Significance
Our study not only reveals a novel role of peroxisome proliferator-activated receptor δ (PPARδ) in colonic inflammation and colitis-associated tumorigenesis, but also provides some rationale for development of PPARδ antagonists as new therapeutic agents in the treatment of inflammatory bowel disease and colitis-associated colorectal cancer. Moreover, our findings indicate that prostaglandin E2 generated by chronic inflammation is a crucial mediator connecting chronic inflammation and colorectal carcinogenesis.
Keywords: colorectal cancer, COX-2/PGE2
Abstract
Although epidemiologic and experimental evidence strongly implicates chronic inflammation and dietary fats as risk factors for cancer, the mechanisms underlying their contribution to carcinogenesis are poorly understood. Here we present genetic evidence demonstrating that deletion of peroxisome proliferator-activated receptor δ (PPARδ) attenuates colonic inflammation and colitis-associated adenoma formation/growth. Importantly, PPARδ is required for dextran sodium sulfate induction of proinflammatory mediators, including chemokines, cytokines, COX-2, and prostaglandin E2 (PGE2), in vivo. We further show that activation of PPARδ induces COX-2 expression in colonic epithelial cells. COX-2–derived PGE2 stimulates macrophages to produce proinflammatory chemokines and cytokines that are responsible for recruitment of leukocytes from the circulation to local sites of inflammation. Our results suggest that PPARδ promotes colonic inflammation and colitis-associated tumor growth via the COX-2–derived PGE2 signaling axis that mediates cross-talk between tumor epithelial cells and macrophages.
Chronic inflammation is clearly associated with increased cancer risk for a number of malignancies, including esophageal, gastric, hepatic, pancreatic, and colorectal cancer (CRC). Indeed, ulcerative colitis (UC), a form of inflammatory bowel disease (IBD), is associated with an increased risk for the development of CRC (1). The common pathological changes associated with IBD include a defect of the innate immune response to microbial agents, diminished epithelial barrier integrity, and increased infiltration of dysregulated immune cells. However, the underlying mechanism(s) responsible for the connection between inflammation and cancer remains of high interest, others have reported that NF-κB signaling and certain cytokines such as IL-6, -17, -22, and -23 are involved in mouse models of colitis-associated CRC (2–4).
Some of the evidence for the link between inflammation and cancer came from epidemiologic and clinical studies showing that use of nonsteroidal anti-inflammatory drugs (NSAIDs) reduced the relative risk for developing CRC by 40–50%. NSAIDs are known to exert one of their anti-inflammatory and antitumor effects by targeting an inducible enzyme cyclooxygenase 2 (COX-2). COX-2 expression is elevated in CRC and is associated with a lower survival of CRC patients (5–7). COX-2–derived prostaglandin E2 (PGE2) is the most abundant prostaglandin found in human CRC (8) and plays a predominant role in promoting tumor growth (9). Similarly, COX-2 and PGE2 levels are elevated in the gastrointestinal (GI) tract of patients with active IBD (10, 11). These results prompted us to ask whether the COX-2–derived PGE2 pathway could be involved in colitis-associated carcinogenesis.
Dietary fat intake is an environmental factor that is associated with some human diseases such as diabetes, obesity, dyslipidemias, and cancer (12, 13). Peroxisome proliferator-activated receptors (PPARs) have been shown to play a central role in regulating the storage and catabolism of dietary fats via complex metabolic pathways, including fatty acid oxidation and lipogenesis (14). PPARδ is a member of PPAR family that belongs to the nuclear hormone receptor superfamily and is also a ligand-dependent transcription factor. PPARδ is expressed in diverse tissues (15), and its expression level is very high in the GI tract compared with other tissues (16). Although PPARδ has been shown to be involved in chronic inflammation and in CRC progression, its role is still unclear and vigorously debated (17). Particularly, its role in colitis-induced carcinogenesis has never really been explored carefully.
Results
PPARδ Is Required for Dextran Sodium Sulfate-Induced Colonic Inflammation.
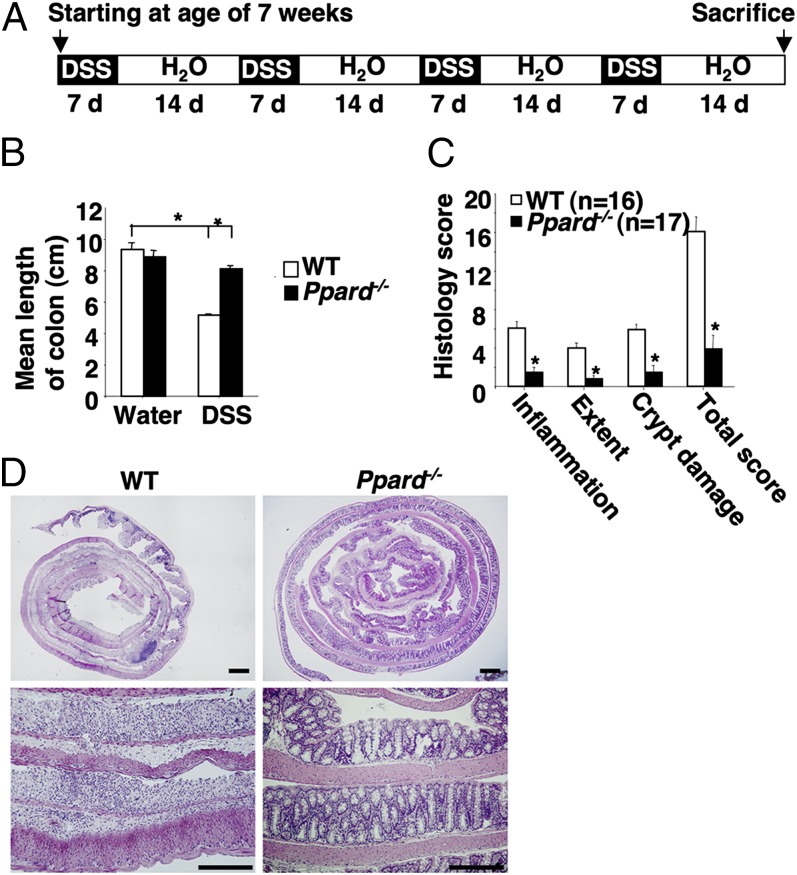
To investigate the biological function of PPARδ in colonic inflammation, we first examined the phenotype of dextran sodium sulfate (DSS)-treated PPARδ-deficient mice generated by deletion of exons 4–5 (18). In this model, PPARδ was deleted in the whole organism. WT mice that repeatedly received DSS as described in Fig. 1A developed a shorter colonic length due to inflammation-induced changes (Fig. 1B) and histologic signs of severe colitis, characterized by inflammation (infiltration of immune cells), extent (depth of inflammation), and crypt damage (Fig. 1 C and D). In contrast, PPARδ-deficient mice exhibited marked resistance to DSS-induced colonic inflammation (Fig. 1 B and D). Water-treated WT or PPARδ-deficient mice showed no clinical and histologic signs of chronic inflammation. Moreover, the absence of PPARδ did not affect DSS-induced intestinal epithelial cell death or regeneration of epithelial cells (Fig. S1). In addition, we evaluated whether loss of PPARδ affected intestinal homeostasis, such as intestinal epithelial cell proliferation, survival, and total number of stem cells. Both WT and PPARδ-deficient mice exhibited the same rates of intestinal epithelial cell proliferation and survival as well as similar levels of Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5)-expressing intestinal stem cells (Fig. S2).
