Significance
Cadmium (Cd), a trace nutrient for marine algae, has a marine distribution that closely matches the macronutrients nitrate and phosphate. Sedimentary microfossil records of Cd provide reconstructions of past ocean nutrient distributions that facilitate understanding the role of the oceans in the carbon cycle and climate change. However, incomplete knowledge of processes that control the addition and removal of Cd in the ocean, and Cd’s variability relative to major nutrients, limit use of the paleoceanographic proxy. We present coupled data of Cd concentration and isotopic composition in seawater and suspended marine particles, indicating direct removal of Cd via coprecipitation with sulfide in oxygen-deficient waters. Thus, the marine Cd cycle may be highly sensitive to the extent of global oceanic oxygen depletion.
Keywords: biogeochemistry, paleoceanography, GEOTRACES, trace elements, marine chemistry
Abstract
Cadmium (Cd) is a micronutrient and a tracer of biological productivity and circulation in the ocean. The correlation between dissolved Cd and the major algal nutrients in seawater has led to the use of Cd preserved in microfossils to constrain past ocean nutrient distributions. However, linking Cd to marine biological processes requires constraints on marine sources and sinks of Cd. Here, we show a decoupling between Cd and major nutrients within oxygen-deficient zones (ODZs) in both the Northeast Pacific and North Atlantic Oceans, which we attribute to Cd sulfide (CdS) precipitation in euxinic microenvironments around sinking biological particles. We find that dissolved Cd correlates well with dissolved phosphate in oxygenated waters, but is depleted compared with phosphate in ODZs. Additionally, suspended particles from the North Atlantic show high Cd content and light Cd stable isotope ratios within the ODZ, indicative of CdS precipitation. Globally, we calculate that CdS precipitation in ODZs is an important, and to our knowledge a previously undocumented marine sink of Cd. Our results suggest that water column oxygen depletion has a substantial impact on Cd biogeochemical cycling, impacting the global relationship between Cd and major nutrients and suggesting that Cd may be a previously unidentified tracer for water column oxygen deficiency on geological timescales. Similar depletions of copper and zinc in the Northeast Pacific indicate that sulfide precipitation in ODZs may also have an influence on the global distribution of other trace metals.
Cadmium has a nutrient-type depth profile in the open ocean, with low concentrations in surface water due to phytoplankton uptake and higher concentrations in deep water where sinking biological material remineralizes, releasing Cd (1–3). Cadmium can act either as a nutrient or a toxin, and therefore influences both phytoplankton growth and community composition (4–7). In addition to the direct impact of Cd on marine microbial communities, dissolved Cd is strongly correlated with the major algal nutrient phosphate  (1, 2), in seawater. A paleoceanographic proxy for dissolved
(1, 2), in seawater. A paleoceanographic proxy for dissolved  therefore takes advantage of Cd incorporation into foraminiferal calcite tests, preserved in the sedimentary record (8, 9). Foraminiferal records of Cd have been used by a number of studies to reconstruct water mass distribution and nutrient utilization in past oceans (8–10).
therefore takes advantage of Cd incorporation into foraminiferal calcite tests, preserved in the sedimentary record (8, 9). Foraminiferal records of Cd have been used by a number of studies to reconstruct water mass distribution and nutrient utilization in past oceans (8–10).
Reliable tracers of paleoceanographic nutrient distributions in the ocean are vital to understanding past climate variability, and thus to predicting the potential impacts of anthropogenic climate change. Successful use of the foraminiferal Cd proxy to reconstruct past macronutrients requires a detailed understanding of Cd cycling and of factors which may influence the Cd: of the ocean. For example, it is well established that correlations between dissolved Cd and
of the ocean. For example, it is well established that correlations between dissolved Cd and 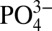 may vary due to regional differences in the rates at which phytoplankton take up Cd and
may vary due to regional differences in the rates at which phytoplankton take up Cd and 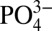 (3, 11). Recent studies (12) of dissolved Cd isotope ratios in the ocean (δ114Cd) indicate a nearly isotopically homogeneous deep ocean (∼0.3‰), which is heavier than crustal δ114Cd (∼0.1‰), indicative of an as-yet-unattributed sink for isotopically light Cd. The extent to which this sink induces local changes in Cd:
(3, 11). Recent studies (12) of dissolved Cd isotope ratios in the ocean (δ114Cd) indicate a nearly isotopically homogeneous deep ocean (∼0.3‰), which is heavier than crustal δ114Cd (∼0.1‰), indicative of an as-yet-unattributed sink for isotopically light Cd. The extent to which this sink induces local changes in Cd: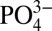 must be understood to correctly interpret paleoceanographic records.
must be understood to correctly interpret paleoceanographic records.
