Abstract
Proton pump inhibitor (PPI)-based triple therapy consisting of PPI, amoxicillin, and clarithromycin, is the recommended first-line treatment for Helicobacter pylori infection. However, the eradication rate of triple therapy has declined over the past few decades. We analyzed the eradication rate and adverse events of triple therapy to evaluate current practices in Korea. A comprehensive literature search was performed up to August 2013 of 104 relevant studies comprising 42,124 patients. The overall eradication rate was 74.6% (95% confidence interval [CI], 72.1%-77.2%) by intention-to-treat analysis and 82.0% (95% CI, 80.8%-83.2%) by per-protocol analysis. The eradication rate decreased significantly from 1998 to 2013 (P < 0.001 for both intention-to-treat and per-protocol analyses). Adverse events were reported in 41 studies with 8,018 subjects with an overall incidence rate of 20.4% (95% CI, 19.6%-21.3%). The available data suggest that the effectiveness of standard triple therapy for H. pylori eradication has decreased to an unacceptable level. A novel therapeutic strategy is warranted to improve the effectiveness of first-line treatment for H. pylori infection in Korea.
Graphical Abstract
Keywords: Helicobacter pylori, Eradication, Triple Therapy
INTRODUCTION
Helicobacter pylori infection causes chronic gastritis and is associated with an increased risk of upper gastrointestinal diseases, such as peptic ulcer disease, gastric cancer, and mucosa-associated lymphoid tissue lymphoma (1, 2). Although its incidence has declined in developed countries, the prevalence of H. pylori remains high in Korea (3, 4, 5, 6). The nationwide seroprevalence surveys performed in 1998 and 2005 among asymptomatic Korean adults reported H. pylori prevalence rates of 66.9% and 59.5% (5).
Proton pump inhibitor (PPI)-based triple therapy, which consists of PPI, amoxicillin, and clarithromycin or metronidazole, is the first-line treatment for H. pylori infection (7). Because metronidazole-resistant H. pylori strains are reported in 40% or more of clinical isolates, triple therapy with PPI standard dose bis in die (bid, twice a day), amoxicillin 1 g bid, and clarithromycin 500 mg bid is the standard regimen for H. pylori eradication in Korea (7, 8, 9). However, even with the current treatment regimens, treatment fails in approximately 20% of patients who remain H. pylori positive (10, 11). In addition, the prevalence of clarithromycin-resistant H. pylori strains is increasing in both Korea and other countries, and the efficacy of PPI-based triple therapies has substantially decreased to 80% or below in most countries in recent decades (11, 12, 13, 14, 15, 16). Consequently, whether standard triple therapy should continue as first-line therapy is currently under debate.
Several observational studies assessing trends in H. pylori eradication over a number of years have been performed in Korea. In some reports, the eradication rate of standard triple therapy was shown to have decreased to 77.5% in recent years (15, 17). In contrast, other studies reported that the eradication rate remained constant over 5-11 yr, although the eradication rate was found to be below 90% in a per-protocol (PP) analysis (18, 19, 20). This discrepancy may be due to geographical differences in antibiotic resistance and to different methods used for determining eradication. We therefore performed a meta-analysis to evaluate the eradication rate and adverse events of first-line triple therapy to verify the effectiveness of current practice in Korea.
MATERIALS AND METHODS
Study selection
A comprehensive literature search was performed to identify relevant studies through August 2013 using computer-assisted bibliographic searches of PubMed, KoreaMed, and KMBASE. Combinations of the following terms were used: H. pylori or H. pylori and eradication, triple, first-line, proton pump inhibitor, PPI, amoxicillin, clarithromycin, or metronidazole. Abstracts and full papers from relevant studies were reviewed, and those meeting the defined selection criteria were considered for further evaluation. Abstracts presented through August 2013 at the following congresses were also hand-searched: Digestive Disease Week, the United European Gastroenterology Week, the Korean Society of Gastroenterology, the Korean College of Helicobacter and Upper Gastrointestinal Research, and the Korean Association of Internal Medicine. Abstracts of articles in each of these multiple searches were reviewed, and those meeting the inclusion criteria were included. Care was taken to avoid obtaining duplicate data by examining the authors' names and affiliations for each publication. Overlapping articles and articles unrelated to our analysis were excluded.
Studies were eligible if they had at least one treatment arm and met all of the following criteria: 1) assessed standard triple therapy (a PPI with amoxicillin and clarithromycin) for H. pylori eradication; 2) included first-line eradication therapy; 3) demonstrated H. pylori infection by at least one high-accuracy diagnostic test (urea breath test, stool antigen test, histological examination, rapid urease test, or culture); 4) confirmed eradication of infection after completion of treatment, based on an appropriate follow-up test; and 5) was the most informative article when multiple articles were published by the same authors or groups.
The quality of each randomized controlled study was assessed by using the Jaded composite scale based on three items: 1) randomization; 2) double blinding; and 3) description of withdrawals and dropouts (21). The selection of studies for this meta-analysis was performed by two independent reviewers. Differences between the two reviewers were resolved by assessment of the full article by a third reviewer. The decision as to whether to include the article was made by consensus.
Data extraction and analysis
Data on the following items were extracted from the selected articles: 1) study design; 2) study period; 3) number of patients enrolled in the study and in each treatment arm; 4) drug regimen, doses, and duration of therapy; 5) diagnostic tests used in the diagnosis of H. pylori infection and confirmation of eradication; 6) indications for H. pylori eradication; 7) number of patients in whom H. pylori infection was successfully eradicated; and 8) number of patients with adverse events and the type of adverse event.
To account for heterogeneity between studies, the overall and yearly eradication rates and their 95% confidence intervals (CIs) were estimated with a random-effect logistic regression model (PROC NLMIXED in SAS software version 9.2, Cary, NC, USA). All reported P values are two-sided, and values of P<0.05 indicated statistical significance.
RESULTS
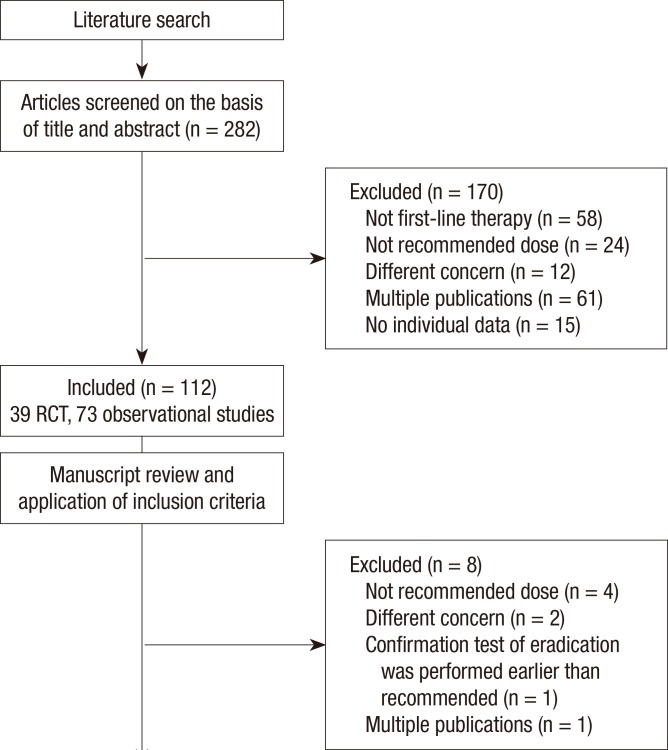
We initially screened 282 studies. Of these, 112 reports of first-line standard triple therapy were included in the analysis (Fig. 1). After systematic review of these studies, a total of 104 studies including 38 randomized controlled trials (RCTs) and 66 observational studies were eligible for meta-analysis. The clinical characteristics of the studies are listed in Tables 1 and 2. A total of 42,124 subjects were included, and the sample size per study ranged from 12 to 4,198 subjects.