Fig. 1.
Loss of PPARδ inhibits DSS-induced chronic colonic inflammation. (A) Schematic of mice treated with 2% (wt/vol) DSS. (B) The average length of mouse colon was measured after completion of the experiments. (C) The histopathologic alterations of the colon were examined on H&E-stained sections, and blinded histological scoring of inflammation in colonic mucosa of mice was performed as described (44). For B and C, data represent mean ± SE. *P < 0.05. (D) Representative H&E-stained sections from WT (Left) and PPARδ-null mice (Right) treated with DSS as described in A are shown. (Scale bars, 250 μm.)
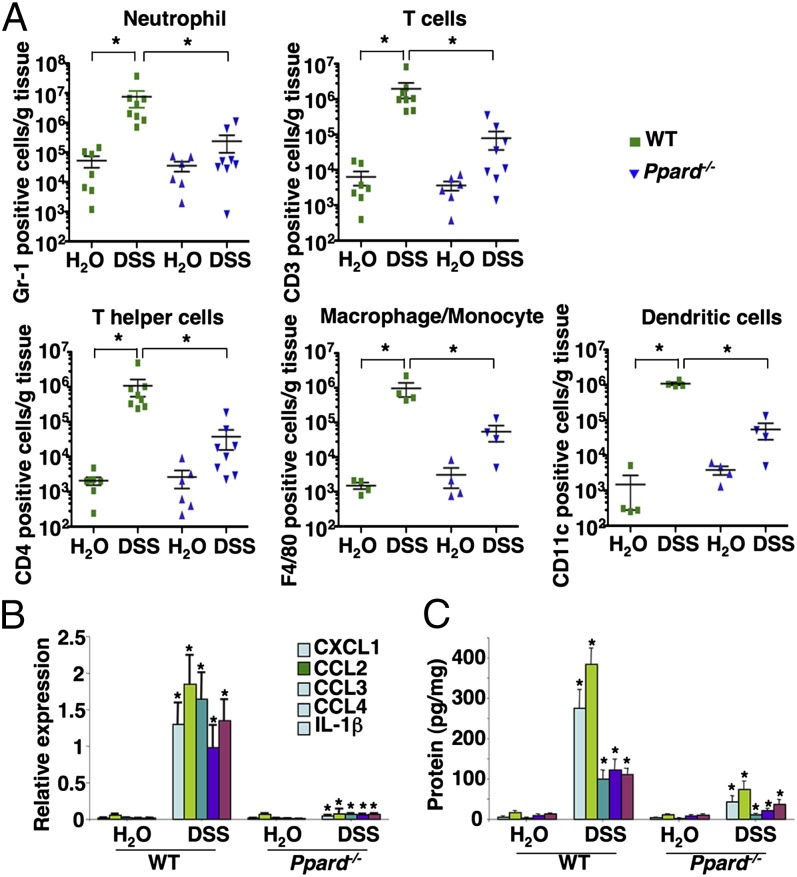
We further quantified the inflammatory response by profiling the type and density of immune cells in the colonic mucosa using flow cytometry. A massive infiltration of neutrophils, T cells, T helper cells, macrophages/monocytes, and dendritic cells (DCs) into the colonic mucosa was observed in the DSS-treated WT mice compared with water-treated WT mice (Fig. 2A). In contrast, the infiltration of immune cells in the colonic mucosa was greatly attenuated in DSS-treated PPARδ-null mice (Fig. 2A). Because certain chemokines are responsible for the recruitment of leukocytes from the circulation to local inflammatory sites and are regulated by proinflammatory cytokines, we measured an array of proinflammatory chemokines and cytokines in the colonic mucosa. We found that loss of PPARδ dramatically reduced DSS induction of certain chemokines and cytokines in colonic mucosa, including CXC ligand 1 (CXCL1), CC ligand 2 (CCL2), CCL3, CCL4, and IL-1β (Fig. 2 B and C). DSS treatment also significantly induced expression of other PPARδ-independent proinflammatory chemokines and cytokines, including IFN-γ, IL-23, and CXCL10. We focused our next studies on the evaluation of PPARδ-dependent proinflammatory mediators. Consistent with the results of massive immune cell infiltration, CXCL1 is a neutrophil chemokine, whereas CCL2, CCL3, and CCL4 are potent chemoattractants for monocytes/macrophages, T cells, and DCs. Together, these results indicate that PPARδ promotes chronic inflammation via induction of proinflammatory chemokines that attract immune cells into the colonic mucosa.
Fig. 2.