Results and Discussion
Cadmium and other trace metals such as copper (Cu) and zinc (Zn) are known to form solid sulfide precipitates in the ocean under conditions of anoxia where sulfide is present. For example, precipitation of Cd sulfide (CdS) (13) leading to dissolved Cd depletion is observed at oxic–anoxic interfaces in stratified basins with permanently or seasonally anoxic waters (14, 15). Here, for the first time to our knowledge, we provide evidence that CdS precipitation may occur in oxic open-ocean waters, operationally defined as containing measureable oxygen (typically <3 μmol kg−1using common methods) (16). At the surface of the ocean, oxygen saturation in seawater is usually 200–300 μmol kg−1. In regions where we observe evidence of CdS precipitation, oxygen concentrations are significantly depleted by heterotrophic respiration, but significantly above detection limits. The northeast subarctic Pacific hosts the world’s most extensive oxygen-deficient zones (ODZ) (Fig. 1), with oxygen depletion (<50 μmol kg−1) extending from 400 to 1,800 m depth and minimum oxygen concentrations of 10–20 μmol kg-1 at ∼1,000 m (Fig. 2) (17). The ODZ in the eastern subtropical North Atlantic underlies the Mauritanian upwelling region and, like the Pacific ODZ, is most intense along the eastern margin (Fig.1), where oxygen deficiency (<75 μmol kg−1) extends from 100 to 750 m depth, with a minimum of 45 μmol kg−1 near 400 m (Fig. 2).
Fig. 1.
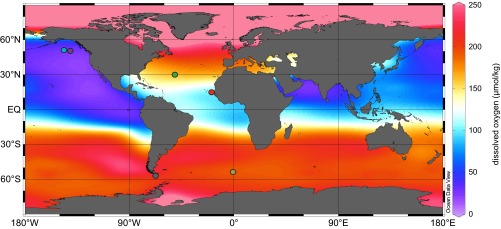
Sampling locations for this study overlain on water column minimum dissolved oxygen concentrations (45). New data are presented here from the eastern North Pacific at station P20 (49.6°N, 139.7°W, purple) and North Atlantic at stations USGT11-14 (27.6°N, 49.6°W, green) and USGT10-9 (17.4°N, 18.25°W, red). Previously published data are presented from station T7/P26 in the eastern North Pacific (50.0°N, 145.0°W, light blue) (46), and Southern Ocean/subantarctic stations 249 (56.1°S, 63.8°W, gray) (47), and PS71-113 (53.0°S, 0.3°W, tan) (21).
Fig. 2.
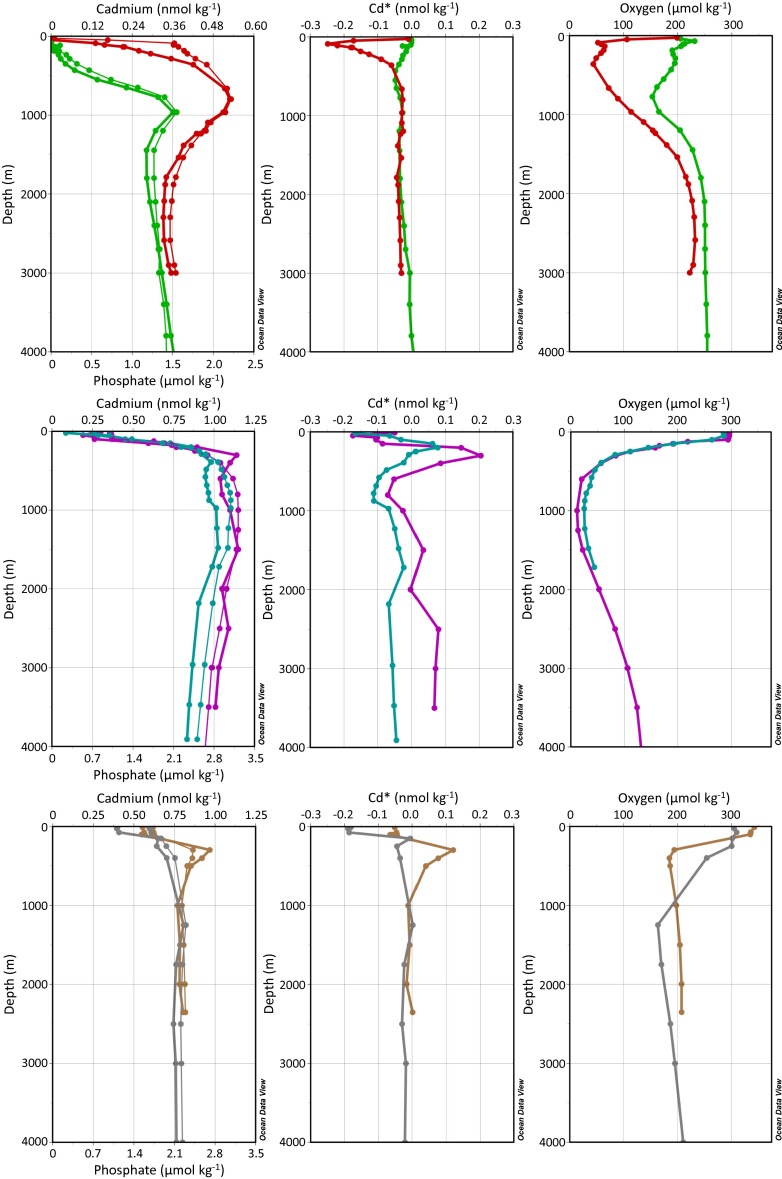
Water column profiles of dissolved oxygen, cadmium, and phosphate concentrations in the North Atlantic, Pacific, and Southern Oceans together with calculated Cd* for each location. (Top) Atlantic data; (Middle) Pacific data, and (Bottom) Southern Ocean–subantarctic data. Trace metal concentrations are shown in thick lines and macronutrients in thin lines, and colors correspond to station locations and studies in Fig. 1.