Fig. 1.
Flowchart of the study design. RCT, randomized controlled trials.
Table 1.
Characteristics of the randomized clinical studies included in the meta-analysis
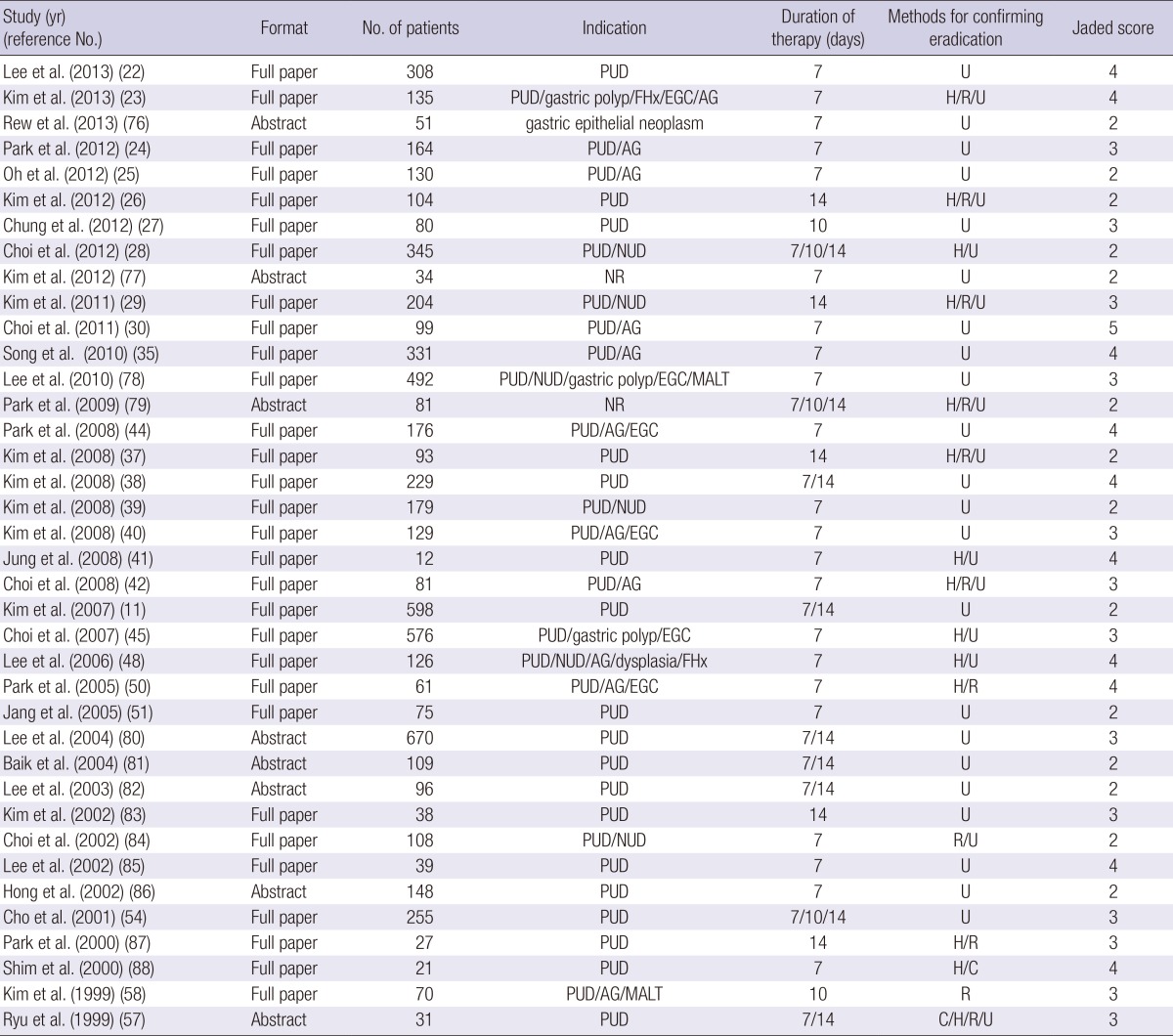
AG, atrophic gastritis; C, culture; EGC, early gastric cancer; FHx, family history of gastric cancer; H, histology; MALT, mucosa-associated lymphoid tumor; NR, not reported; NUD, non-ulcer dyspepsia; PPI, proton pump inhibitor; PUD, peptic ulcer disease; R, rapid urease test; S, serology; U, urea breath test.
Table 2.
Characteristics of the observational studies included in the meta-analysis
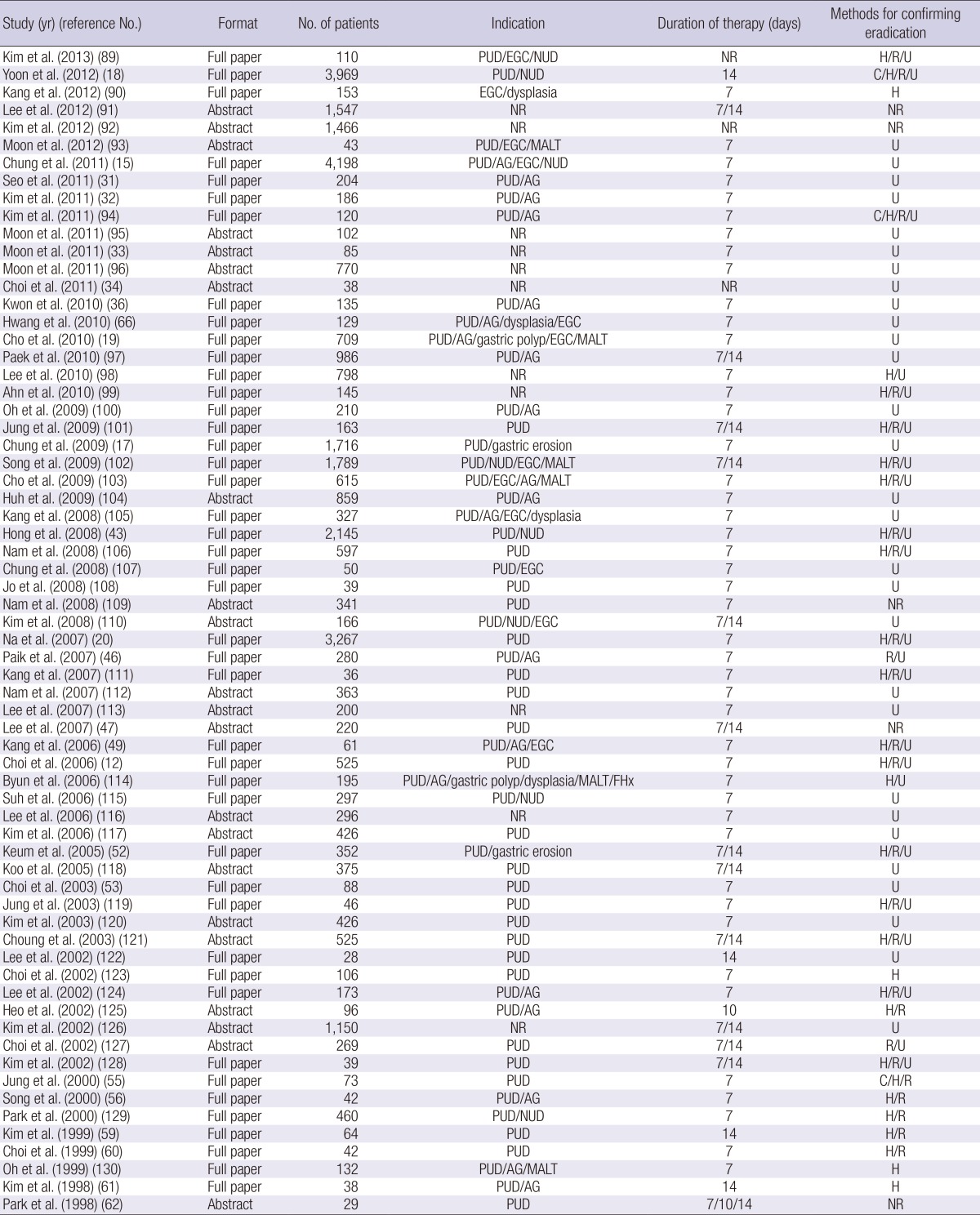
AG, atrophic gastritis; C, culture; EGC, early gastric cancer; FHx, family history of gastric cancer; H, histology; MALT, mucosa-associated lymphoid tumor; NR, not reported; NUD, non-ulcer dyspepsia; PPI, proton pump inhibitor; PUD, peptic ulcer disease; R, rapid urease test; S, serology; U, urea breath test.
