D) Blood flow was measured by laser Doppler imaging before surgery (preop) to set the baseline, immediately after (postop) and 7 and 28 d later. Results were expressed as the ratio between measured flow in the ischemic and the contralateral nonoperated hind limb. (E) The number of collateral arteries was determined histologically in the adductor muscles after 4 wk (mean ± SEM per field; n = 5–10 fields per group); *P < 0.05 vs. negative control, δP < 0.05 vs. the 0.5 μg/mL condition.
To determine the general applicability of this approach, we further investigated whether the optimized delivery of fibrin-bound α2-PI1–8-VEGF164 could promote functional improvement in a distinct ischemic wound-healing model. Based on the results described in Fig. 4, showing that the threshold between normal and aberrant angiogenesis in ischemic tissue lies between 0.5 μg/mL and 5 μg/mL α2-PI1–8-VEGF164, a concentration of 2 μg/mL was used in the optimized fibrin-matrix composition as the therapeutic condition, with an empty gel of the same composition as negative control. Histological analysis (Fig. S8 A and B) and VLD quantification (Fig. S8 C and E) showed that, after 7 d, treatment with α2-PI1–8-VEGF164 significantly increased dermis vascularization both in nonischemic and ischemic wounds. Microvessels were associated with NG2+ pericytes in all groups (Fig. S8 A and B). Furthermore, α-SMA+ mural cells were found associated with both microvessels and small-caliber arterioles and venules (arrowheads in Fig. S8 A and B) because, contrary to muscle tissue, in the skin, a proportion of capillary pericytes also express α-SMA (25). The α2-PI1–8-VEGF164–treated tissues displayed a greater density of regularly shaped larger vessels (>15 μm) with the features of arterioles (covered by a regular smooth-muscle layer and homogeneous in size), consistently with the previously described effect of VEGF to cause arteriolization of preexisting vessels (26). Laser Doppler imaging showed that the increased vessel density in the α2-PI1–8-VEGF164–treated wounds correlated with significantly improved tissue perfusion both in the nonischemic and ischemic sites by 7 d (Fig. S8 D, F, and G) (P < 0.05).
To evaluate the functional effects of increased angiogenesis and perfusion in the treated tissues, wound healing was analyzed. Under nonischemic conditions (Fig. S9A), the control-treated tissues showed signs of very mild inflammation with hyper/parakeratosis, without clearly identifiable dermis beneath the epidermis and with bleeding areas. The underlying muscle layer was fully infiltrated by inflammatory cells, and several muscle fibers displayed signs of partial necrosis, evidenced by infiltration of mononuclear inflammatory cells inside the myofibers. α2-PI1–8-VEGF164 treatment slightly reduced epidermal hyperplasia, above which a layer of keratinized tissue with several apoptotic bodies was visible, whereas the stratus corneum was partially present, indicating that the α2-PI1–8-VEGF164–treated tissue had reached a more advanced stage of regeneration of the physiological skin structure. Further, α2-PI1–8-VEGF164 treatment reduced the inflammatory infiltrate in the underlying muscle layer and completely prevented myofiber damage. Under ischemic conditions (Fig. S9B), control tissues displayed moderate hyperplasia of the epidermis, which was covered with a thick keratinized tissue full of apoptotic bodies. Both the dermis and the underlying muscle layer were prominently infiltrated with inflammatory cells, the muscle fibers were completely disorganized, many were invaded by monocytes, and several degenerative vacuoles were visible within the muscle layer. However, in the α2-PI1–8-VEGF164–treated wounds, both epidermal hyperplasia and the thickness of the keratinized tissue with apoptotic bodies were reduced compared with controls. Further, α2-PI1–8-VEGF164 treatment reduced the inflammatory infiltrate in the muscle layer and prevented myofiber damage, avoiding tissue necrosis. Therefore, histological analysis showed that treatment with α2-PI1–8-VEGF164 significantly improved tissue regeneration and restoration of the physiological structure. Consistently, the quantification of wound-healing rate showed that α2-PI1–8-VEGF164 promoted a significant acceleration of ischemic wound closure 7 d after gel implantation (Fig. S9D). Nonischemic wounds were also smaller both 3 d and 7 d after α2-PI1–8-VEGF164 treatment although the differences were not statistically significant (Fig. S9C).