In the Pacific and Atlantic ODZs we observe a decoupling between concentrations of dissolved Cd and 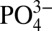 , compared with more oxygenated sites in the Southern Ocean and subantarctic South Atlantic, and the central North Atlantic (Figs. 1 and 2), where Cd and
, compared with more oxygenated sites in the Southern Ocean and subantarctic South Atlantic, and the central North Atlantic (Figs. 1 and 2), where Cd and 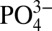 are well correlated throughout deep waters with average Cd:
are well correlated throughout deep waters with average Cd: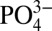 ratios of 0.33 ± 0.03 nmol μmol−1 and 0.23 ± 0.04 nmol μmol−1, respectively (1σ SD) (3). However, within the northeast Pacific and Mauritanian ODZs, dissolved Cd decreases relative to
ratios of 0.33 ± 0.03 nmol μmol−1 and 0.23 ± 0.04 nmol μmol−1, respectively (1σ SD) (3). However, within the northeast Pacific and Mauritanian ODZs, dissolved Cd decreases relative to 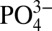 beginning near the point where dissolved O2 falls below about 75 µmol kg−1. In the northeast Pacific this is observed beginning at a depth of 400 m; whereas
beginning near the point where dissolved O2 falls below about 75 µmol kg−1. In the northeast Pacific this is observed beginning at a depth of 400 m; whereas 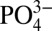 continues to increase in a nutrient-like fashion to a maximum at 1,000 m, Cd concentrations do not (Fig. 2). In the eastern North Atlantic, the decoupling between
continues to increase in a nutrient-like fashion to a maximum at 1,000 m, Cd concentrations do not (Fig. 2). In the eastern North Atlantic, the decoupling between 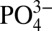 and Cd is observed as a much slower rate of increase in Cd concentrations below 80 m, compared with the rate at which
and Cd is observed as a much slower rate of increase in Cd concentrations below 80 m, compared with the rate at which 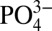 increases. The departure of Cd from expected concentrations within the ODZs, based on average deepwater Cd:
increases. The departure of Cd from expected concentrations within the ODZs, based on average deepwater Cd: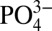 , can be visualized by plotting the variable Cd* (Methods), which is the excess or depletion of Cd compared with
, can be visualized by plotting the variable Cd* (Methods), which is the excess or depletion of Cd compared with 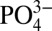 . Depletions in Cd* of up to 100 pmol kg-1and 250 pmol kg−1 are observed within the ODZs at the northeast Pacific sites and eastern North Atlantic sites, respectively. At depths where the water column is more oxygenated, Cd* depletions are generally <50 pmol kg−1 (Fig. 2).
. Depletions in Cd* of up to 100 pmol kg-1and 250 pmol kg−1 are observed within the ODZs at the northeast Pacific sites and eastern North Atlantic sites, respectively. At depths where the water column is more oxygenated, Cd* depletions are generally <50 pmol kg−1 (Fig. 2).
Suspended particles provide additional evidence of Cd precipitation within the North Atlantic ODZ (Fig. 3). The concentrations of both particulate Cd and P, like surface primary productivity, are higher in the eastern basin compared with the central basin. In the central North Atlantic where oxygen concentrations are higher, suspended particulate Cd and P are both high in surface waters where phytoplankton grow, and decrease monotonically with depth. Whereas suspended particulate P in the eastern basin also follows this trend, suspended particulate Cd concentrations and the Cd:P ratio reach a maximum within the ODZ. Elevated particulate Cd concentration and Cd:P ratios in the ODZ, compared with overlying waters including the chlorophyll maximum, suggests a source of particulate Cd within the ODZ in addition to sinking biogenic material.
Fig. 3.
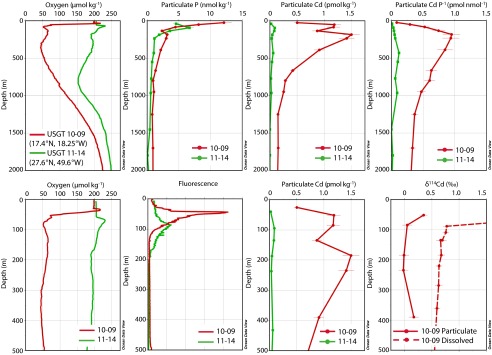
Particulate cadmium and phosphorus concentrations and cadmium stable isotope ratios (δ114Cd) from the US GEOTRACES North Atlantic Transect, shown with oxygen concentrations and fluorescence. Station USGT11-14 (27.6°N, 49.6°W) is shown in green and station USGT10-9 (17.4°N, 18.25°W) is shown in red. (Upper) Depth range of 0–2,000 m; (Lower) ODZ range from 0 to 500 m.