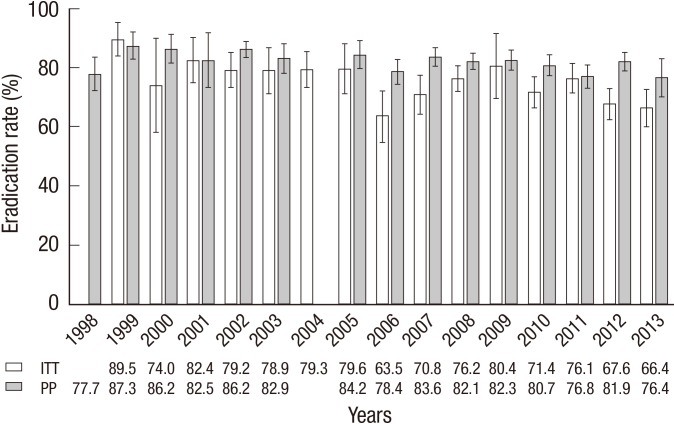
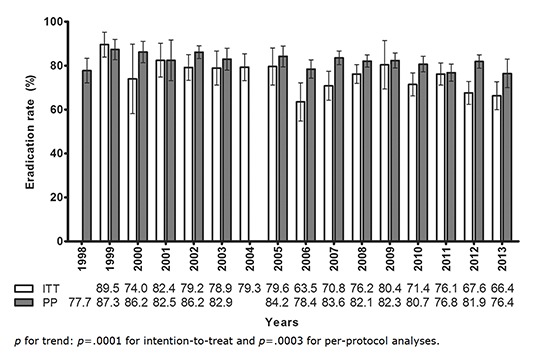
The overall eradication rate with standard triple therapy was 74.6% (95% CI, 72.1%-77.2%) for intention-to-treat (ITT) analysis and 82.0% (95% CI, 80.8%-83.2%) for PP analysis. The eradication rate showed a decreasing tendency from the years 1998 to 2013 based on ITT and PP analysis (P<0.001 and P=0.0003, respectively) (Fig. 2). The eradication rates of the 7-day and 14-day treatments were 81.1% (95% CI, 79.8%-82.3%) and 85.3% (95% CI, 83.5%-87.1%) for PP analysis, respectively.
Fig. 2.
Change in the eradication rate of the standard triple therapy by year. A decreasing trend was seen in the eradication rate during the last 16 yr (P < 0.001 for ITT analysis and P = 0.0003 for PP analysis). ITT, intention-to-treat; PP, per-protocol.
Adverse events were reported in 41 studies (24 RCTs and 17 observational studies) with 8,018 subjects treated with standard triple therapy (22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62). The incidence of adverse events in these studies was 20.4% (95% CI, 19.6%-21.3%). Taste alteration was the most common adverse event, followed by loose stool or diarrhea, abdominal discomfort, and nausea. The percentage of patients who stopped medication due to adverse events was 1.81% (35/1,930), suggesting that most patients could tolerate adverse events.
DISCUSSION
In our current study, we performed an up-to-date meta-analysis on the eradication rate of first-line PPI-based triple therapy. The results of our meta-analysis suggest that the efficacy of triple therapy for H. pylori infection is lower than desired, with a pooled eradication rate of 74.6% in ITT analysis and 82.0% in PP analysis. In addition, the eradication rate has significantly decreased over the last 16 yr.
Since 1998, PPI-based triple therapy has been recommended as the first-line therapy for H. pylori eradication in Korea. However, eradication rates have been declining, and, as of 2000, decreased to below 80% (11, 48), thereby failing to meet the requirements of the Asia-Pacific Consensus Guidelines, which state that a success rate of >80% in ITT analysis and >90% in PP analysis is needed for a regimen to be considered suitable for first-line eradication therapy (19, 42, 63).
There are several known factors that affect the eradication of H. pylori, such as antibiotic resistance, geographical area, patient age, smoking status, compliance, duration of therapy, bacterial density, Cag A, gastric acid concentration, individual response to PPI, and the presence of CYP2C19 polymorphism (10, 37, 64, 65). Among these factors, the main cause of the low eradication rate is presumed to be clarithromycin resistance. In Korea, the rate of resistance to clarithromycin was 5.9% before 2000, but has rapidly increased to 38.5% in the period of 2007 to 2009 (9, 16, 66, 67, 68). These rates are much higher than in other countries, such as Italy, and the main reason for this discrepancy may be the wide national and regional variations in the prevalence of antibiotic resistance (69). Clarithromycin resistance has been associated with cross-resistance caused by previous treatment with macrolides (70). In clinical practice, such antibiotics are usually prescribed for respiratory infections and are widely used for H. pylori eradication treatment. In addition, antibiotic treatment interruption due to gastrointestinal adverse events, such as diarrhea, nausea, vomiting, and abdominal discomfort, may be another reason for increased antibiotic resistance. Whereas an eradication rate higher than 75% is still achieved despite metronidazole resistance, clarithromycin resistance lowers the eradication rate by 20%, making it difficult to achieve an 80% eradication rate (71, 72). Potential approaches to overcome this resistance problem include patient-tailored, sequential, or concomitant therapies.