Loss of PPARδ attenuates DSS-induced massive infiltration of immune cells and proinflammatory gene expression in the colonic mucosa. (A) Cells isolated from the colonic mucosa of indicated genotypic mice treated with either DSS or water as described in SI Materials and Methods were incubated with antibodies against indicated cell-surface markers to characterize the subpopulations by flow cytometry. Values are reported as the number of Gr-1, CD3, CD4, F4/80, and CD11c positive cells per gram of each colon tissue, respectively. *P < 0.05. (B and C) The mRNA (B) and protein (C) levels of indicated genes in colonic mucosa were analyzed by q-PCR and ELISA from a DSS-treated cohort of 12 mice for each genotype and a water-treated cohort of seven mice for each genotype. For mRNA, data represent the mean ± SE of relative expression of target gene. The relative expression of each target gene represents the averages of triplicates that are normalized against the transcription levels of mGapdh. For protein, equal total proteins from each sample were subjected to ELISA. Data represent the mean ± SE of protein concentration (picograms per milligram of tissue weight). *P < 0.05.
PPARδ Is Required for Colitis-Associated Tumorigenesis.
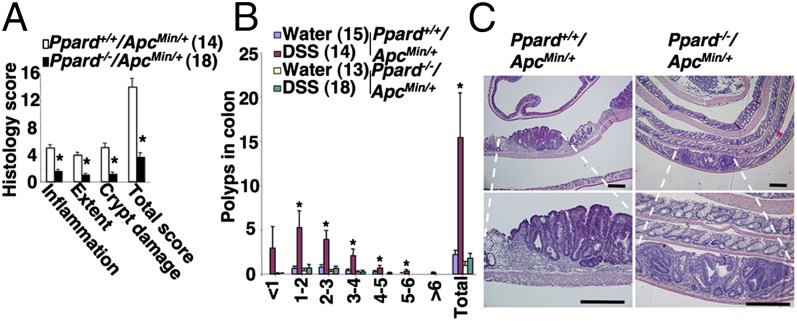
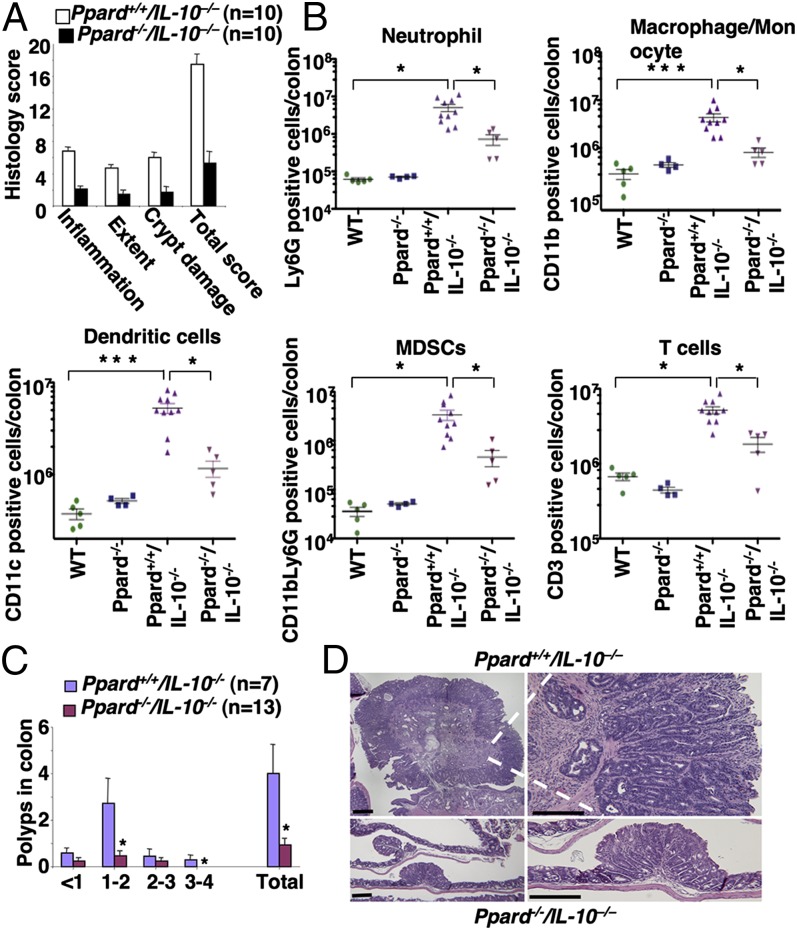
We first investigated the role of PPARδ in DSS-treated ApcMin/+ mice. Mice were treated with DSS as described in Fig. 1A. Consistent with the above results, DSS-treated Ppard+/+/ApcMin/+ mice exhibited higher levels of these genes in colonic mucosa with a massive infiltration of the immune cells compared with water-treated mice (Fig. S3). In contrast, loss of PPARδ attenuated the ability of DSS to induce these genes and markedly reduced the infiltration of immune cells in the colonic mucosa of ApcMin/+ mice (Fig. S3). In particular, deletion of PPARδ impaired DSS induction of cytokines that are involved in promotion of colitis-associated tumorigenesis, such as IL-6, -17A, and -22 (Fig. S3C). Indeed, the absence of PPARδ significantly reduced DSS-induced chronic inflammation and colonic tumor burden in the ApcMin/+ mice (Fig. 3 A and B). We found that the severity of chronic inflammation directly correlated with the level of colonic tumor burden. Histological analysis showed that a massive infiltration of immune cells was observed in all adenomas taken from DSS-treated Ppard+/+/ApcMin/+ mice, but not in all tumors taken from DSS-treated Ppard−/−/ApcMin/+ mice (Fig. 3C). To further confirm the role of PPARδ in promoting colonic inflammation and colitis-associated carcinogenesis, another mouse model of colitis-associated tumorigenesis was examined. Deletion of Ppard attenuated chronic inflammation in azoxymethane (AOM)-treated Il-10−/− mice compared with their control littermates (Ppard+/+/Il-10−/−) (Fig. 4A). Similarly, Il-10−/− mice contained a much more massive infiltration of immune cells in colonic mucosa compared with WT or PPARδ-deficient mice (Fig. 4B). In contrast, PPARδ-deficient IL-10–null mice had significantly less infiltration of immune cells within the colonic mucosa compared with littermate controls (Ppard+/+/Il-10−/−) (Fig. 4B). Moreover, loss of PPARδ significantly reduced AOM-induced colitis-associated tumor burden in Il-10−/− mice compared with their control littermates (Fig. 4C). Similarly, histological analysis showed that tumors taken from AOM-treated Ppard+/+/Il-10−/− mice had a massive infiltration of immune cells into their mucosa compared with AOM-treated Ppard−/−/Il-10−/− mice (Fig. 4D). Together, these results provide, to our knowledge, the first genetic evidence showing that PPARδ is required for colonic inflammation and colitis-associated colonic tumor formation and growth.