Particulate δ114Cd within the ODZ is consistent with CdS precipitation (Fig. 3). Because Cd is nearly quantitatively removed by phytoplankton growing within the euphotic zone, we expect the biogenic particulate δ114Cd flux out of the surface ocean to match the upward mixing flux of dissolved δ114Cd into the euphotic zone. Thus, assuming that the suspended biogenic particulate δ114Cd accurately reflects the biogenic flux, it should be +0.77‰, which is equivalent to dissolved δ114Cd at the base of the euphotic zone (89 m). Instead we observe particulate δ114Cd from −0.01 to +0.35‰, significantly lighter than the expected biogenic signature. Isotopically lighter particulate δ114Cd compared with seawater is consistent with CdS precipitation, assuming nonquantitative precipitation of seawater Cd with a preferential precipitation of lighter Cd isotopes into sulfides, as observed in low-temperature hydrothermal systems (18). The highest δ114Cd (+0.35‰) is observed just below the chlorophyll a maximum, perhaps reflecting a greater contribution of biogenic particulate Cd at this depth. The lowest δ114Cd values (−0.01‰ at 185 m and −0.02‰ at 235 m) are found within the ODZ at the same depths where we observe a maximum in particulate Cd:P. Together, the lighter δ114Cd of particles compared with seawater, and the increasing particulate [Cd] with depth within the ODZ, support a local dissolved source of Cd to particles rather than gradual remineralization of sinking biogenic Cd.
Several hypotheses have been advanced to explain the decoupling of Cd and P concentrations in the global ocean, although none seems appropriate to describe the decoupling observed in ODZs. Differences in the remineralization with depth of Cd and P are inconsistent with the relatively similar remineralization rates of Cd and P observed in a majority of existing vertical profiles (3). Biological uptake rates of Cd compared with 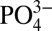 have been observed to vary with species composition, irradiance, trace metal concentration, and carbon dioxide availability (19). One consequence of this is that preformed dissolved Cd:
have been observed to vary with species composition, irradiance, trace metal concentration, and carbon dioxide availability (19). One consequence of this is that preformed dissolved Cd: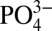 is lower in waters which ventilate in the Southern Ocean (20). Recent measurements of dissolved Cd:
is lower in waters which ventilate in the Southern Ocean (20). Recent measurements of dissolved Cd: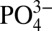 and δ114Cd suggest that Cd and
and δ114Cd suggest that Cd and 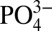 mix conservatively along isopycnals in the well-oxygenated ocean interior (21), meaning that some portion of the lowered Cd:
mix conservatively along isopycnals in the well-oxygenated ocean interior (21), meaning that some portion of the lowered Cd: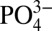 we observe in the northeastern subarctic Pacific may reflect mixing with low Cd:
we observe in the northeastern subarctic Pacific may reflect mixing with low Cd: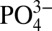 subantarctic and Antarctic waters. However, Cd:
subantarctic and Antarctic waters. However, Cd: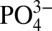 values at similar densities in the Southern Ocean are not low enough to account for the observed minimum Northeast Pacific Cd:
values at similar densities in the Southern Ocean are not low enough to account for the observed minimum Northeast Pacific Cd: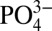 values. The low Cd:
values. The low Cd: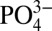 North Atlantic ODZ waters do not outcrop in the Southern Ocean and therefore cannot be explained by mixing of low Cd:
North Atlantic ODZ waters do not outcrop in the Southern Ocean and therefore cannot be explained by mixing of low Cd: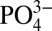 Southern Ocean water. Particulate Cd and δ114Cd in the North Atlantic ODZ also cannot be accounted for by mixing of water masses.
Southern Ocean water. Particulate Cd and δ114Cd in the North Atlantic ODZ also cannot be accounted for by mixing of water masses.
We have considered the possibility that low Cd: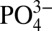 anomalies observed in the ocean interior could reflect lateral advection of waters modified by contact with reducing shelf sediments, which tend to concentrate Cd (22, 23), and to release P (24). However, we believe that these signals represent a water column process, rather than a sedimentary process, based on comparison with dissolved Fe stable isotope data (δ56Fe). Low δ56Fe at station USGT10-9 is a sensitive tracer of reduced Fe input, demonstrating that sedimentary inputs are strongest at the base of the water column near the sediments, rather than at shallower depths where the water column minimum in O2 coincides with low Cd*, high particulate Cd/P, and low particulate δ114Cd (25). Additionally, using maximum Cd accumulation rates into reducing sediments of 10 ng Cd cm−2⋅y−1 (23) the time necessary to form observed volume-specific Cd anomalies along an isopycnal would be ∼104 y, an order of magnitude greater than ocean circulation time. Ocean mixing would therefore be expected to obscure low Cd:
anomalies observed in the ocean interior could reflect lateral advection of waters modified by contact with reducing shelf sediments, which tend to concentrate Cd (22, 23), and to release P (24). However, we believe that these signals represent a water column process, rather than a sedimentary process, based on comparison with dissolved Fe stable isotope data (δ56Fe). Low δ56Fe at station USGT10-9 is a sensitive tracer of reduced Fe input, demonstrating that sedimentary inputs are strongest at the base of the water column near the sediments, rather than at shallower depths where the water column minimum in O2 coincides with low Cd*, high particulate Cd/P, and low particulate δ114Cd (25). Additionally, using maximum Cd accumulation rates into reducing sediments of 10 ng Cd cm−2⋅y−1 (23) the time necessary to form observed volume-specific Cd anomalies along an isopycnal would be ∼104 y, an order of magnitude greater than ocean circulation time. Ocean mixing would therefore be expected to obscure low Cd: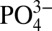 signals formed along ocean margins well before they could reach the ocean interior.
signals formed along ocean margins well before they could reach the ocean interior.
