Abstract
This study was conducted to evaluate age-specific seroprevalence of pertussis in Korea and to formulate a strategy to prevent and reduce the incidence of pertussis. Residual serum samples of healthy adolescents and adults 11 yr of age or older were collected between July 2012 and December 2012, and anti-pertussis toxin (PT) IgG titers were measured using a commercial ELISA kit. We compared the mean anti-PT IgG titers and seroprevalence of pertussis of the six age groups: 11-20, 21-30, 31-40, 41-50, 51-60, and ≥ 61 yr. A total of 1,192 subjects were enrolled. The mean anti-PT IgG titer and pertussis seroprevalence were 35.53 ± 62.91 EU/mL and 41.4%, respectively. The mean anti-PT IgG titers and seroprevalence were not significantly different between the age groups. However, the seroprevalence in individuals 51 yr of age or older was significantly higher than in individuals younger than 51 yr (46.5% vs 39.1%, P = 0.017). Based on these results, a new pertussis prevention strategy is necessary for older adults.
Graphical Abstract
Keywords: Pertussis Vaccine, Prevalence, Korea, Vaccination
INTRODUCTION
The incidence of pertussis decreased with the introduction of the diphtheria-tetanus-whole cell pertussis (DTwP) vaccination in children around the world (1), and a decrease in pertussis was also observed in Korea where the DTwP vaccination has been universally recommended for infants and children since 1954 (2). However, pertussis began to rise in the 1990s in Europe and North America, especially in adolescents (1, 3, 4, 5), and it has been also observed since the 2000s in Korea (2). The increased incidence of pertussis was assumed to be caused by a waning vaccine effect after previous vaccinations, a decreased boosting effect by natural pertussis infections in a community, the emergence of mutated Bordetella pertussis species that differ from the vaccine strain, and improved diagnostic modalities (3, 4, 5, 6, 7). In the United States, since 2006, Tdap booster vaccinations at adolescence have been recommended to control the increasing pertussis incidence based on cost-effectiveness studies (8, 9, 10). An adolescent Tdap booster vaccination at 11-12 yr of age was also introduced in 2009 in Korea because the incidence of pertussis began to rise in the 2000s (2). Pertussis in adolescents and adults often presents with atypical manifestations, such as asymptomatic infections and a chronic cough, rather than typical pertussis symptoms, and because of this, the actual infection rates may be higher than the incidence and prevalence based on clinical diagnosis (11, 12, 13). Therefore, repeated seroepidemiological studies are necessary to understand pertussis epidemiology and seroprevalence in communities. We conducted this seroepidemiological study to evaluate the current status and periodic changes of pertussis prevalence in Korea after 2002 and 2008 (14).
MATERIALS AND METHODS
Serum collection
Residual serum samples were collected from healthy adults and adolescents 11 yr of age or older, who visited the Health Promotion Center of Seoul St. Mary's Hospital, Seoul and St. Vincent Hospital, Suwon, Korea between July and December 2012.
Anti-pertussis toxin IgG enzyme-linked immunosorbent assay
IgG antibody titer against pertussis toxin (PT) which is a specific B. pertussis antigen was determined in the collected serum samples using a commercial enzyme-linked immunosorbent assay (ELISA) kit (IBL International GmbH, Hamburg, Germany) according to the manufacturer's recommendations. In brief, each serum sample was diluted with a dilution buffer in a 1:101 ratio, and 100 µL of the diluted solution was pipetted into each well. The well was covered with adhesive foil, and the diluted solution was incubated for 60 min at room temperature. The incubated solution was discarded, and each well was washed three times with 300 µL of wash buffer. Next, 100 µL of enzyme conjugate was put into each well, and the wells were covered with adhesive foil. The wells were incubated for 30 min, and each well was washed three times. Next, 100 µL substrate solution was added to each well, and then the samples were incubated for 20 min in the dark. Then, 100 µL of a stop solution was added, gently mixed, and an optical density at 450 nm was determined. The antibody titer was calculated from the optical density using a standard curve. Seropositivity was defined as anti-PT IgG titer>24 EU/mL according to the manufacturer's recommendation.