Increasing the duration of treatment may prove effective in curing infection, but this hypothesis remains to be validated. The majority of recently recommended eradication therapy regimens are 7-days in duration, which conveys advantages with respect to compliance and medical cost, while maintaining a similar eradication rate to that of longer regimens (73). The Asia-Pacific Consensus Guidelines and Japanese Consensus reports recommend a 7-day PPI-based triple therapy, whilst the Korean College of Helicobacter and Upper Gastrointestinal Research recommend 7-day or 14-day PPI-based triple therapy (74, 75). In Korea, 7-day PPI-based triple therapy has a PP eradication rate of 90%, which is not inferior to that of the 10-day or 14-day therapy (55, 60). However, in a recent study, although the 7-day PPI-based triple therapy was not inferior to the 14-day therapy, neither of the treatment durations provided an acceptable eradication rate of 90% in the PP analysis (11, 38). These studies, which were performed in Korea, were unable to provide conclusive evidence that a prolonged treatment for 2 weeks could counteract resistance to clarithromycin. Moreover, if the cause of treatment failure is antibiotic resistance, extension of the treatment period would not be expected to increase the eradication rate. Therefore, other strategies that achieve higher H. pylori eradication rates are needed, in addition to prolonged treatment duration, to increase the eradication rate in Korea.
Our present study is the largest study to date examining H. pylori eradication in Korea; however, there are several limitations. First, although we tried to include data from all over Korea, our data may not be representative of the entire Korean population. Second, we could not assess the antibiotic resistance rates and precise compliance rates in each study population. Therefore, there may be other factors not included in our analysis that influence the eradication rate of H. pylori.
In conclusion, conflicting results have been reported worldwide with regard to H. pylori eradication with standard triple therapy. Our data support the evidence for a decreased eradication rate of H. pylori, suggesting that a novel therapeutic strategy is warranted to improve first-line treatment for H. pylori infection in Korea.
Footnotes
The authors have no competing conflicts of interest to disclose.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.McColl KE. Clinical practice: helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 3.Jung JH, Choi KD, Han S, Jung HY, Do MY, Chang HS, Choe JW, Lee GH, Song HJ, Kim DH, et al. Seroconversion rates of Helicobacter pylori infection in Korean adults. Helicobacter. 2013;18:299–308. doi: 10.1111/hel.12043. [DOI] [PubMed] [Google Scholar]
- 4.Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, Yim JY, Kim HU, Baik GH, Seo GS, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013;13:104. doi: 10.1186/1471-230X-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Kim HY, Kim NY, Kim SW, Kim JG, Kim JJ, Roe IH, Seo JK, Sim JG, Ahn H, et al. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001;16:969–975. doi: 10.1046/j.1440-1746.2001.02568.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009;54:269–278. doi: 10.4166/kjg.2009.54.5.269. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004;48:4843–4847. doi: 10.1128/AAC.48.12.4843-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS, Graham DY, Kwon DH. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001;47:459–461. doi: 10.1093/jac/47.4.459. [DOI] [PubMed] [Google Scholar]
- 10.Calvet X, García N, López T, Gisbert JP, Gené E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:603–609. doi: 10.1046/j.1365-2036.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim BG, Lee DH, Ye BD, Lee KH, Kim BW, Kim SG, Kim SW, Kim SK, Kim JJ, Kim HY, et al. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: neither treatment duration provides acceptable eradication rate in Korea. Helicobacter. 2007;12:31–35. doi: 10.1111/j.1523-5378.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 12.Choi YS, Cheon JH, Lee JY, Kim SG, Kim JS, Kim N, Lee DH, Kim JM, Jung HC, Song IS. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006;48:156–161. [PubMed] [Google Scholar]
- 13.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Kim N, Kim SJ, Baik GH, Kim GH, Kim JM, Nam RH, Kim HB, Lee DH, Jung HC, et al. Regional difference of antibiotic resistance of Helicobacter pylori strains in Korea. Korean J Gastroenterol. 2011;57:221–229. doi: 10.4166/kjg.2011.57.4.221. [DOI] [PubMed] [Google Scholar]
- 15.Chung JW, Lee GH, Han JH, Jeong JY, Choi KS, Kim DH, Jung KW, Choi KD, Song HJ, Jung HY, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011;58:246–250. [PubMed] [Google Scholar]
- 16.Kim JM, Kim JS, Jung HC, Kim N, Song IS. Antibiotic resistance of Helicobacter pylori isolated from Korean patients in 2003. Korean J Gastroenterol. 2004;44:126–135. [PubMed] [Google Scholar]
- 17.Chung WC, Lee KM, Paik CN, Lee JR, Jung SH, Kim JD, Han SW, Chung IS. Inter-departmental differences in the eradication therapy for Helicobacter pylori infection: a single center study. Korean J Gastroenterol. 2009;53:221–227. [PubMed] [Google Scholar]
- 18.Yoon JH, Baik GH, Sohn KM, Kim DY, Kim YS, Suk KT, Kim JB, Kim DJ, Kim JB, Shin WG, et al. Trends in the eradication rates of Helicobacter pylori infection for eleven years. World J Gastroenterol. 2012;18:6628–6634. doi: 10.3748/wjg.v18.i45.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho DK, Park SY, Kee WJ, Lee JH, Ki HS, Yoon KW, Cho SB, Lee WS, Joo YE, Kim HS, et al. The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy. Korean J Gastroenterol. 2010;55:368–375. doi: 10.4166/kjg.2010.55.6.368. [DOI] [PubMed] [Google Scholar]
- 20.Na HS, Hong SJ, Yoon HJ, Maeng JH, Ko BM, Jung IS, Ryu CB, Kim JO, Cho JY, Lee JS, et al. Eradication rate of first-line and second-line therapy for Helicobacter pylori infection, and reinfection rate after successful eradication. Korean J Gastroenterol. 2007;50:170–175. [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee HJ, Kim JI, Cheung DY, Kim TH, Jun EJ, Oh JH, Chung WC, Kim BW, Kim SS, Park SH, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 2013;208:1123–1130. doi: 10.1093/infdis/jit287. [DOI] [PubMed] [Google Scholar]