We propose that CdS precipitation occurs in response to sulfide generated within low-oxygen microenvironments associated with sinking organic matter (26), in analogy to the precipitation of barite (27). Differences in the oxygen threshold below which CdS precipitates in the Atlantic and Pacific may therefore be related to both the quantity and the composition of sinking organic particles. Microbial sulfide production in oxic waters of the Pacific ODZ is supported by genomic evidence for the presence of both heterotrophic sulfate reducing bacteria and sulfur oxidizing autotrophs (26, 28) and isotopically labeled incubation experiments showing sulfate reduction rates of up to 0.5–1 nmol L−1⋅S⋅h−1 in the core of the nearly anoxic eastern tropical South Pacific ODZ (28). CdS may also precipitate in waters with more extreme oxygen depletion such as the eastern tropical South Pacific ODZ, where sulfide concentrations of 1–5 µmol L−1 have been found throughout an extensive region (19). CdS is supersaturated at these sulfide concentrations, allowing Cd to be rapidly scavenged from the water column (13).
The enrichment of Cd in oxygen-depleted sediments has previously led to the suggestion that CdS precipitation from sediment porewaters is the primary sink for Cd from the oceans (22, 23). Based on the areal extent of low oxygen sediments in the modern ocean and calculated Cd flux into the sediments, Rosenthal et al. (22) calculated a removal rate of 0.6–2.3 × 107 mol y−1, which is similar to calculated Cd input from the atmosphere and world rivers (0.6–2.5 × 107 mol y−1). Because the Pacific ODZ is the largest worldwide, we use the observed Cd depletion from the northeast Pacific ODZ (∼100 pmol L−1) and the volume of the global ocean with dissolved O2 < 50 µmol L−1 (1.3–6.0 × 1019 L) (29), to estimate a global depletion of 0.1–0.5 × 1010 mol dissolved Cd from sulfide precipitation. Assuming that this depletion occurs on a timescale as short as the residence time of water in an ODZ (∼100 y) or as long as that of ocean mixing (∼103 y) yields a Cd removal rate range from 0.1 × 107 mol Cd y−1 to 0.5 × 108 mol Cd y−1. This calculation suggests that CdS precipitation in ODZs is an important vector for Cd delivery to suboxic sediments, possibly the primary sink for Cd from the oceans. As Cd associated with sinking particles is known to be the primary route to burial in suboxic sediments (30), the formation of particulate CdS species which are stable in oxic water (31) allows for distributed Cd burial in sediments and may represent a significant contribution to the sedimentary Cd enrichments seen in oxygen-depleted sediments.
A globally significant water column sink for Cd via Cd sulfide precipitation suggests that the distributions of other biologically used metals disposed to forming highly insoluble sulfides, such as Cu and Zn, may be similarly affected. Although particle and isotope data are not available for Cu and Zn, we note that Cu and Zn are depleted relative to Si for the same depths at which Cd is depleted relative to phosphate within the Northeast Pacific ODZ (Fig. 4), suggesting they may also be precipitated as sulfides. Cu and Zn are known to influence biological production and community composition (32–37) through taxon-specific requirements and toxic sensitivity. Indeed, removal of these metals relative to other bioessential trace metals like iron (Fe) and cobalt (Co) in the ocean interior could influence biogeochemical cycling through metal–metal antagonisms and colimitation when deep waters are brought to the surface in ocean upwelling zones. For example, within oxygen-deficient upwelling regions observations have been made of Zn limitation in eukaryotic phytoplankton (38), and of an increased biogeochemical significance of prokaryotes (39), perhaps reflecting Zn deficiency in upwelling waters combined with the higher Zn requirements of eukaryotic plankton compared with prokaryotic plankton (40, 41).
Fig. 4.
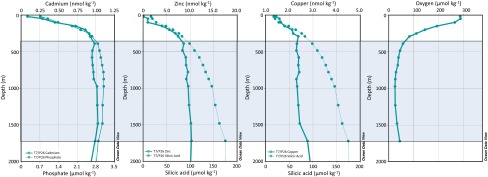
Concurrent decoupling of cadmium, zinc, and copper from corresponding macronutrients in the northeast subarctic Pacific. The trace-metal–macronutrient pairs of Cd-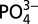 , Zn–Si, and Cu–Si all show decoupling in the northeast subarctic Pacific ODZ (station P26, 50.0°N 145.0°W). Whereas macronutrients (shown in thin lines) continue to increase monotonically throughout the ODZ, the trace metals (shown in thick lines) reach stable values or decrease slightly. The onset of decoupling, which begins near the depth where oxygen concentrations drop below ∼50 µmol kg−1 (shaded region), shows strong agreement between the three trace metal–macronutrient pairs.
, Zn–Si, and Cu–Si all show decoupling in the northeast subarctic Pacific ODZ (station P26, 50.0°N 145.0°W). Whereas macronutrients (shown in thin lines) continue to increase monotonically throughout the ODZ, the trace metals (shown in thick lines) reach stable values or decrease slightly. The onset of decoupling, which begins near the depth where oxygen concentrations drop below ∼50 µmol kg−1 (shaded region), shows strong agreement between the three trace metal–macronutrient pairs.