Fig. 3.
Loss of PPARδ reduced DSS-induced colonic inflammation and colitis-associated tumor growth in ApcMin/+ mice. (A and B) Mice with different genotypes were treated with DSS or water as described in Fig. 1A. (A) At the end of the experiments, the histological scoring of inflammation in colonic mucosa was performed as described in Fig. 1C, and the number and size of polyps in colon were measured. (B) Data are expressed as means ± SE of polyp number. *P < 0.05. (C) Representative H&E-stained sections of colonic adenomas from Ppard+/+/ApcMin/+ (Left) and Ppard−/−/ApcMin/+ (Right) mice treated with DSS are shown. (Scale bars, 250 μm.)
Fig. 4.
The effect of PPARδ loss on colonic inflammation and colitis-associated tumor growth in AOM-treated IL-10–null mice. Mice with different genotypes were treated with AOM as described in SI Materials and Methods. (A) At the end of the experiments, the histological scoring of inflammation in colonic mucosa was performed as described in Fig. 1C. (B) The profiles of immune cells in the colon mucosa of indicated genotypic mice were determined as described in Fig. 2A. (C) The number and size of polyps in colon were measured as described in Fig. 3B. *P < 0.05. (D) Representative H&E-stained sections of colonic adenomas from AOM-treated Ppard+/+/Il-10−/− and Ppard−/−/Apc−/− mice are shown. (Scale bars, 250 μm.)
COX-2 Is a Downstream Target of PPARδ.
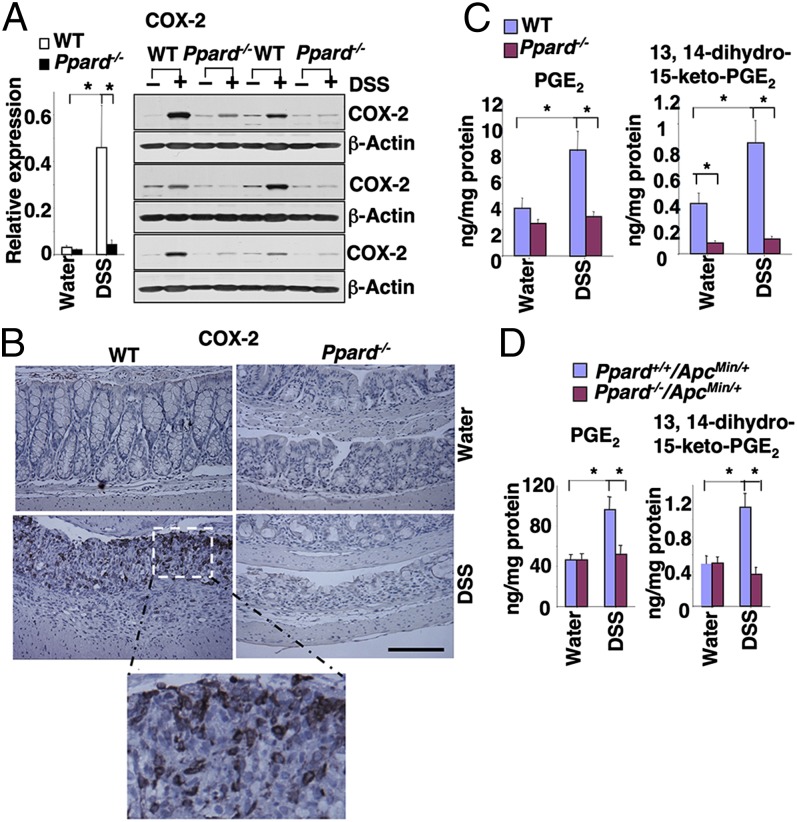
Because the levels of COX-2 and PGE2 are elevated in inflamed mucosa of IBD patients, we examined whether COX-2–derived PGE2 signaling was affected during colonic inflammation. Indeed, DSS treatment led to increased COX-2 expression in colonic mucosa taken from WT mice, but not in the samples taken from PPARδ-null mice (Fig. 5A). Interestingly, COX-2 was expressed in both epithelial and stromal cells in the colonic ulcerative areas of DSS-treated WT mice (Fig. 5B). Moreover, the results from immunofluorescent staining of COX-2, EpCAM (epithelial call marker), and CD45 (immune cell marker) further confirmed that COX-2 is expressed in both epithelial and immune cells (Fig. S4). In contrast, even in the markedly reduced ulcerative areas of PPARδ-deficient mice, no COX-2 staining was observed (Fig. 5B), demonstrating that PPARδ is required for induction of COX-2 expression in inflamed mucosa following DSS treatment. Similarly, the levels of PGE2 and its metabolic product (13,14-dihydro-15-keto-PGE2) were elevated in the colonic mucosa of the DSS-treated WT and ApcMin/+ mice, but not in PPARδ-null mice (Fig. 5 C and D). These results reveal that the COX-2–derived PGE2 signaling is one of the downstream pathways of PPARδ in the context of these experiments.
Fig. 5.