Data analysis and statistical analysis
Subjects were divided into six age groups: 11-20 yr, 21-30 yr, 31-40 yr, 41-50 yr, 51-60 yr, and ≥61 yr. A mean anti-PT IgG titer was calculated for each age group, and the mean titers were compared using the one-way analysis of variance (ANOVA). Seroprevalence, defined as the proportion of seropositive subjects of each age group, was compared using a chi-square test. Statistical analysis was performed with SPSS Statistics 17.0 software (SPSS Inc., Chicago, IL, USA), and statistical significance was defined as a two-tailed P value<0.05.
Ethics statement
This study was approved by the institutional review board (IRB) of the Seoul St. Mary Hospital (IRB No. KC12TNSI0283) and also each participating hospital. The requirement for informed consent of subjects was waived by the board.
RESULTS
During the study period, 1,192 residual sera were collected from adults and adolescents from 11 to 85 yr of age, the subjects consisted of 600 males (50.3%) and 592 females (49.7%). The number of subjects in each age group was as follows: 198 in 11-20 yr, 211 in 21-30 yr, 213 in 31-40 yr, 198 in 41-50 yr, 191 in 51-60 yr, and 181 in ≥61 yr. The gender ratios of all of the age groups were not different significantly (Table 1).
Table 1.
Anti-pertussis toxin IgG titer and pertussis seroprevalence in each age group
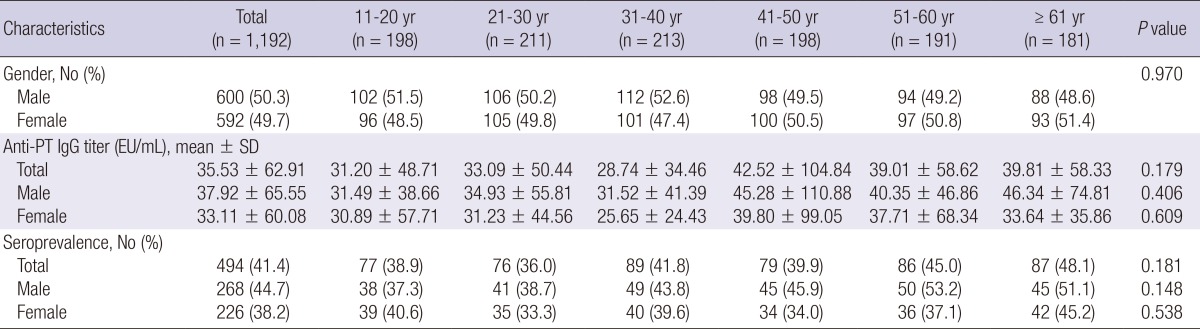
PT, pertussis toxin; SD, standard deviation; EU, ELISA unit.
Anti-pertussis toxin IgG titer
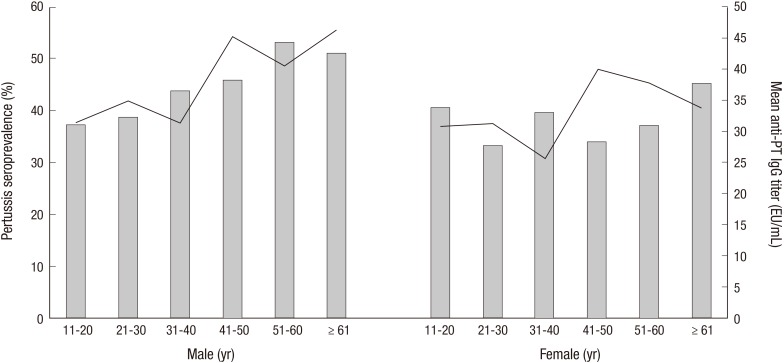
The anti-PT IgG titers ranged from 1.78 to 975.62 EU/mL, and the mean anti-PT IgG titer was 35.53±62.91 EU/mL. The mean anti-PT IgG titer was highest in the 41-50 yr age group and lowest in the 31-40 yr age group. The mean titers of all the age groups were not significantly different (Table 1). The mean IgG titers for males and females were 37.92±65.55 EU/mL and 33.11±60.08 EU/mL, respectively, and these titers were not significantly different (Fig. 1, P=0.187). The mean IgG titers were not significantly different based on gender, according to age groups (Table 1).
Fig. 1.
Mean anti-PT IgG titer (line) and pertussis seroprevalence (bar) according to the age groups in each gender.