Conclusions
The implications for decoupling of Cd concentrations from macronutrient concentrations in ODZs span a wide range of timescales. On geological timescales, it suggests that in situ water column precipitation is at least a significant sink, if not the predominant sink of Cd from the oceans. Consequently, global variability in marine dissolved Cd concentrations and δ114Cd will be crucially dependent on the extent of ODZs in the world’s oceans over geological timescales. For studies investigating macronutrient distributions on glacial–interglacial timescales, our results suggest that the commonly used foraminiferal Cd proxy must be interpreted with caution in oxygen-depleted regions. In the future, the predicted expansion of oxygen depletion in the global oceans (42) means that water column sulfide precipitation may be an increasingly important sink for Cd as well as Cu and Zn from the ocean, with significant consequences for marine biogeochemical cycles due to the dual roles of these metals as both toxins and essential micronutrients.
Methods
Northeast Pacific Cd samples were collected along an onshore–offshore transect from the coast of Vancouver Island, BC, Canada to Ocean Station P (50°N, 145°W, depth 4,220 m) February 12–18, 2005, on board the CCGS J.P. Tully (43). Filtered (0.2 μm, Millipore Opticap) samples from 0 to 50 m were collected using a Teflon bellows pump. Samples from 50 to 600 m depth were collected using X-Niskin or GO-FLO (General Oceanics) bottles on a Kevlar line and were filtered immediately after collection through a 0.2-μm filter (Millipore Opticap). Samples below 600 m were collected on a standard metal frame rosette equipped with Niskin bottles and were not filtered. Duplicate samples were collected from the nontrace metal clean rosette and clean bottles on the Kevlar line to confirm that the standard rosette was of sufficient cleanliness for analysis (43). Samples were acidified with 12 mol L−1 ultrapure HCl (SEASTAR Chemicals Inc.) within 48 h of collection. Cadmium was analyzed using the 1-pyrrolidine dithiocarbamate–diethylammoniumdiethyldithiocarbamate organic extraction method followed by isotope dilution inductively coupled plasma mass spectrometry, and nutrients were determined with standard colorimetric techniques (43).
North Atlantic samples were collected as part of the US GEOTRACES A03 North Atlantic transect at stations USGT10-9 (17.4°N, 18.3°W) and USGT11-14 (27.6°N, 49.6°W). Dissolved samples (0.2-μm filtered) were collected using the US GEOTRACES trace-metal clean sampling system, and particulate samples were collected onto 0.8-μm Supor filters using in situ large-volume pumps. Seawater samples were acidified onshore with 1 mL 12 mol L−1 ultrapure HCl (VWR) and allowed to sit for several months before processing. Dissolved Cd was extracted from seawater onto Nobias PA-1 chelating resin, purified by an anion exchange chromatographic technique, and analyzed by Thermo Neptune multicollector inductively coupled plasma mass spectrometer at the Center for Elemental Mass Spectrometry at the University of South Carolina according to previously published methods (12). Particulate δ114Cd was determined following a pH 8 oxalate-EDTA (0.1–0.05 mol L−1) leach of particles; purification and analysis was performed according to the same procedures used for seawater. The stable isotope values for Cd are reported as δ114Cd relative to the National Institute of Standards and Technology Standard Reference Material 3108 standard by
 |
Cd* was calculated by
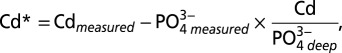 |
where 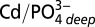 represents the average deepwater ratio in either the Pacific or Atlantic samples. A value of 0.25 is used for the deepwater ratio for Atlantic samples and 0.35 is used for Pacific and Southern Ocean–subantarctic samples. These values represent a best average composition for our North Atlantic and northeast Pacific samples and show agreement with published values. Because the absolute value of Cd* will vary depending on the value chosen for deepwater Cd:
represents the average deepwater ratio in either the Pacific or Atlantic samples. A value of 0.25 is used for the deepwater ratio for Atlantic samples and 0.35 is used for Pacific and Southern Ocean–subantarctic samples. These values represent a best average composition for our North Atlantic and northeast Pacific samples and show agreement with published values. Because the absolute value of Cd* will vary depending on the value chosen for deepwater Cd: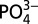 , we focus mainly on trends in Cd* within profiles and basins, which illustrate relative enrichments or depletions of Cd compared with
, we focus mainly on trends in Cd* within profiles and basins, which illustrate relative enrichments or depletions of Cd compared with 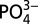 independent of the Cd:
independent of the Cd: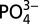 used. Figures in the manuscript were produced using Ocean Data View (44).
used. Figures in the manuscript were produced using Ocean Data View (44).