- 23.Kim SY, Lee SW, Hyun JJ, Jung SW, Koo JS, Yim HJ, Park JJ, Chun HJ, Choi JH. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple "concomitant" therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013;47:21–24. doi: 10.1097/MCG.0b013e3182548ad4. [DOI] [PubMed] [Google Scholar]
- 24.Park HG, Jung MK, Jung JT, Kwon JG, Kim EY, Seo HE, Lee JH, Yang CH, Kim ES, Cho KB, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment Pharmacol Ther. 2012;35:56–65. doi: 10.1111/j.1365-2036.2011.04902.x. [DOI] [PubMed] [Google Scholar]
- 25.Oh HS, Lee DH, Seo JY, Cho YR, Kim N, Jeoung SH, Kim JW, Hwang JH, Park YS, Lee SH, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012;27:504–509. doi: 10.1111/j.1440-1746.2011.06922.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim SY, Jung SW, Kim JH, Koo JS, Yim HJ, Park JJ, Chun HJ, Lee SW, Choi JH. Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br J Clin Pharmacol. 2012;73:140–143. doi: 10.1111/j.1365-2125.2011.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung JW, Jung YK, Kim YJ, Kwon KA, Kim JH, Lee JJ, Lee SM, Hahm KB, Lee SM, Jeong JY, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012;27:1675–1680. doi: 10.1111/j.1440-1746.2012.07249.x. [DOI] [PubMed] [Google Scholar]
- 28.Choi HS, Chun HJ, Park SH, Keum B, Seo YS, Kim YS, Jeen YT, Um SH, Lee HS, Kim CD, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012;18:2377–2382. doi: 10.3748/wjg.v18.i19.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YS, Kim SJ, Yoon JH, Suk KT, Kim JB, Kim DJ, Kim DY, Min HJ, Park SH, Shin WG, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011;34:1098–1105. doi: 10.1111/j.1365-2036.2011.04843.x. [DOI] [PubMed] [Google Scholar]
- 30.Choi KH, Chung WC, Lee KM, Paik CN, Kim EJ, Kang BK, Oak JH, Jung SH. Efficacy of levofloxacin and rifaximin based quadruple therapy in Helicobacter pylori associated gastroduodenal disease: a double-blind, randomized controlled trial. J Korean Med Sci. 2011;26:785–790. doi: 10.3346/jkms.2011.26.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo JY, Kim MJ, Ko KH, Kim DH, Lim DS, Chon HR. Efficacy of ecabet sodium for Helicobacter pylori eradication, combined with lansoprazole-based triple regimen: a prospective study. Korean J Med. 2011;80:546–552. [Google Scholar]
- 32.Kim JY, Lee DH, Son JH, Kim JY, Kwon JE, Park YS, Kim N, Shin CM, Jung HC, Song IS. Effect of additional ecabet sodium on conventional triple therapy for Helicobacter pylori eradication in Korea. Korean J Gastrointest Endosc. 2011;42:349–355. [Google Scholar]
- 33.Moon B, Lim H, Lee S, Han K, Chung J, Lee Y. Efficacy of concomitant nonbithmuth-based quadruple therapy as first-line treatment for eradication of Helicobacter pylori [Abstract] Helicobacter. 2011;16:131. [Google Scholar]
- 34.Choi C, Lee D, Chon I, Park H, Kim N, Jeoung S, Kim J, Hwang J. Concomitant therapy was more effective than PPI-based triple therapy in Korea: a preliminary report [Abstract] Helicobacter. 2011;16:136. [Google Scholar]
- 35.Song MJ, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter. 2010;15:206–213. doi: 10.1111/j.1523-5378.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 36.Kwon JH, Lee DH, Song BJ, Lee JW, Kim JJ, Park YS, Kim N, Jeong SH, Kim JW, Lee SH, et al. Ten-day sequential therapy as first-line treatment for Helicobacter pylori infection in Korea: a retrospective study. Helicobacter. 2010;15:148–153. doi: 10.1111/j.1523-5378.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim SY, Lee SW, Jung SW, Koo JS, Yim HJ, Park JJ, Chun HJ, Lee HS, Choi JH, Kim CD, et al. Comparative study of Helicobacter pylori eradication rates of twice-versus four-times-daily amoxicillin administered with proton pump inhibitor and clarithromycin: a randomized study. Helicobacter. 2008;13:282–287. doi: 10.1111/j.1523-5378.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim N, Park SH, Seo GS, Lee SW, Kim JW, Lee KJ, Shin WC, Kim TN, Park MI, Park JJ, et al. Lafutidine versus lansoprazole in combination with clarithromycin and amoxicillin for one versus two weeks for Helicobacter pylori eradication in Korea. Helicobacter. 2008;13:542–549. doi: 10.1111/j.1523-5378.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim MN, Kim N, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Kim JS, Jung HC, et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter. 2008;13:261–268. doi: 10.1111/j.1523-5378.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim HW, Kim GH, Cheong JY, Yang US, Park SK, Song CS, Kang DH, Song GA. Helicobacter pylori eradication: a randomized prospective study of triple therapy with or without ecabet sodium. World J Gastroenterol. 2008;14:908–912. doi: 10.3748/wjg.14.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung JM, Shim KN, Oh HJ, Na YJ, Jung HS, Jung SA, Yoo K. Role of anti-secretory treatment in addition to Helicobacter pylori eradication triple therapy in the treatment of peptic ulcer. Korean J Gastroenterol. 2008;51:11–18. [PubMed] [Google Scholar]
- 42.Choi WH, Park DI, Oh SJ, Baek YH, Hong CH, Hong EJ, Song MJ, Park SK, Park JH, Kim HJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 2008;51:280–284. [PubMed] [Google Scholar]
- 43.Hong EJ, Park DI, Oh SJ, Song MJ, Choi WH, Hong CH, Park JH, Kim HJ, Cho YK, Shon CI, et al. Comparison of Helicobacter pylori eradication rate in patients with non-ulcer dyspepsia and peptic ulcer diseases according to proton pump inhibitors. Korean J Gastroenterol. 2008;52:80–85. [PubMed] [Google Scholar]
- 44.Park SK, Park DI, Choi JS, Kang MS, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. The effect of probiotics on Helicobacter pylori eradication. Hepatogastroenterology. 2007;54:2032–2036. [PubMed] [Google Scholar]
- 45.Choi HS, Park DI, Hwang SJ, Park JS, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. Double-dose, new-generation proton pump inhibitors do not improve Helicobacter pylori eradication rate. Helicobacter. 2007;12:638–642. doi: 10.1111/j.1523-5378.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 46.Paik WH, Kim YJ, Kim IK, Lee JK, Lee CH, Chung GE, Hong KS, Park YS, Hwang JH, Kim JW, et al. Comparison of the eradication rates of one-week low-dose triple therapy with standard-dose triple therapy for Helicobacter pylori infection. Korean J Gastrointest Endosc. 2007;35:1–5. [Google Scholar]
- 47.Lee K, Kim T, Jang B, Lee H, Eun J, Kim K, Lee S, Choi J, Park Y, Moon H, et al. Comparison of the first-line triple therapy for Helicobacter pylori eradication according to the duration of the treatment and trends of eradication rate [Abstract] Korean J Gastroenterol. 2007;1:304. [Google Scholar]