Pertussis seroprevalence
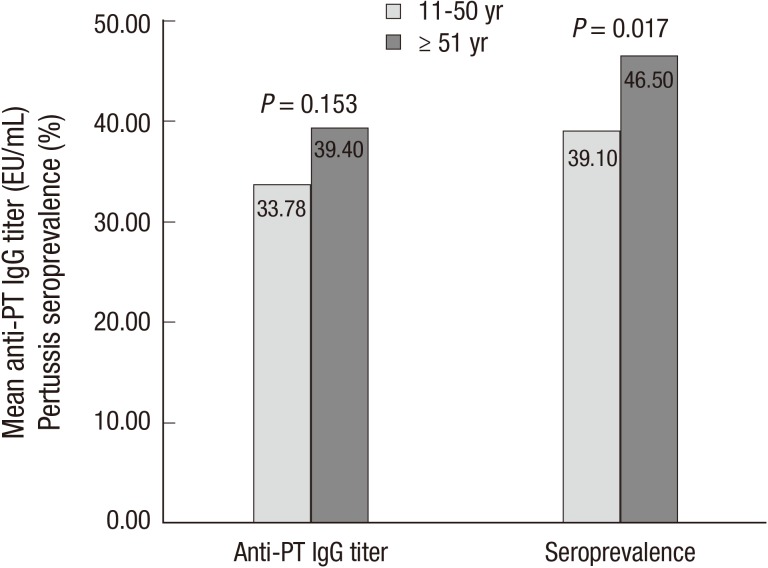
Among 1,192 subjects, 494 individuals (41.4%) were seropositive (anti-PT IgG titer>24 EU/mL), and there were 268 seropositive males (54.3%) and 226 seropositive females (45.7%, P=0.023). Although the seroprevalence of each age group was not significantly different (Table 1), the seroprevalence of subjects aged 51 or older was significantly higher than the seroprevalence of subjects younger than 51 yr (46.5% vs. 39.1%, P=0.017, Fig. 2). In each gender, there was no significant difference for seroprevalence according to the age groups (Table 1).
Fig. 2.
Comparison of mean anti-PT IgG titer and pertussis seroprevalence between individuals 51 yr of age or older and individuals less than 51 yr of age.
DISCUSSION
This study was the third surveillance of pertussis seroprevalence based on anti-PT IgG titers after 2002 and 2008 (14). The mean anti-PT IgG titer of the whole population in this study was 35.53 EU/mL, which was lower than the titer of a previous study, conducted in 2008, which reported a mean anti-PT titer of 56.16 EU/mL (14). In addition, the mean titer of each age group was lower in this study than in the 2008 study: 11-20 yr, 31.20 vs 46.91 EU/mL; 21-30 yr, 33.09 vs 40.34 EU/mL; 31-40 yr, 28.74 vs 38.52 EU/mL; 41-50 yr, 42.52 vs 42.57 EU/mL; 51-60 yr, 39.01 vs 44.65 EU/mL; ≥61 yr, 39.81 vs 43.59 EU/mL. Pertussis seroprevalence was also lower in this study than in the 2008 study in each age group: 11-20 yr, 38.9% vs 53.0%; 21-30 yr, 36.0% vs 55.0%; 31-40 yr, 41.8% vs 47.0%; 41-50 yr, 39.9% vs 59.0%; 51-60 yr, 45.0% vs 57.0%; ≥61 yr, 48.1% vs 55.0%. Although the decreases in the mean anti-PT IgG titer and pertussis seroprevalence might be attributed to an overall reduction of pertussis in communities, efforts have not been made to control pertussis besides an introduction of Tdap booster vaccination at 11-12 yr of age in 2009 in Korea. Therefore, regional differences in incidence and prevalence of pertussis should be considered because these factors also affect the mean IgG titers and seroprevalence. The 2008 study was conducted in Incheon, Gangwon-do, Chungcheongnam-do and Gyeongsangnam-do (14), and the incidence of pertussis in each region in 2008 was 0.07, 0.00, 0.05, and 0.00 per 100,000 persons, respectively (15). The current study was conducted in Seoul and Gyeonggi-do, and the incidences of pertussis in 2008 were 0.01 and 0.03 per 100,000 persons, respectively (15). In 2011, the incidences of pertussis in Incheon, Gangwon-do, Chungcheongnam-do, Gyeongsangnam-do, Seoul and Gyeonggi-do were≥0.3, 0.20-0.29, ≥0.3, 0.00, 0.20-0.29, and 0.10-0.19 per 100,000 persons, respectively (16). In short, the pertussis incidence in regions where the 2008 study was conducted was higher than the incidence in regions where this study was conducted in both 2008 and 2011. Therefore, we suggest that the higher mean anti-PT IgG titer and higher pertussis seroprevalence in the 2008 study were caused by regional differences of pertussis incidence between the two studies.