DSS induction of COX-2 expression depends on PPARδ in colon. (A, Left) The levels of COX-2 mRNA in the same samples from the experiments as described in Fig. 2B were analyzed by q-PCR. (Right) Western blot analysis with anti–COX-2 antibody was performed on cell lysates from mouse colon tissue taken from a DSS-treated cohort of six mice for each genotype and a water-treated cohort of six mice for each genotype. (B) Sections of formalin-fixed and paraffin-embedded colon tissues from a cohort of eight mice for each group were immunostained with anti–COX-2 antibody. A set of representative images is shown. (Scale bars, 250 μm.) (C) The levels of PGE2 and its metabolite (13,14-dihydro-15-keto-PGE2) in the same samples from the experiments as described in Fig. 2B were quantified by mass spectrometry. *P < 0.05. (D) The levels of PGE2 and 13,14-dihydro-15-keto-PGE2 in the same samples from the experiments as described in Fig. S4 B and C were quantified by mass spectrometry. *P < 0.05.
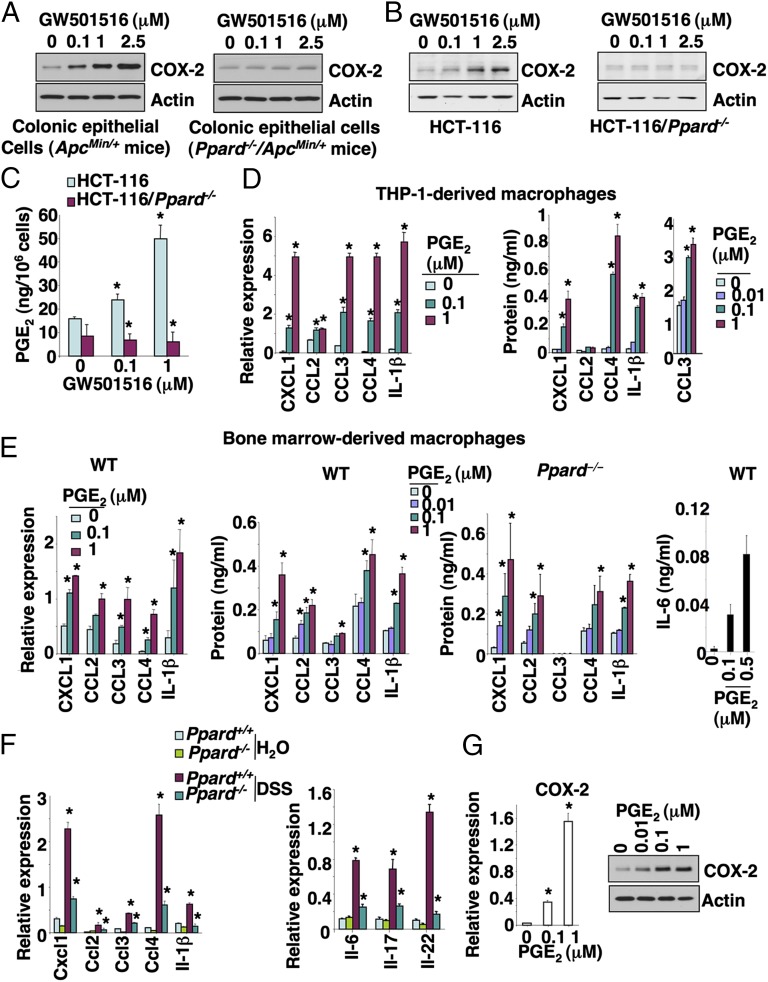
Because COX-2 is mainly expressed in colonic epithelial cells and macrophages of inflamed mucosa and colorectal carcinoma tissues, we examined whether activation of PPARδ induces COX-2 expression in these cells. As expected, activation of PPARδ by its agonist (GW501516) induced COX-2 expression in colonic tumor epithelial cells isolated from ApcMin/+ mice (Fig. 6A) and HCT-116 colorectal carcinoma cells (Fig. 6B), but not in PPARδ-deficient mouse colonic tumor epithelial cells or PPARδ-deficient HCT-116 cells (Fig. 6 A and B). Similarly, GW501516 induced PGE2 production in HCT-116 cells, but not in PPARδ-deficient HCT-116 cells (Fig. 6C). In addition, overexpression of PPARδ alone resulted in elevation of COX-2 expression compared with vector control cells, but treatment of PPARδ-overexpressing HCT-116 cells with GW501516 did not further induce COX-2 expression (Fig. S5A). These results demonstrate that the effect of GW501516 on induction of COX-2 and PGE2 is most likely due to specific activation of PPARδ nuclear receptor. Moreover, activation of PPARδ also induced COX-2 expression in other colorectal carcinoma cell lines and young adult mouse colonic epithelial cells (Fig. S5B).
Fig. 6.
The activation of PPARδ induced COX-2 expression in tumor epithelial cells, and PGE2 stimulated macrophages to secrete proinflammatory mediators. (A) The primary colonic tumor epithelial cells isolated from Ppard+/+/ApcMin/+ and Ppard−/−/ApcMin/+ mice were treated with the indicated dose of GW501516 for 24 h after serum starvation for 24 h. (B and C) The parental and PPARδ-deficient HCT-116 cells were cultured in medium with 0.5% fat-free FBS for 24 h and then treated with the indicated dose of GW501516 for 24 (B) or 72 h (C) for measuring COX-2 (B) and PGE2 (C) levels, respectively. COX-2 protein expression and PGE2 levels were measured as described in Fig. 5. (D and E) THP-1–derived macrophages (D) and BMMs (E) were treated with the indicated dose of PGE2 for 24 h for mRNA expression (Left) and 48 h for secreted proteins (Right) after serum starvation for 24 h, respectively. (D) The levels of indicated genes at mRNA levels (Left) and secreted protein levels (Right) were quantified by q-PCR and ELISA or Bio-Plex assays. (E) Left panel represents the gene mRNA levels and the rest of panels represents protein levels. Data are represented as the mean ± SE of relative expression for mRNA or protein concentration from three independent experiments. (F) The colonic macrophages were isolated from a cohort of five mice for each genotype treated with either 2% DSS or water as described in Fig. 1A and pooled together. A total of 1 × 105 pooled colonic macrophages from each indicated group was subjected to q-PCR. Data represent the mean ± SD of relative expression for mRNA. (G) THP-1–derived macrophages were treated with PGE2 as described in D. The COX-2 expression at the mRNA (Left) and protein (Right) levels was quantified by q-PCR and Western blotting. *P < 0.05.