- 48.Lee JH, Hong SP, Kwon CI, Phyun LH, Lee BS, Song HU, Ko KH, Hwang SG, Park PW, Rim KS, et al. The efficacy of levofloxacin based triple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2006;48:19–24. [PubMed] [Google Scholar]
- 49.Kang MS, Park DI, Yun JW, Oh SY, Yoo TW, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, et al. Levofloxacin-azithromycin combined triple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2006;47:30–36. [PubMed] [Google Scholar]
- 50.Park SH, Park DI, Kim SH, Kim HJ, Cho YK, Sung IK, Sohn CI, Jeon WK, Kim BI, Keum DK. Effect of high-dose aspirin on Helicobacter pylori eradication. Dig Dis Sci. 2005;50:626–629. doi: 10.1007/s10620-005-2547-3. [DOI] [PubMed] [Google Scholar]
- 51.Jang HJ, Choi MH, Kim YS, Seo YA, Baik KH, Baik IH, Eun CS, Kim JB, Kae SH, Kim DJ, et al. Effectiveness of triple therapy and quadruple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2005;46:368–372. [PubMed] [Google Scholar]
- 52.Keum B, Lee SW, Kim SY, Kim JM, Choung RS, Yim HJ, Jeen YT, Lee HS, Chun HJ, Um SH, et al. Comparison of Helicobacter pylori eradication rate according to different PPI-based triple therapy: omeprazole, rabeprazole, esomeprazole and lansoprazole. Korean J Gastroenterol. 2005;46:433–439. [PubMed] [Google Scholar]
- 53.Choi BK, Yang SY, Park ET, Jang YS, Lee YJ, Lee SH, Seol SY, Chung JM. A prospective study on rabeprazole-based triple therapy for Helicobacter pylori eradication in patients with peptic ulcer. Korean J Gastroenterol. 2003;42:102–107. [PubMed] [Google Scholar]
- 54.Cho YJ, Chun HJ, Kim ST, Koh DW, Park JH, Park DK, Park CH, Lee SJ, Jeen YT, Lee HS, et al. Analysis of eradication rate of Helicobacter pylori according to treatment duration by using 13C-urea breath test comparison of OAC 7, 10 or 14 days regimen. Korean J Gastrointest Endosc. 2001;23:207–212. [Google Scholar]
- 55.Jung IS, Hong SJ, Kim JO, Cho JY, Lee MS, Shim CS. The effect of Helicobacter pylori eradication of triple therapy with omeprazole, amoxicillin and clarithromycin. Korean J Med. 2000;58:626–631. [Google Scholar]
- 56.Song HJ, Yang YS, Lee IS, Lee KM, Lee DS, Kim SW, Kim SS, Han SW, Choi KY, Chung IS, et al. Efficacy and tolerability of pantoprazole-based triple therapy in eradication of Helicobacter pylori in patients with peptic ulcer and/or gastritis. Korean J Gastroenterol. 2000;36:185–191. [Google Scholar]
- 57.Ryu KH, Kim YH, Lee KT, Lee JK, Lee JH, Rhee PL, Kim JJ, Koh KC, Paik SW, Rhee JC, et al. Comparison of the efficacy of triple therapy with omeprazole, amoxicillin and clarithromycin in Helicobacter pylori eradication according to the duration in patients with pepric ulcer disease [Abstract] Korean J Med. 1999;57:86. [Google Scholar]
- 58.Kim JH, Lee KT, Lee SM, Kim SH, Lee BS, Kim NJ, Jeong HY, Lee HY, Kim SY, Kim YK. Efficacy of ten days of clarithromycin, amoxicillin and omeprazole in eradicating Helicobacter pylori infection. Korean J Med. 1999;56:581–589. [Google Scholar]
- 59.Kim JI, Chung IS, Bhang CS, Park SH, Choi MG, Kim JK, Han SW, Sun HS, Park DH, Chang ED. Factors influencing eradication of Helicobacter pylori in patients with peptic ulcer disease. Korean J Gastroenterol. 1999;33:624–634. [Google Scholar]
- 60.Choi IJ, Lee WJ, Kim YS, Kim JS, Jung HC, Song IS, Kim CY. Efficacy of 1-week pantoprazole-based triple therapy in eradicating Helicobacter pylori without additional acid suppression therapy. Korean J Gastroenterol. 1999;34:724–732. [Google Scholar]
- 61.Kim BS, Chung IS, Park DH, Yang YS, Lee DS, Kim SW, Byun BH, Choi JY. The therapeutic effect of triple therapy in Helicobacter pylori infection. Korean J Gastroenterol. 1998;32:32–37. [Google Scholar]
- 62.Park YJ, Yi JS, Kwon KS, Cho HK, Choi W, Lee DH, Kim PS, Kim H, Shin YW, Kim YS. Efficacy of triple therapy with omeprazole, amoxicillin and clarithromycin in Helicobacter pylori eradication according to the treatment duration [Abstract] Korean J Med. 1998;55:219. [Google Scholar]
- 63.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–931. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 65.Graham DY, Lew GM, Malaty HM, Evans DG, Evans DJ, Jr, Klein PD, Alpert LC, Genta RM. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 1992;102:493–496. doi: 10.1016/0016-5085(92)90095-g. [DOI] [PubMed] [Google Scholar]
- 66.Hwang TJ, Kim N, Kim HB, Lee BH, Nam RH, Park JH, Lee MK, Park YS, Lee DH, Jung HC, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H: pylori in a single center of Korea. J Clin Gastroenterol. 2010;44:536–543. doi: 10.1097/MCG.0b013e3181d04592. [DOI] [PubMed] [Google Scholar]
- 67.Bang SY, Han DS, Eun CS, Kim JE, Ahn SB, Sohn JH, Jeon YC, Kang JO. Changing patterns of antibiotic resistance of Helicobacter pylori in patients with peptic ulcer disease. Korean J Gastroenterol. 2007;50:356–362. [PubMed] [Google Scholar]
- 68.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 69.Kim N, Kim JM, Kim CH, Park YS, Lee DH, Kim JS, Jung HC, Song IS. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006;40:683–687. doi: 10.1097/00004836-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Xia HX, Buckley M, Keane CT, O'Morain CA. Clarithromycin resistance in Helicobacter pylori: prevalence in untreated dyspeptic patients and stability in vitro. J Antimicrob Chemother. 1996;37:473–481. doi: 10.1093/jac/37.3.473. [DOI] [PubMed] [Google Scholar]
- 71.Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 72.Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: the QUADRATE Study. Gastroenterology. 2002;123:1763–1769. doi: 10.1053/gast.2002.37051. [DOI] [PubMed] [Google Scholar]
- 73.Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–562. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 74.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 75.Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1–20. doi: 10.1111/j.1523-5378.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 76.Rew JS, Park SY, Ki HS, Jun CH, Park CH, Kim HS, Choi SK. An antimicrobial susceptibility-guided versus standard triple therapy for Helicobacter pylori eradication in patients with gastric epithelial neoplasm [Abstract] Gastroenterology. 2013;144:S331. [Google Scholar]
- 77.Kim N, Nam RH, Kim JY, Lee MK, Lee JW. Comparison of ten-day, fifteen-day sequential therapy and proton-pump inhibitor-based triple therapy in Korea: a prospective randomized study [Abstract] Gastroenterology. 2012;142:S484. [Google Scholar]