Supplementary Material
Acknowledgments
We thank Angela Rosenberg and Brandi Revels for technical assistance with analysis at the University of South Carolina. We thank the Captain and crew of the R/V Knorr, the dissolved and particle sampling teams on the US GEOTRACES North Atlantic Zonal Transect for collecting samples, and specifically Phoebe Lam for providing particulate samples. We thank the Captain and crew of the CCGS J.P. Tully, Line P program Chief Scientist Marie Robert, and the trace metal sampling team. S.G.J. and T.M.C. were funded by National Science Foundation Grant OCE-1131387. J.T.C. and T.F.P. were funded by Natural Sciences and Engineering Research Council (Canada).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Boyle EA, Sclater F, Edmond JM. On the marine geochemistry of cadmium. Nature. 1976;263(5563):42–44. [Google Scholar]
- 2.Bruland KW, Knauer GA, Martin JH. Cadmium in northeast Pacific waters. Limnol Oceanogr. 1978;23(4):618–625. [Google Scholar]
- 3.de Baar HJW, Saager PM, Notling RF, van der Meer J. Cadmium versus phosphate in the world ocean. Mar Chem. 1994;46(3):261–281. [Google Scholar]
- 4.Lane TW, Morel FMM. A biological function for cadmium in marine diatoms. Proc Natl Acad Sci USA. 2000;97(9):4627–4631. doi: 10.1073/pnas.090091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JG, Roberts SB, Morel FMM. Cadmium: A nutrient for the marine diatom Thalassiosira weissflogii. Limnol Oceanogr. 1995;40(6):1056–1063. [Google Scholar]
- 6.Price NM, Morel FMM. Cadmium and cobalt substitution for zinc in a marine diatom. Nature. 1990;344(6267):658–660. [Google Scholar]
- 7.Xu Y, Feng L, Jeffrey PD, Shi YG, Morel FMM. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature. 2008;452(7183):56–61. doi: 10.1038/nature06636. [DOI] [PubMed] [Google Scholar]
- 8.Boyle EA. Cadmium: Chemical tracer of deepwater paleoceanography. Paleoceanog. 1988;3(4):471–489. [Google Scholar]
- 9.Elderfield H, Rickaby REM. Oceanic Cd/P ratio and nutrient utilization in the glacial Southern Ocean. Nature. 2000;405(6784):305–310. doi: 10.1038/35012507. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal Y, Boyle EA, Labeyrie L. Last glacial maximum paleochemistry and deepwater circulation in the Southern Ocean: Evidence from foraminiferal cadmium. Paleoceanog. 1997;12(6):787–796. [Google Scholar]
- 11.Cullen JT. On the nonlinear relationship between dissolved cadmium and phosphate in the modern global ocean: Could chronic iron limitation of phytoplankton growth cause the kink? Limnol Oceanogr. 2006;51(3):1369–1380. [Google Scholar]
- 12.Conway TM, Rosenberg AD, Adkins JF, John SG. A new method for precise determination of iron, zinc and cadmium stable isotope ratios in seawater by double-spike mass spectrometry. Anal Chim Acta. 2013;793:44–52. doi: 10.1016/j.aca.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Al-Farawati R, van den Berg CMG. Metal-sulfide complexation in seawater. Mar Chem. 1999;63(3-4):331–352. [Google Scholar]
- 14.Jacobs L, Emerson S. Trace-metal solubility in an anoxic fjord. Earth Planet Sci Lett. 1982;60(2):237–252. [Google Scholar]
- 15.Jacobs L, Emerson S, Skei J. Partitioning and transport of metals across the O2/H2S interface in a permanently anoxic basin - Framvaren Fjord, Norway. Geochim Cosmochim Acta. 1985;49(6):1433–1444. [Google Scholar]
- 16.Berner RA. A new geochemical classification of sedimentary environments. J Sediment Petrol. 1981;51(2):359–365. [Google Scholar]
- 17.Whitney FA, Freeland HJ, Robert M. Persistently declining oxygen levels in the interior waters of the eastern subarctic Pacific. Prog Oceanogr. 2007;75(2):179–199. [Google Scholar]
- 18.Schmitt AD, Galer SJG, Abouchami W. Mass-dependent cadmium isotopic variations in nature with emphasis on the marine environment. Earth Planet Sci Lett. 2009;277(1-2):262–272. [Google Scholar]
- 19.Xu Y, Morel FMM. In: Cadmium in Marine Phytoplankton. Cadmium: From Toxicity to Essentiality, Metal Ions in Life Sciences. Sigel A, Sigel H, Sigel RKO, editors. Vol 11. Dordrecht, The Netherlands: Springer Netherlands; 2013. pp. 509–528. [DOI] [PubMed] [Google Scholar]
- 20.Frew RD. Antarctic bottom water formation and the global cadmium to phosphorus relationship. Geophys Res Lett. 1995;22(17):2349–2352. [Google Scholar]
- 21.Abouchami W, et al. Biogeochemical cycling of cadmium isotopes in the Southern Ocean along the Zero Meridian. Geochim Cosmochim Acta. 2013;127:348–367. [Google Scholar]