Next, we examined whether Wnt and PPARδ signaling cooperatively induced COX-2 expression. Treatment of HCT-116 cells with Wnt3a did not affect COX-2 expression or further enhance PPARδ induction of COX-2 (Fig. S5C). In contrast, GW501516 treatment had no effect on COX-2 expression in the mouse bone marrow-derived macrophages (BMMs) (Fig. S5D) or other macrophages such as RAW264.7 and THP-1–derived macrophages. These results demonstrate that activation of PPARδ induces COX-2 expression in colonic epithelial cells, but not in the macrophages we evaluated.
COX-2–Derived PGE2 Induces the Expression of Proinflammatory Mediators in Macrophages.
Because our in vivo results showed that elevation of CXCL1, CCL2, CCL3, CCL4, and IL-1β in colonic mucosa depends on the presence of PPARδ, it was conceivable that activation of PPARδ could directly induce these genes in colonic epithelial cells and/or macrophages. However, GW501516 failed to induce these genes in mouse colonic tumor epithelial cells, mouse BMMs, RAW264.7 macrophage cells, and THP-1–derived macrophages. Even in PPARδ-overexpressing HCT-116 cells, GW501516 treatment did not affect these proinflammatory genes (Fig. S5E). These results suggest that activation of PPARδ does not directly regulate these genes in both epithelial cells and macrophages. Because COX-2–derived PGE2 signaling is downstream of PPARδ (Figs. 5 and 6), we postulated that PGE2 mediates the effects of PPARδ on induction of these genes. Indeed, PGE2 induced the expression of CXCL1, CCL2, CCL3, CCL4, and IL-1β in THP-1–derived macrophages (Fig. 6D), in WT mouse BMMs, and PPARδ-deficient BMMs (Fig. 6E). These results indicate that PGE2 is a downstream effector of PPARδ in vivo. Moreover, PGE2 stimulates WT BMMs to secrete IL-6 that promotes colitis-associated tumorigenesis (Fig. 6 E, Right). However, we did not detect IL-22 and -17 proteins in the supernatants from BMMs in the absence or presence of PGE2 treatment. Analysis of quantitative PCR (q-PCR) revealed that all four prostaglandin E receptors (EP) were expressed in BMMs (Fig. S5F). These in vitro findings were supported by in vivo results showing that the mRNA levels of CXCL1, CCL2, CCL3, CCL4, IL-1β, -6, -17, and -22 in the colonic macrophages isolated from the DSS-treated WT mice were much higher than DSS-treated PPARδ-deficient mice (Fig. 6F). Importantly, treatment of THP-1–derived macrophages with PGE2 also induced COX-2 expression (Fig. 6G). These results demonstrate that PGE2 stimulates macrophages to secrete proinflammatory chemokines and cytokines as well as to induce COX-2 expression in both an autocrine and paracrine fashion.
Discussion
Despite emerging evidence showing that PPARδ is involved in the pathogenesis of IBD and CRC, its roles in pathobiology are still hotly debated. Administration of a PPARδ agonist exacerbated colitis in IL-10–deficient mice and accelerated intestinal tumor growth in ApcMin/+ mice (19–21). Studies from two independent groups revealed that loss of PPARδ by deletion of its exons 4–5 or exon 4 reduced intestinal adenoma burden in both ApcMin/+ and AOM-treated mice without exposure to DSS (22, 23). A recent report described a role of PPARδ in Helicobacter pylori-associated gastric carcinogenesis, which represents another example of its effects in a proinflammatory pathway (24). These results suggest that PPARδ has proinflammatory and protumor effects. However, one group reported conflicting results showing that deletion of PPARδ (at exon 8) significantly aggravated colitis in the DSS-treated mice and enhanced adenoma growth in ApcMin/+ and AOM-treated mice in the absence of DSS treatment (25, 26). Their results suggest that PPARδ exerts anti-inflammatory and antitumor effects. The reason for this discrepancy may be due to the use of different deletion strategies to remove PPARδ. The deletion of PPARδ exons 4–5, which encodes an essential portion of the DNA binding domain, is thought to totally disrupt PPARδ function as a nuclear transcriptional factor, whereas deletion of exon 8, the last exon of the PPARδ gene, is postulated to generate a hypomorphic allele, which retains some aporeceptor function. Here, to our knowledge, we provide the first evidence demonstrating that deletion of PPARδ at exons 4–5 attenuated chronic colonic inflammation and colitis-associated tumor growth in two different mouse models (Figs. 1–4). These results strongly support the notion that PPARδ promotes chronic colonic inflammation and colitis-associated tumorigenesis.
A massive infiltration of neutrophils, macrophages, and CD4+ T cells was found in the inflamed tissues of IBD patients, and the levels of proinflammatory chemokines such as CXCL1, CCL2, CCL3, and CCL4 also correlate with the severity of disease in IBD patients (27). Moreover, genetic and pharmacologic studies provide evidence showing that CCL2, CCL3, or CCL4 signaling promotes inflammation in models of injurious agent-induced experimental colitis (28–30). Similarly, proinflammatory cytokines such as IL-6, -17, and -22 are known to contribute to colitis-associated tumorigenesis. To our knowledge, our in vivo results demonstrate for the first time that PPARδ is required for elevation of these chemokines and cytokines as well as leukocyte infiltration during colonic inflammation and colitis-associated tumorigenesis (Figs. 2 and 4 as well as Fig. S3). These results indicate that these PPARδ-dependent chemokines attract immune cells into colonic mucosa.