- 78.Lee JH, Jung HY, Choi KD, Song HJ, Lee GH, Kim JH. The influence of CYP2C19 polymorphism on eradication of Helicobacter pylori: a prospective randomized study of lansoprazole and rabeprazole. Gut Liver. 2010;4:201–206. doi: 10.5009/gnl.2010.4.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park S, Chun H, Kim E, Park S, Jung E, Lee S, Jang J, Kwon Y, Keum B, Seo Y, et al. The 10-day sequential therapy for Helicobacter pylori eradication in Korea: less effective than expected [Abstract] Gastroenterology. 2009;136:A339–A340. [Google Scholar]
- 80.Lee D, Lee S, Chun H, Jung H, Kim J. Two weeks triple regimens with PPI, clarithromycin and amoxicillin is more effective in H. pylori eradication than one week triple regimens in patients with peptic ulcer disease in South Korea [Abstract] Helicobacter. 2004;9:570. [Google Scholar]
- 81.Baik GH, Kim DJ, Kim JB, Seo YA, Kae SH, Jang HJ, Kim KH, Park CH, Kim HY. Efficacy of rabeprazole-based 7-day and 14-day triple therapy in patients with Helicobacter pylori-related peptic ulcer [Abstract] Korean J Gastroenterol. 2004;1:285. [Google Scholar]
- 82.Lee EJ, Lee JE, Choe JW, Kim GH, O HJ, O HA, Lee HC, Kim JH, Kim TN, Jeong MG. Comparison of one-week and two-week triple therapy in Helicobacter pylori eradication [Abstract] Korean J Gastroenterol. 2003;44:546. [Google Scholar]
- 83.Kim JI, Park SH, Kim JK, Chung IS, Chung KW, Sun HS. The effects of nocturnal acid breakthrough on Helicobacter pylori eradication. Helicobacter. 2002;7:331–336. doi: 10.1046/j.1523-5378.2002.00105.x. [DOI] [PubMed] [Google Scholar]
- 84.Choi IJ, Jung HC, Choi KW, Kim JH, Ahn DS, Yang US, Rew JS, Lee SI, Rhee JC, Chung IS, et al. Efficacy of low-dose clarithromycin triple therapy and tinidazole-containing triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:145–151. doi: 10.1046/j.1365-2036.2002.01130.x. [DOI] [PubMed] [Google Scholar]
- 85.Lee SW, Kim KH, Oh HJ, Kim TD, Lee EJ, Jang BI, Kim TN, Chung MN. Ulcer healing effect of Helicobacter pylori eradication in patients with Helicobacter pylori-associated peptic ulcer. Korean J Med. 2002;63:134–140. [Google Scholar]
- 86.Hong SJ, Jung IS, Ryu CB, Kim JO, Cho JY, Lee JS, Lee MS, Shim CS, Kim BS. Efficacy of one-week esomeprazole-based triple therapy in Helicobacter pylori eradication in patients with duodenal ulcer [Abstract] Korean J Med. 2002;63:83. [Google Scholar]
- 87.Park S, Cho SH, Choi KY, Chung IS, Chung KW, Sun HS, Park DH. Effect of single-dose vs. divided-dose drug administration on eradication of Helicobacter pylori in patients with peptic ulcers. Korean J Gastroenterol. 2000;35:23–31. [Google Scholar]
- 88.Shim SG, Kim JJ, Kim YH, Sung IK, Son HJ, Lee KT, Rhee PL, Koh KC, Paik SW, Rhee JC, et al. One-week triple therapy for Helicobacter pylori-a prospective, randomized study. Korean J Gastroenterol. 2000;35:16–22. [Google Scholar]
- 89.Kim T, Song HJ, Shin SY, Kim JH, Na SY, Boo SJ, Choi EK, Cho YK, Kim HU, Song BC. Clarithromycin-resistant Helicobacter pylori associated with 23S rRNA point mutations in Jeju Island. Korean J Gastroenterol. 2013;61:252–258. doi: 10.4166/kjg.2013.61.5.252. [DOI] [PubMed] [Google Scholar]
- 90.Kang HY, Kim SG, Lee MK, Kim JS, Jung HC, Song IS. Effect of Helicobacter pylori eradication according to the IL-8-251 polymorphism in Koreans. J Korean Med Sci. 2012;27:1202–1207. doi: 10.3346/jkms.2012.27.10.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee S, Yang C. Comparison of 7-day and 14 day triple therapy for Helicobacter pylori eradication and change of eradication rate [Abstract] Helicobacter. 2012;17:103. [Google Scholar]
- 92.Kim HI, Shim KN, Yoon SY, Song EM, Kwon KJ, Cho WY, Kim SE, Jung HK, Jung SA. Trends of the eradication rates of first- and second-line therapy for Helicobacter pylori in recent ten years [Abstract] Korean J Helicobacter Up Gastrointest Res. 2012;12:152. [Google Scholar]
- 93.Moon JS, Park KS, Kwak CH, Kim EJ, Kim YS, Kim JN. The detection and eradication rates of clarithromycin-resistant Helicobacter pylori: efficacy of Panplex TM ClaR-H. pylori (pilot study) [Abstract] Korean J Helicobacter Up Gastrointest Res. 2012;12:159. [Google Scholar]
- 94.Kim JY, Kim N, Park HK, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Primary antibiotic resistance of Helicobacter pylori strains and eradication rate according to gastroduodenal disease in Korea. Korean J Gastroenterol. 2011;58:74–81. doi: 10.4166/kjg.2011.58.2.74. [DOI] [PubMed] [Google Scholar]
- 95.Moon BS, An B, Kim H, Lim HC, Lee YC, Lee G, Kim S, Park M. Antibiotic resistance and eradication rate of Helicobacter pylori strains isolated in Korean patients [Abstract] Helicobacter. 2011;16:119. [Google Scholar]
- 96.Moon B, Lee S, Han K, Chan YY, Chung J, Chon C, Lim H, Lee Y. Influence of clinical demographic factors in successful eradication of Helicobacter pylori [Abstract] Helicobacter. 2011;16:134. [Google Scholar]
- 97.Paek NY, Lim YJ, Lee JH, Kang JH, Park JB, Lee JH. Recent eradication rates of first-line triple regimens for H. pylori infection. Korean J Gastrointest Endosc. 2010;41:5–9. [Google Scholar]
- 98.Lee JH, Sung IK, Kim JH, Lee SY, Hong SN, Park HS, Shim CS, Jin CJ, Han HS, Kim KH. Impact of Clarithromycin resistance on the outcome of standard triple Helocibacter pylori eradication therapy. Korean J Helicobacter Up Gastrointest Res. 2010;10:14–20. [Google Scholar]
- 99.Ahn JY, Jung HY, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Choi KD, Song HJ, Lee GH, et al. Is antimicrobial susceptibility testing necessary in the first Helicobacter pylori eradication? Korean J Helicobacter Up Gastrointest Res. 2010;10:21–26. [Google Scholar]
- 100.Oh JH, Dong MS, Choi MG, Yoo HW, Lee SB, Park YI, Chung IS. Effects of CYP2C19 and MDR1 genotype on the eradication rate of Helicobacter pylori infection by triple therapy with pantoprazole, amoxycillin and clarithromycin. J Gastroenterol Hepatol. 2009;24:294–298. doi: 10.1111/j.1440-1746.2008.05605.x. [DOI] [PubMed] [Google Scholar]
- 101.Jung SW, Lee SW, Hyun JJ, Kim DI, Koo JS, Yim HJ, Park JJ, Lee HS, Chun HJ, Um SH, et al. Efficacy of Helicobacter pylori eradication therapy in chronic liver disease. Dig Liver Dis. 2009;41:134–140. doi: 10.1016/j.dld.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 102.Song JG, Lee SW, Park JY, Nam SJ, Kim SY, Ahn JH, Kim JN, Park SM, Kim JH, Kim DI, et al. Trend in the eradication rates of Helicobacter pylori infection in the last 11 years. Korean J Med. 2009;76:303–310. [Google Scholar]