- 22.Rosenthal Y, Lam P, Boyle EA, Thomson J. Authigenic cadmium enrichments in suboxic sediments: Precipitation and postdepositional mobility. Earth Planet Sci Lett. 1995;132(1-4):99–111. [Google Scholar]
- 23.van Geen A, McCorkle DC, Klinkhammer GP. Sensitivity of the phosphate-cadmium-carbon isotope relation in the ocean to cadmium removal by suboxic sediments. Paleoceanog. 1995;10(2):159–169. [Google Scholar]
- 24.Van Cappellen P, Ingall ED. Redox stabilization of the atmosphere and oceans by phosphorus-limited marine productivity. Science. 1996;271(5248):493–496. doi: 10.1126/science.271.5248.493. [DOI] [PubMed] [Google Scholar]
- 25.Conway TM, John SG. Sources of Fe to the North Atlantic: Insights from Fe isotopes. Mineral Mag. 2013;77(5):912. [Google Scholar]
- 26.Wright JJ, Konwar KM, Hallam SJ. Microbial ecology of expanding oxygen minimum zones. Nat Rev Microbiol. 2012;10(6):381–394. doi: 10.1038/nrmicro2778. [DOI] [PubMed] [Google Scholar]
- 27.Bishop JKB. The barite-opal-organic carbon association in oceanic particulate matter. Nature. 1988;332(6162):341–343. [Google Scholar]
- 28.Canfield DE, et al. A cryptic sulfur cycle in oxygen-minimum-zone waters off the Chilean coast. Science. 2010;330(6009):1375–1378. doi: 10.1126/science.1196889. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi D, Dunne JP, Sarmiento JL, Galbraith ED. Data-based estimates of suboxia, denitrification, and N2O production in the ocean and their sensitivities to dissolved O2. Global Biogeochem Cycles. 2012;26(2):GB2009. [Google Scholar]
- 30.Klinkhammer GP, Palmer MR. Uranium in the oceans - where it goes and why. Geochim Cosmochim Acta. 1991;55(7):1799–1806. [Google Scholar]
- 31.Luther GW, Rickard DT. Metal sulfide cluster complexes and their biogeochemical importance in the environment. J Nanopart Res. 2005;7(4-5):389–407. [Google Scholar]
- 32.Sunda WG, Huntsman SA. Feedback interactions between zinc and phytoplankton in seawater. Limnol Oceanogr. 1992;37(1):25–40. [Google Scholar]
- 33.Tortell PD, Price NM. Cadmium toxicity and zinc limitation in centric diatoms of the genus Thalassiosira. Mar Ecol Prog Ser. 1996;138:245–254. [Google Scholar]
- 34.Saito MA, Goepfert TJ. Zinc-cobalt colimitation of Phaeocystis antarctica. Limnol Oceanogr. 2008;53(1):266–275. [Google Scholar]
- 35.Mann EL, Ahlgren N, Moffett JW, Chisholm SW. Copper toxicity and cyanobacteria ecology in the Sargasso Sea. Limnol Oceanogr. 2002;47(4):976–988. [Google Scholar]
- 36.Peers G, Price NM. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature. 2006;441(7091):341–344. doi: 10.1038/nature04630. [DOI] [PubMed] [Google Scholar]
- 37.Morel FMM, et al. Zinc and carbon co-limitation of marine phytoplankton. Nature. 1994;369(6483):740–742. [Google Scholar]
- 38.Franck VM, Bruland KW, Hutchins DA, Brzezinski MA. Iron and zinc effects on silicic acid and nitrate uptake kinetics in three high-nutrient, low-chlorophyll (HNLC) regions. Mar Ecol Prog Ser. 2003;252:15–33. [Google Scholar]
- 39.Stukel MR, Decima M, Selph KE, Taniguchi DAA, Landry MR. The role of Synechococcus in vertical flux in the Costa Rica upwelling dome. Prog Oceanogr. 2013;112:49–59. [Google Scholar]
- 40.Twining BS, Baines SB. The trace metal composition of marine phytoplankton. Annu Rev Mar Sci. 2013;5:191–215. doi: 10.1146/annurev-marine-121211-172322. [DOI] [PubMed] [Google Scholar]
- 41.Dupont CL, Yang S, Palenik B, Bourne PE. Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc Natl Acad Sci USA. 2006;103(47):17822–17827. doi: 10.1073/pnas.0605798103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stramma L, Schmidtko S, Levin LA, Johnson GC. Ocean oxygen minima expansions and their biological impacts. Deep Sea Res Part I Oceanogr Res Pap. 2010;57(4):587–595. [Google Scholar]
- 43.Kramer D, Cullen JT, Christian JR, Johnson WK, Pedersen TF. Silver in the subarctic northeast Pacific Ocean: Explaining the basin scale distribution of silver. Mar Chem. 2011;123(1-4):133–142. [Google Scholar]
- 44.Schlitzer R. 2014. Ocean Data View (version 4.5.7). Available at http://odv.awi.d. Accessed April 2014.
- 45.Garcia H, et al. 2010. World Ocean Atlas 2009, Volume 3: Dissolved Oxygen, Apparent Oxygen Utilization, and Oxygen Saturation. NOAA Atlas NESDIS 70, ed Levitus S (US Government Printing Office, Washington, DC)
- 46.Martin JH, Gordon RM, Fitzwater S, Broenkow WW. VERTEX: Phytoplankton/iron studies in the Gulf of Alaska. Deep-Sea Res. 1989;36(5):649–680. [Google Scholar]
- 47.Xue Z, et al. Cadmium isotope variations in the Southern Ocean. Earth Planet Sci Lett. 2013;382(0):161–172. [Google Scholar]