COX-2 is an immediate–early response gene normally absent from most cells, but it is found in high levels at sites of inflammation in response to inflammatory stimuli (31, 32). To our knowledge, here we provide the first in vivo evidence showing that COX-2 is a downstream target of PPARδ (Fig. 5), although PPARδ has previously been shown to induce COX-2 expression in liver and lung carcinoma cells in vitro (33, 34). Although no peroxisome-proliferator response element has been identified in the COX-2 promoter, PPARδ is known to mediate its transcriptional activity via interaction with other transcriptional factors, including NF-κB and C/EBP (35, 36). It is well established that COX-2 expression is regulated by various transcription factors such as NF-κB, C/EBP, CREB, NFAT, and AP-1. Thus, PPARδ could up-regulate COX-2 expression via NF-κB and C/EBP. Because PGE2 promotes tumor growth in vivo (9), our results indicate that PGE2, at least in part, mediates the effect of PPARδ on promotion of colitis-associated tumorigenesis in the animal models we studied. In addition to COX-2–derived PGE2 signaling, it is possible that other pathways may also mediate the effects of PPARδ on promotion of inflammation and colitis-associated tumorigenesis. Further studies are needed to investigate whether other PPARδ downstream targets mediate the proinflammatory and protumor effects of PPARδ.
In experimental IBD models, COX-2–deficient mice suffer increased sensitivity to DSS-induced colitis (37), suggesting that COX-2 may be critical for healing of colonic injury by stimulation of epithelial cell proliferation and other wound-healing pathways. Conversely, dietary administration of nimesulide (a somewhat selective COX-2 inhibitor) effectively suppressed the development of colonic tumors induced by AOM/DSS (38), suggesting that elevation of COX-2 resulting from chronic inflammation contributes to tumorigenesis. Similarly, basal physiological levels of PGE2 are required for protection against DSS-induced or inflammation-associated epithelial barrier injury by enhancement of epithelial cell survival and regeneration of epithelial barrier (39), whereas high levels of PGE2 exacerbate the inflammatory process (40). However, our results demonstrate that loss of PPARδ only reduced inflammation-elevated COX-2 expression and PGE2 production to the physiologic levels (water-treated WT mice) but did not totally block COX-2 expression and PGE2 production (Fig. 5). These results may explain why loss of PPARδ attenuated DSS-induced chronic inflammation and colitis-associated tumorigenesis.
Our in vitro results (Fig. 6) suggest that PGE2 secreted from colonic tumor epithelial cells via PPARδ induction of COX-2 stimulates macrophages to produce proinflammatory mediators in vivo. These findings may also explain why recruited macrophages secrete proinflammatory mediators in vivo (41) and why COX-2 is highly expressed in colonic mucosal macrophages (Fig. 5B). Moreover, our previous data showing that PGE2 induced the expression of CXCL1, CCL3, CCL4, and CCL5 in human CRC cells (42) indicate that PGE2 may induce these chemokines in both epithelial cells and macrophages as well as other stromal cells. Further work is necessary to answer this question.
In conclusion, this study not only reveals novel functions of PPARδ in colonic inflammation and colitis-associated tumorigenesis, but also provides a rationale for development of PPARδ antagonists as potential new therapeutic agents in treatment of IBD and colitis-associated CRC. Moreover, we found a novel function of COX-2–derived PGE2 signaling in mediating cross-talk between colonic tumor epithelial cells and macrophages. Our results indicate that both PPARδ and COX-2–derived PGE2 signaling coordinately promotes colonic inflammation and colitis-associate tumorigenesis and is likely to be clinically relevant because the elevation of both PPARδ and COX-2 in tumor tissues correlates with a poor prognosis in CRC patients (43).
Materials and Methods
Animals.
PPARδ-null mice and their littermate control mice as well as PPARδ-deficient ApcMin/+ mice and their littermate controls were generated as described (22) and fed with standard mouse diet in the Animal Care Facility according to National Institutes of Health and institutional guidelines. Information describing the animal experiments is presented in SI Materials and Methods.
Cell Culture and Reagents.
Human CRC cell lines and a monocytic cell line (THP-1) were obtained from the ATCC, and HCA-7 cells were a gift from Susan Kirkland (University of London, London). Additional information on culture of all cancer cells, THP-derived macrophages, BMMs, and primary colonic tumor epithelial cells as well as isolation of colonic tumor epithelial cells, macrophages, and reagents is provided in SI Materials and Methods.
Analysis of Flow Cytometry.
For multicolor flow-cytometry immunotypic analysis, cells were stained with the indicated monoclonal antibodies and analyzed on BD LSRII system (BD Biosciences) to determine the percentage of positive cells. Information on antibodies and a description of experimental procedures are presented in SI Materials and Methods.
q-PCR.
The procedure describing the q-PCR assay is included in SI Materials and Methods.
ELISA and Bio-Plex Assays.
Information on extraction of total proteins from colon tissues and ELISA kits as well as Bio-Plex assay is presented in SI Materials and Methods.
Western Blot Analysis.
Detailed information about Western blotting assay and treatment of indicated cells with indicated reagents is provided in SI Materials and Methods.
Immunohistochemical Staining.
The procedure describing the immunohistochemical staining is included in SI Materials and Methods.
Analysis of PGE2.
The levels of PGE2 and its metabolite (13,14-dihydro-15-keto-PGE2) in the colon tissues and cells were quantified by using an Agilent 6460 Triple Quadrupole tandem mass spectrometry configured with the Agilent 1200 Series liquid chromatography separation system.
Statistical Analysis.
Each experiment was performed at least three times, and data are presented as the mean ± SE. Statistical significance was determined by using Student t test or one- or two-factor ANOVA, where applicable. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Peiying Yang (MD Anderson Cancer Center) for measurement of PGE2 levels. This work was supported in part by the National Institutes of Health (NIH) MERIT Award R37 DK47297 (to R.N.D.); NIH Grants R01 DK 62112 (to R.N.D.) and R01 AT004821 (to K.T.W.); National Cancer Institute Grant P01 CA77839 (to R.N.D.); and Cancer Prevention Research Institute of Texas Grant RP100960 (to R.N.D.). The National Colorectal Cancer Research Alliance provided generous support (to R.N.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324233111/-/DCSupplemental.