Discussion
Here, we found that the combination of fibrin-bound α2-PI1–8-VEGF164 with the fibrin-bound fibrinolysis inhibitor aprotinin-α2-PI1–8 provides a highly tunable platform to precisely control the VEGF dose delivered to tissues and efficiently induce stable and functional angiogenesis. Incorporation of aprotinin-α2-PI1–8 at an optimal concentration is a key requirement to efficiently induce normal angiogenesis in vivo through its control of fibrinolysis and thus release of α2PI1–8VEGF164. Although differing release rates of the same α2-PI1–8-VEGF164 concentration could cause disparate effects, from none to aberrant angiogenesis, optimal gel degradation rates enabled fibrin-bound α2-PI1–8-VEGF164 to reliably induce exclusively normal, mature, stable, and functionally perfused microvascular networks over a 500-fold range of concentrations in normal muscle tissue. Remarkably, newly induced vessels did not regress for at least 3 mo whereas the implanted gels were almost completely consumed by 4 wk, demonstrating that they achieved independence from exogenous VEGF signaling for their survival and suggesting that they could persist indefinitely. This finding is particularly relevant for the therapeutic potential of this approach because one of the main limitations of recombinant protein delivery for therapeutic angiogenesis has been the insufficient duration of factor release to achieve persistent effects (20). On the other hand, aberrant angiogenesis induced by 100 µg/mL α2-PI1–8-VEGF164 failed to recruit physiological pericyte coverage by 4 wk, when gel-bound factor was still present, consistently with the described function of VEGF as a negative regulator of pericyte function through formation of nonfunctional VEGFR-2/PDGFR-β complexes and consequent inhibition of PDGFR-β phosphorylation (27). When exogenous α2-PI1–8-VEGF-A164 became exhausted, more than 60% of the vascular structures induced by this high dose disappeared, indicating that they could not stabilize despite sustained VEGF release for at least 4 wk.
Therefore, incorporation of 56 μg/mL aprotinin-α2-PI1–8 ensured an optimal gel degradation rate in vivo, which is the key parameter controlling both the duration and the effective dose of factor delivery. Under these optimized conditions, the angiogenic outcome was solely controlled by α2-PI1–8-VEGF164 concentration, with aberrant vascular structures being induced by levels ≥25 μg/mL and physiological functional capillary networks resulting from a wide range of doses 0.01–5 μg/mL. The high tunability of the fibrin-bound delivery platform allowed us to span an extremely wide range of VEGF doses, including those determining the transition between normal and aberrant angiogenesis, which is a key feature for the design of preclinical dose-escalation studies. It should be noted that we chose to deliver the syngenic mouse recombinant factor mVEGF164 instead of the clinically used human homolog hVEGF165 because the experiments were performed in a mouse model and we recently found that the dose-dependent effects of VEGF are species-specific (28).
The concentration of α2-PI1–8-VEGF164 did not affect the amount of induced vasculature because already the lowest dose caused the maximum increase in VLD, which was maintained over a 500-fold range. However, the dose of α2-PI1–8-VEGF164 influenced the size of induced vessels, which remained unaffected by concentrations up to 0.1 μg/mL but was significantly increased by 5 μg/mL. These results are relevant for the therapeutic potential of α2-PI1–8-VEGF164 delivery. In fact, we and others have previously shown that the size of newly induced vessels is key to determine the efficacy of therapeutic angiogenesis approaches (29, 30) as a similar increase in number without increase in size provided no therapeutic benefit (29). Based on these considerations, two different α2-PI1–8-VEGF164 concentrations in the upper range of normal angiogenesis, 0.5 μg/mL and 5 μg/mL, were chosen to investigate dose-dependent functional improvement in a hind-limb ischemia model. Results showed that 0.5 μg/mL was effective to induce functional improvement as it caused a twofold increase in the amount of normal capillaries and significantly increased blood flow in ischemic tissue compared with controls. However, 5 μg/mL α2-PI1–8-VEGF164, which induced only normal and stable angiogenesis in nonischemic muscle, actually stimulated the growth of aberrant vascular structures that failed to improve blood flow and collateral arteriogenesis in ischemic tissue. This disparate effect of the same concentration of α2-PI1–8-VEGF164 may be attributable to the elevated levels of inflammatory cells and proteases during ischemia, which accelerate gel degradation and thus the effective rate of growth factor release. These results highlight the need to determine the therapeutic window of fibrin-bound α2-PI1–8-VEGF164 delivery specifically for each envisioned clinical application as the unique biological variables associated with different pathologies can influence the effective degradation rate, factor release, and angiogenic outcome.