This study identified a significantly higher seroprevalence in adults aged 51 or older compared to other age groups. This result was different from the result of the 2008 study, which showed a decrease in the seroprevalence after the age of 41 yr (14). We expected that the highest pertussis seroprevalence would be observed in the 11-20 yr age group because this group should had received Tdap booster vaccinations, recommended at 11-12 yr of age by the National Immunization Program of the Korean government (17). However, the highest prevalence was observed in the ≥61 yr age group. This finding indicates that natural pertussis infection is endemic in older adults and that Tdap booster vaccination rates at 11-12 yr of age may be insufficient. Reports from Israel and the Netherlands have already indicated that the highest pertussis seroprevalence was in older adults (13, 18). Because protective immunity against pertussis may last for 4-12 yr after a primary DTaP vaccination series (19, 20), natural pertussis infection could occur in older adults even after previous vaccinations. However, pertussis in older adults may present with atypical manifestations (12, 21, 22), and the pertussis might not be diagnosed and treated early. The adults with pertussis infection might transmit pertussis, which could allow the disease to persist endemically in older adults and also affect unvaccinated infants (3, 23, 24, 25). Even if, adolescent Tdap booster vaccinations are administered extensively, natural pertussis infection may continue in older adults due to the waning effect of the Tdap vaccination (5, 11, 19, 20). Therefore, it will be necessary to develop a strategy that reduces natural pertussis infection in older adults after the adolescent Tdap vaccination. First, doctors should work to diagnose and treat patients with pertussis infection with atypical manifestations early. The necessity of an additional pertussis booster vaccination for adults after the adolescent Tdap vaccination should also be considered. However, additional studies are needed to determine the duration of protective immunity after a pertussis vaccination in adults, the amount of reactogenicity that will occur with repeated pertussis vaccinations in adults and the optimal pertussis vaccination interval for adults (26).
Both the mean anti-PT IgG titer and pertussis seroprevalence in this study were higher in males than in females without a statistical significance. One previous study reported higher seroprevalence of pertussis in females compared to males (13), while other studies usually reported higher seroprevalence in males (18, 27, 28). However, these differences in pertussis seroprevalence between males and females were not statistically significant. In Korea, tetanus seroprevalence in adult males is higher than in females because tetanus vaccines are administered to most adult males during their obligatory military service (29). In addition, more social and occupational activities in males compared to females may result in more natural infection of tetanus, and vaccination for tetanus prophylaxis and consequently higher seroprevalence of tetanus (30). Considering that pertussis vaccination is not given to adults in Korea, higher pertussis seroprevalence in adult males compared to females may be caused by more natural infection in males who have more social and occupational activities than females. However, patients aged 10 or more reported with pertussis in Korea were 17 males and 28 females between 2009 and 2011 (16), and this female dominance is contrary to the male dominance of pertussis seroprevalence. Because the estimated incidence of pertussis in Korean people older than 10 yr was low, about 0.013 per 100,000 males and 0.022 per 100,000 females (16), the sex difference in pertussis incidence may be not significant. Because we cannot estimate the number of pertussis patients who were not diagnosed or not reported, the sex difference in the incidence of natural pertussis infection cannot be defined, and a multicenter or nationwide study is required.
This study was conducted using the same commercial ELISA kit as the 2008 study, however, the regions that participated in the two studies were different. Therefore, we might be unable to directly compare the mean anti-PT IgG titer and seroprevalence of pertussis. Additionally, the anti-PT IgG titer, >24 EU/mL, might not reflect the actual seroprevalence because the ELISA methods for the anti-PT IgG titer have not been standardized and the anti-PT IgG titer cut-off value to discriminate between recent and remote infections has not yet been determined in the Korean population. However, the changing trend of anti-PT seroprevalence according to the age groups should be meaningful.