References
- 1.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 2.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Grivennikov SI, Karin M. Implications of anti-cytokine therapy in colorectal cancer and autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii100–ii103. doi: 10.1136/annrheumdis-2012-202201. [DOI] [PubMed] [Google Scholar]
- 5.Ogino S, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res. 2008;14(24):8221–8227. doi: 10.1158/1078-0432.CCR-08-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhart CE, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 7.Sheehan KM, et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999;282(13):1254–1257. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 8.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122(5):518–523. [PubMed] [Google Scholar]
- 9.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauritsen K, Laursen LS, Bukhave K, Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology. 1986;91(4):837–844. doi: 10.1016/0016-5085(86)90684-0. [DOI] [PubMed] [Google Scholar]
- 11.Singer II, et al. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115(2):297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 12.Schmid A. The role of meat fat in the human diet. Crit Rev Food Sci Nutr. 2011;51(1):50–66. doi: 10.1080/10408390903044636. [DOI] [PubMed] [Google Scholar]
- 13.Woutersen RA, Appel MJ, van Garderen-Hoetmer A, Wijnands MV. Dietary fat and carcinogenesis. Mutat Res. 1999;443(1-2):111–127. doi: 10.1016/s1383-5742(99)00014-9. [DOI] [PubMed] [Google Scholar]
- 14.Berger JP, Akiyama TE, Meinke PT. PPARs: Therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26(5):244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: Complex stories. Nat Rev Cancer. 2004;4(1):61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 16.Higashiyama H, Billin AN, Okamoto Y, Kinoshita M, Asano S. Expression profiling of peroxisome proliferator-activated receptor-delta (PPAR-delta) in mouse tissues using tissue microarray. Histochem Cell Biol. 2007;127(5):485–494. doi: 10.1007/s00418-007-0279-5. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, DuBois RN. Therapeutic potential of peroxisome proliferator-activated receptors in chronic inflammation and colorectal cancer. Gastroenterol Clin North Am. 2010;39(3):697–707. doi: 10.1016/j.gtc.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadra K, et al. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Mol Cell Biol. 2006;26(8):3266–3281. doi: 10.1128/MCB.26.8.3266-3281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JW, et al. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology. 2007;133(1):108–123. doi: 10.1053/j.gastro.2007.03.113. [DOI] [PubMed] [Google Scholar]
- 20.Gupta RA, et al. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med. 2004;10(3):245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6(3):285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, et al. Crosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progression. Proc Natl Acad Sci USA. 2006;103(50):19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo X, et al. Targeted genetic disruption of peroxisome proliferator-activated receptor-delta and colonic tumorigenesis. J Natl Cancer Inst. 2009;101(10):762–767. doi: 10.1093/jnci/djp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagy TA, et al. β-Catenin and p120 mediate PPARδ-dependent proliferation induced by Helicobacter pylori in human and rodent epithelia. Gastroenterology. 2011;141(2):553–564. doi: 10.1053/j.gastro.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harman FS, et al. Peroxisome proliferator-activated receptor-delta attenuates colon carcinogenesis. Nat Med. 2004;10(5):481–483. doi: 10.1038/nm1026. [DOI] [PubMed] [Google Scholar]
- 26.Hollingshead HE, et al. PPARbeta/delta protects against experimental colitis through a ligand-independent mechanism. Dig Dis Sci. 2007;52(11):2912–2919. doi: 10.1007/s10620-006-9644-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Dubois RN, Richmond A. The role of chemokines in intestinal inflammation and cancer. Curr Opin Pharmacol. 2009;9(6):688–696. doi: 10.1016/j.coph.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan WI, et al. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G803–G811. doi: 10.1152/ajpgi.00069.2006. [DOI] [PubMed] [Google Scholar]
- 29.Andres PG, et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: Lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164(12):6303–6312. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 30.Tokuyama H, et al. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int Immunol. 2005;17(8):1023–1034. doi: 10.1093/intimm/dxh284. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128(5):1445–1461. doi: 10.1053/j.gastro.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 32.Dubois RN, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12(12):1063–1073. [PubMed] [Google Scholar]
- 33.Glinghammar B, Skogsberg J, Hamsten A, Ehrenborg E. PPARdelta activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2003;308(2):361–368. doi: 10.1016/s0006-291x(03)01384-6. [DOI] [PubMed] [Google Scholar]
- 34.Pedchenko TV, Gonzalez AL, Wang D, DuBois RN, Massion PP. Peroxisome proliferator-activated receptor beta/delta expression and activation in lung cancer. Am J Respir Cell Mol Biol. 2008;39(6):689–696. doi: 10.1165/rcmb.2007-0426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di-Poï N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell. 2002;10(4):721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 36.Hoque A, et al. Increased 5-lipoxygenase expression and induction of apoptosis by its inhibitors in esophageal cancer: A potential target for prevention. Carcinogenesis. 2005;26(4):785–791. doi: 10.1093/carcin/bgi026. [DOI] [PubMed] [Google Scholar]
- 37.Morteau O, et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest. 2000;105(4):469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohno H, Suzuki R, Sugie S, Tanaka T. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 2005;5:46. doi: 10.1186/1471-2407-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang GL, et al. The prevention of colitis by E Prostanoid receptor 4 agonist through enhancement of epithelium survival and regeneration. J Pharmacol Exp Ther. 2007;320(1):22–28. doi: 10.1124/jpet.106.111146. [DOI] [PubMed] [Google Scholar]
- 40.Sheibanie AF, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23—>IL-17 axis. J Immunol. 2007;178(12):8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 41.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6(1):21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203(4):941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshinaga M, et al. The expression of both peroxisome proliferator-activated receptor delta and cyclooxygenase-2 in tissues is associated with poor prognosis in colorectal cancer patients. Dig Dis Sci. 2011;56(4):1194–1200. doi: 10.1007/s10620-010-1389-9. [DOI] [PubMed] [Google Scholar]
- 44.Dieleman LA, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114(3):385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.