Considering the results obtained in the normal and ischemic hind limb, a concentration of 2 μg/mL was chosen to test the functional efficacy of fibrin-bound α2-PI1–8-VEGF164 in an ischemic wound-healing model. The treatment with α2-PI1–8-VEGF164 stimulated an 80% increase in normal angiogenesis, which significantly improved tissue perfusion both in nonischemic and ischemic tissues. The angiogenic stimulus induced a significant improvement of the wound closure in ischemic conditions and a positive trend toward improvement in nonischemic tissues. Interestingly, in control conditions, ischemia significantly slowed wound healing compared with normal tissue (55% vs. 38% still-open wound size by 7 d), and α2-PI1–8-VEGF164 treatment restored healing in ischemia to the nonischemic level (36% still-open wound size by 7 d). In nonischemic tissue, α2-PI1–8-VEGF164 treatment provided a further benefit (28% still-open wound size by 7 d), but the magnitude of the difference was insufficient to reach statistical significance. These data suggest that, in the absence of ischemia, tissue repair proceeds already at physiological speed and that increased blood flow could improve it only marginally whereas, in ischemic conditions, impaired perfusion is the critical factor limiting wound healing, and α2-PI1–8-VEGF164 treatment may unfold its therapeutic effect. Consistently with this concept, it has been previously found that skin wounding in normal conditions leads to a transient localized ischemia due to microvascular damage and a six- to sevenfold up-regulation of endogenous VEGF expression, but this up-regulation is not further increased if wounding is carried out under conditions of ischemia (31).
In ischemic tissue, maximum blood supply is limited, and the opening of collateral arteries (arteriogenesis) is required to restore physiological flow levels to meet the metabolic demands of regenerating tissue. Microvascular angiogenesis by VEGF can induce arteriogenesis by increasing blood flow and shear stress (1) and generating upstream responses through retrograde conduction along vessel walls via intercellular gap junctions (2). However, in chronic ischemia, spontaneous angiogenesis is insufficient to restore physiological flow, and we have previously found that VEGF doses higher than the maximal up-regulation achieved by the endogenous response are necessary to significantly increase both the amount and size of microvascular networks, induce collateral arteriogenesis, and achieve therapeutic benefit (29). Our results suggest that such doses can be effectively achieved in ischemic tissue by optimized delivery of fibrin-bound α2-PI1–8-VEGF164. However, as the specific conditions prevalent in different tissues and pathologic states dictate the actual degradation rate and therefore the effective growth factor release in vivo, it is impossible to define a general therapeutic window. In this respect, the high tunability of the optimized platform developed in this study, with normal and stable angiogenesis being induced in healthy skeletal muscle over a 500-fold range of α2-PI1–8-VEGF164 concentrations, provides a key enabling tool for specific dose-finding studies for each envisioned clinical application, such as peripheral or coronary artery disease, or chronic wounds, so that dosage can be carefully determined and adapted.
Methods
Detailed information is provided in SI Methods.
Recombinant α2PI1–8-VEGF164 Production and Purification.
The transglutaminase substrate sequence NQEQVSPL (α2-PI1–8) was fused to the N terminus of the mouse VEGF-A164 cDNA by PCR. The fusion protein was expressed into Escherichia coli strain BL21 (Dε3) pLys (Novagen) and isolated as described previously (32) and in SI Methods.
Intramuscular Fibrin-Gel Implantation.
Fifty microliters of fibrin hydrogel, prepared as described in SI Methods, was aspirated with an insulin syringe with integrated 30G needle (Becton Dickinson) and injected into the gastrocnemius muscle of 6- to 8-wk-old immunodeficient SCID CB.17 mice (Charles River), previously anesthetized by 3% isofluorane inhalation.
Hind-Limb Ischemia.
Hind-limb ischemia experiments were performed on female CD1-mice (Charles River) by unilateral femoral artery ligation and excision, as detailed in SI Methods. Blood flow was measured by a laser Doppler imaging system (Moor Instruments), and results were presented as a ratio of the flow in the ischemic to that in the contralateral normally perfused hind limbs to account for variables, including ambient light and temperatures, as previously described (24) and as detailed in SI Methods.
Ischemic Wound Healing.
An ischemic wound-healing model was performed on male Sprague–Dawley rats (Harlan-Winkelmann; n = 6 per group), based on a modification of a previously described epigastric flap model (33) and as detailed in SI Methods.
Supplementary Material
Acknowledgments
We thank Celine Dessibourg (Ecole Polytechnique Fédérale de Lausanne) for preparation of the recombinant proteins and Sabine Pfeifer, Tatjana Morton, and the technical staff at the Ludwig Boltzmann Institute for invaluable assistance with in vivo imaging and wound-healing experiments. This work was supported in part by Swiss National Science Foundation Grants 127426 and 143898 (to A.B.) and by European Union FP7 Grant ANGIOSCAFF (CP-IP 214402) (to A.B., J.A.H., H.R., and M.E.).
Footnotes
Conflict of interest statement: The fibrin gel immobilization scheme is the subject of patents upon which J.A.H. is named as inventor and has been licensed by a company in which J.A.H. is a shareholder.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404605111/-/DCSupplemental.