The results of this study indicated that the pertussis seroprevalence was higher in older adults than in younger adults and adolescents, which contradicted results of previous studies. We also identified the necessity of a new strategy to prevent and reduce pertussis infection in older adults. Additional pertussis booster vaccination in adults may be a strategy, however, further studies that examine optimal vaccination schedules and a cost-effectiveness of the strategy are needed.
Footnotes
This study was funded by a grant from the Korea Centers for Disease Control and Prevention (grant number 2012E3200200).
There is no conflict of interest for all authors.
References
- 1.Hewlett EL, Edwards KM. Clinical practice: pertussis-not just for kids. N Engl J Med. 2005;352:1215–1222. doi: 10.1056/NEJMcp041025. [DOI] [PubMed] [Google Scholar]
- 2.Choe YJ, Park YJ, Jung C, Bae GR, Lee DH. National pertussis surveillance in South Korea 1955-2011: epidemiological and clinical trends. Int J Infect Dis. 2012;16:e850–e854. doi: 10.1016/j.ijid.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Brooks DA, Clover R. Pertussis infection in the United States: role for vaccination of adolescents and adults. J Am Board Fam Med. 2006;19:603–611. doi: 10.3122/jabfm.19.6.603. [DOI] [PubMed] [Google Scholar]
- 4.Güriş D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Wharton M, Livengood JR. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin Infect Dis. 1999;28:1230–1237. doi: 10.1086/514776. [DOI] [PubMed] [Google Scholar]
- 5.Pebody RG, Gay NJ, Giammanco A, Baron S, Schellekens J, Tischer A, Olander RM, Andrews NJ, Edmunds WJ, Lecoeur H, et al. The seroepidemiology of Bordetella pertussis infection in Western Europe. Epidemiol Infect. 2005;133:159–171. doi: 10.1017/s0950268804003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassiday P, Sanden G, Heuvelman K, Mooi F, Bisgard KM, Popovic T. Polymorphism in Bordetella pertussis pertactin and pertussis toxin virulence factors in the United States, 1935-1999. J Infect Dis. 2000;182:1402–1408. doi: 10.1086/315881. [DOI] [PubMed] [Google Scholar]
- 7.Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet. 2006;367:1926–1936. doi: 10.1016/S0140-6736(06)68848-X. [DOI] [PubMed] [Google Scholar]
- 8.Caro JJ, Getsios D, El-Hadi W, Payne K, O'Brien JA. Pertussis immunization of adolescents in the United States: an economic evaluation. Pediatr Infect Dis J. 2005;24:S75–S82. doi: 10.1097/01.inf.0000160918.72953.51. [DOI] [PubMed] [Google Scholar]
- 9.Tan T, Halperin S, Cherry JD, Edwards K, Englund JA, Glezen P, Greenberg D, Rothstein E, Skowronski D. Pertussis immunization in the global pertussis initiative North American region: recommended strategies and implementation considerations. Pediatr Infect Dis J. 2005;24:S83–S86. doi: 10.1097/01.inf.0000160919.94330.1a. [DOI] [PubMed] [Google Scholar]
- 10.Van Rie A, Hethcote HW. Adolescent and adult pertussis vaccination: computer simulations of five new strategies. Vaccine. 2004;22:3154–3165. doi: 10.1016/j.vaccine.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 11.Campins-Martí M, Cheng HK, Forsyth K, Guiso N, Halperin S, Huang LM, Mertsola J, Oselka G, Ward J, Wirsing von König CH, et al. Recommendations are needed for adolescent and adult pertussis immunisation: rationale and strategies for consideration. Vaccine. 2001;20:641–646. doi: 10.1016/s0264-410x(01)00393-0. [DOI] [PubMed] [Google Scholar]
- 12.Cherry JD, Grimprel E, Guiso N, Heininger U, Mertsola J. Defining pertussis epidemiology: clinical, microbiologic and serologic perspectives. Pediatr Infect Dis J. 2005;24:S25–S34. doi: 10.1097/01.inf.0000160926.89577.3b. [DOI] [PubMed] [Google Scholar]
- 13.Rendi-Wagner P, Tobias J, Moerman L, Goren S, Bassal R, Green M, Cohen D. The seroepidemiology of Bordetella pertussis in Israel-estimate of incidence of infection. Vaccine. 2010;28:3285–3290. doi: 10.1016/j.vaccine.2010.02.104. [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Choi UY, Kim JS, Ahn JH, Choi JH, Ma SH, Park JS, Kim HM, Kang JH. Immunoassay of pertussis according to ages. Korean J Pediatr Infect Dis. 2012;19:55–60. [Google Scholar]
- 15.Korea Centers for Disease Control and Prevention. Communicable diseases surveillance yearbook 2008. Seoul: Korea Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 16.Korea Centers for Disease Control and Prevention. Infectious diseases surveillance yearbook 2011. Seoul: Korea Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 17.Choi KM, Kim KH, Kim YJ, Kim JH, Park SE, Lee HJ, Eun BW, Jo DS, Choi EH, Hong YJ. Recommendation for the use of newly introduced Tdap vaccine in Korea. Korean J Pediatr. 2011;54:141–145. doi: 10.3345/kjp.2011.54.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Greeff SC, de Melker HE, van Gageldonk PG, Schellekens JF, van der Klis FR, Mollema L, Mooi FR, Berbers GA. Seroprevalence of pertussis in the Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS One. 2010;5:e14183. doi: 10.1371/journal.pone.0014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkinson D. Duration of effectiveness of pertussis vaccine: evidence from a 10 year community study. Br Med J (Clin Res Ed) 1988;296:612–614. doi: 10.1136/bmj.296.6622.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. 2005;24:S58–S61. doi: 10.1097/01.inf.0000160914.59160.41. [DOI] [PubMed] [Google Scholar]
- 21.Senzilet LD, Halperin SA, Spika JS, Alagaratnam M, Morris A, Smith B Sentinel Health Unit Surveillance System Pertussis Working Group. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clin Infect Dis. 2001;32:1691–1697. doi: 10.1086/320754. [DOI] [PubMed] [Google Scholar]
- 22.Wright SW, Edwards KM, Decker MD, Zeldin MH. Pertussis infection in adults with persistent cough. JAMA. 1995;273:1044–1046. [PubMed] [Google Scholar]
- 23.Kwon HJ, Yum SK, Choi UY, Lee SY, Kim JH, Kang JH. Infant pertussis and household transmission in Korea. J Korean Med Sci. 2012;27:1547–1551. doi: 10.3346/jkms.2012.27.12.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson JD. The changing epidemiology of pertussis in young infants: the role of adults as reservoirs of infection. Am J Dis Child. 1978;132:371–373. doi: 10.1001/archpedi.1978.02120290043006. [DOI] [PubMed] [Google Scholar]
- 25.Wendelboe AM, Hudgens MG, Poole C, Van Rie A. Estimating the role of casual contact from the community in transmission of Bordetella pertussis to young infants. Emerg Themes Epidemiol. 2007;4:15. doi: 10.1186/1742-7622-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsyth K, Tan T, von König CH, Caro JJ, Plotkin S. Potential strategies to reduce the burden of pertussis. Pediatr Infect Dis J. 2005;24:S69–S74. doi: 10.1097/01.inf.0000160917.29723.03. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Qi, Zheng H, Liu M, Han K, Shu J, Wu C, Xu N, He Q, Luo H. The seroepidemiology of immunoglobulin G antibodies against pertussis toxin in China: a cross sectional study. BMC Infect Dis. 2012;12:138. doi: 10.1186/1471-2334-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai FY, Thoon KC, Ang LW, Tey SH, Heng D, Cutter JL, Phoon MC, Chow VT. Comparative seroepidemiology of pertussis, diphtheria and poliovirus antibodies in Singapore: waning pertussis immunity in a highly immunized population and the need for adolescent booster doses. Vaccine. 2012;30:3566–3571. doi: 10.1016/j.vaccine.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Kim JS, Ahn JH, Choi JH, Ma SH, Park JS, Kim HM, Kang JH. Immunoassay of diphtheria and tetanus according to ages. Infect Chemother. 2012;44:62–66. [Google Scholar]
- 30.Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761–766. doi: 10.1056/NEJM199503233321201. [DOI] [PubMed] [Google Scholar]