References
- 1.Rissanen TT, et al. Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation. 2005;112(25):3937–3946. doi: 10.1161/CIRCULATIONAHA.105.543124. [DOI] [PubMed] [Google Scholar]
- 2.Pries AR, Höpfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: Control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10(8):587–593. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105(8):724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ylä-Herttuala S, Markkanen JE, Rissanen TT. Gene therapy for ischemic cardiovascular diseases: Some lessons learned from the first clinical trials. Trends Cardiovasc Med. 2004;14(8):295–300. doi: 10.1016/j.tcm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Karvinen H, Ylä-Herttuala S. New aspects in vascular gene therapy. Curr Opin Pharmacol. 2010;10(2):208–211. doi: 10.1016/j.coph.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa CR, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4(12):1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dor Y, et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21(8):1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tafuro S, et al. Inducible adeno-associated virus vectors promote functional angiogenesis in adult organisms via regulated vascular endothelial growth factor expression. Cardiovasc Res. 2009;83(4):663–671. doi: 10.1093/cvr/cvp152. [DOI] [PubMed] [Google Scholar]
- 11.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21(32-33):3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 12.Banfi A, von Degenfeld G, Blau HM. Critical role of microenvironmental factors in angiogenesis. Curr Atheroscler Rep. 2005;7(3):227–234. doi: 10.1007/s11883-005-0011-7. [DOI] [PubMed] [Google Scholar]
- 13.Breen A, O’Brien T, Pandit A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng Part B Rev. 2009;15(2):201–214. doi: 10.1089/ten.TEB.2008.0527. [DOI] [PubMed] [Google Scholar]
- 14.Schense JC, Bloch J, Aebischer P, Hubbell JA. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat Biotechnol. 2000;18(4):415–419. doi: 10.1038/74473. [DOI] [PubMed] [Google Scholar]
- 15.Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem. 1999;10(1):75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 16.Ehrbar M, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res. 2004;94(8):1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 17.Schmoekel HG, et al. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol Bioeng. 2005;89(3):253–262. doi: 10.1002/bit.20168. [DOI] [PubMed] [Google Scholar]
- 18.Lorentz KM, Yang L, Frey P, Hubbell JA. Engineered insulin-like growth factor-1 for improved smooth muscle regeneration. Biomaterials. 2012;33(2):494–503. doi: 10.1016/j.biomaterials.2011.09.088. [DOI] [PubMed] [Google Scholar]
- 19.Traub S, et al. The promotion of endothelial cell attachment and spreading using FNIII10 fused to VEGF-A165. Biomaterials. 2013;34(24):5958–5968. doi: 10.1016/j.biomaterials.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 20.Ehrbar M, et al. The role of actively released fibrin-conjugated VEGF for VEGF receptor 2 gene activation and the enhancement of angiogenesis. Biomaterials. 2008;29(11):1720–1729. doi: 10.1016/j.biomaterials.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Lorentz KM, Kontos S, Frey P, Hubbell JA. Engineered aprotinin for improved stability of fibrin biomaterials. Biomaterials. 2011;32(2):430–438. doi: 10.1016/j.biomaterials.2010.08.109. [DOI] [PubMed] [Google Scholar]
- 22.Armulik A, Genové G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Masaki I, et al. Angiogenic gene therapy for experimental critical limb ischemia: Acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res. 2002;90(9):966–973. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
- 24.Couffinhal T, et al. Mouse model of angiogenesis. Am J Pathol. 1998;152(6):1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 25.Sundberg C, et al. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol. 2001;158(3):1145–1160. doi: 10.1016/S0002-9440(10)64062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Springer ML, et al. Localized arteriole formation directly adjacent to the site of VEGF-induced angiogenesis in muscle. Mol Ther. 2003;7(4):441–449. doi: 10.1016/s1525-0016(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg JI, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mujagic E, et al. Induction of aberrant vascular growth, but not of normal angiogenesis, by cell-based expression of different doses of human and mouse VEGF is species-dependent. Hum Gene Ther Methods. 2013;24(1):28–37. doi: 10.1089/hgtb.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Degenfeld G, et al. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. FASEB J. 2006;20(14):2657–2659. doi: 10.1096/fj.06-6568fje. [DOI] [PubMed] [Google Scholar]
- 30.Korpisalo P, et al. Capillary enlargement, not sprouting angiogenesis, determines beneficial therapeutic effects and side effects of angiogenic gene therapy. Eur Heart J. 2011;32(13):1664–1672. doi: 10.1093/eurheartj/ehq433. [DOI] [PubMed] [Google Scholar]
- 31.Corral CJ, et al. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg. 1999;134(2):200–205. doi: 10.1001/archsurg.134.2.200. [DOI] [PubMed] [Google Scholar]
- 32.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF: Fibrin matrices for endothelialization. J Control Release. 2001;72(1-3):101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 33.Michlits W, Mittermayr R, Schäfer R, Redl H, Aharinejad S. Fibrin-embedded administration of VEGF plasmid enhances skin flap survival. Wound Repair Regen. 2007;15(3):360–367. doi: 10.1111/j.1524-475X.2007.00238.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.