- 103.Cho HJ, Bae RC, Lee SH, Jang SI, Lee HS, Choi SY, Shin KY, Heo J, Kim SK, Jung MK, et al. The trend in the eradication rates of first- and second-line therapy for Helicobacter pylori infection in Daegu and Kyungpook provinces: a single center experience for the most recent 9 years. Korean J Med. 2009;76:186–192. [Google Scholar]
- 104.Huh K, Kim Y, Kim S, Lee T, Im E, Choi Y, Kang Y. Is the conventional 7-day triple therapy sufficient for eradication of Helicobacter pylori [Abstract]? Gastroenterology. 2009;136:A496. [Google Scholar]
- 105.Kang JM, Kim N, Lee DH, Park YS, Kim JS, Chang IJ, Song IS, Jung HC. Effect of the CYP2C19 polymorphism on the eradication rate of Helicobacter pylori infection by 7-day triple therapy with regular proton pump inhibitor dosage. J Gastroenterol Hepatol. 2008;23:1287–1291. doi: 10.1111/j.1440-1746.2008.05392.x. [DOI] [PubMed] [Google Scholar]
- 106.Nam TM, Lee DH, Kang KP, Lee JH, Chung JI, Choi HC, Lee SH, Park YS, Hwang JH, Kim JW, et al. Clinical factors that potentially affect the treatment outcome of Helicobacter pylori eradication therapy with using a standard triple regimen in peptic ulcer patients. Korean J Gastrointest Endosc. 2008;36:200–205. [Google Scholar]
- 107.Chung JW, Jung HY, Choi KD, Choi KS, Kim DH, Jung KW, Song HJ, Lee GH, Kim JH. Influence of CYP2C19 polymorphism on eradication of Helicobacter pylori: comparison between pantoprazole based first-line and rabeprazole based second-line therapy. Korean J Helicobacter Up Gastrointest Res. 2008;8:15–19. [Google Scholar]
- 108.Jo HJ, Lee DH, Kang SJ, Kim MN, Kim SH, Park JM, Choi MS, Jung HC, Song IS, Kim N, et al. Comparison of the efficacy of bismuth containing PPI-based quadruple therapy with PPI-based triple therapy only as first-line treatment for Helicobacter pylori infection. Korean J Gastrointest Endosc. 2008;37:259–264. [Google Scholar]
- 109.Nam T, Lee D, Kim N, Jung S, Kim J, Hwang J, Park Y, Lee S, Jung H, Song I. Histological factors that affect treatment outcome of Helicobacter pylori eradication in peptic ulcers in Korea: high grade of gastric atrophy was associated with low eradication rate [Abstract] Gastroenterology. 2008;134:A333. [Google Scholar]
- 110.Kim D, Kim G, Kim I, Jung W, Jeong K, Heo J, Song G. Efficacy of preceding propon pump inhibitor therapy on successful eradication of Helicobacter pylori [Abstract] Korean J Helicobacter Up Gastrointest Res. 2008;8:80. [Google Scholar]
- 111.Kang MJ, Shim KN, Baik SJ, Oh HJ, Jung JM, Na YJ, Kim SE, Jung SA, Yoo K. Efficacy and safety of standard triple therapy of Helicobacter pylori eradication in patients with chronic kidney disease. Korean J Helicobacter Up Gastrointest Res. 2007;7:20–25. [Google Scholar]
- 112.Nam Y, Lee D, Kim N, Jung S, Kim J, Hwang J. The efficacy of adding eupatilin to proton pump inhibitor-based triple therapy for Helicobacter pylori eradication in peptic ulcer in Korea [Abstract] Helicobacter. 2007;12:435. [Google Scholar]
- 113.Lee C, Paik W, Lee J, Chung G, Kim N, Lee D. The role of eupatilin in Helicobacter pylori eradication [Abstract] Helicobacter. 2007;12:434. [Google Scholar]
- 114.Byun YH, Jo YJ, Kim SC, Lee JS, Shin WY, Park YS, Kim SH, Lee HH, Song MH. Clinical factors that predicts successful eradication of Helicobacter pylori. Korean J Gastroenterol. 2006;48:172–179. [PubMed] [Google Scholar]
- 115.Suh SO, Lee DH, Park YS, Hwang JH, Kim JW, Kim N, Jung H, Song IS. Difference in Helicobacter pylori eradication rates in patients with peptic ulcer and non-ulcer dyspepsia. Korean J Med. 2006;70:505–510. [Google Scholar]
- 116.Lee CH, Lee JK, Kim JW, Park YS. The effectiveness of bismuth and eupatilin along with proton-pump inhibitor-based triple regimen in eradication of Helicobacter pylori [Abstract] Helicobacter. 2006;11:392. [Google Scholar]
- 117.Kim D. Efficacy of esomeprazole and rabeprazole for Helicobacter pylori eradication in patients with peptic ulcer [Abstract] Helicobacter. 2006;11:394. [Google Scholar]
- 118.Koo JS, Lee SW, Jung WW, Han WS, Lee JS, Kim MJ, Lee HS, Choi JH, Kim CD, Ryum HS, et al. Helicobacter pylori eradication rate in patients with scar stage of peptic ulcer [Abstract] Korean J Med. 2005;69:193. [Google Scholar]
- 119.Jung HK, Jin KJ, You MA, Bae KS, Kwon JM, Lee JS, Kim DY, Moon IH. Eradication rate of lansoprazole-based triple therapy in peptic ulcer patients with Helicobacter pylori and efficacy of urea breath test in evaluating Helicobater pylori eradication. Infect Chemother. 2003;35:154–159. [Google Scholar]
- 120.Kim J, Kim J, Lee D, Lee J, Rhee P, Rhee C. Efficacy of esomeprazole and rabeprazole for Helicobacter pylori eradication in patients with peptic ulcer [Abstract] Helicobacter. 2003;8:469. [Google Scholar]
- 121.Choung R, Lee S, Kim M, Jang Y, Kim J, Lee H. The eradication rate of Helicobacter pylori in complicated peptic ulcer patients [Abstract] Helicobacter. 2003;8:472. [Google Scholar]
- 122.Lee JH, Rhee PL, Hyun JG, Choe WH, Lim YJ, Ahn BH, Lee YW, Kim YH, Kim JJ, Koh KC, et al. Eradication of Helicobacter pylori in patients with S-2 stage duodenal ulcer scar an interim report. Korean J Gastrointest Endosc. 2002;24:71–75. [Google Scholar]
- 123.Choi HS, Kim JH, Kim MC, Jeong TH. Eradication rate of one-week triple therapy for peptic ulcer with Helicobacter pylori and clinical characteristics of patients with failed eradication. J Korean Acad Fam Med. 2002;23:60–67. [Google Scholar]
- 124.Lee JH, Kim HY, Park JK, Shim JH, Kim JW, Hwang JH, Kim BG, Chang DK, Kim JW, Kim NY, et al. Current effectiveness of Helicobacter pylori eradication treatment in primary care setting in Korea. Korean J Med. 2003;65:422–425. [Google Scholar]
- 125.Heo JH, Nam SW, Roe IH, Yang MR, Kim JT, Song IH, Lim CY, Kim JW, Shin JH. Correspondence between H. pylori eradication failure rate and clarithromycin resistance rate [Abstract] Korean J Gastroenterol. 2002 [Google Scholar]
- 126.Kim KO, Chun HJ, Jeong RS, Kim YS, Kim YS, Park CH, Jeen YT, Lee HS, Lee SW, Um S, et al. Comparison of 7-day and 14-day triple therapy in Helicobacter pylori eradication in elderly subjects [Abstract] Korean J Med. 2002;63:83. [Google Scholar]
- 127.Choi KD, Kim JS, Jung HC, Song IS. Efficacy of rabeprazole-based triple therapy regimen for the eradication of Helicobacter pylori in patients with peptic ulcer disease [Abstract] Korean J Helicobacter Up Gastrointest Res. 2003 [Google Scholar]
- 128.Kim YJ, Lee SY, Choi SH, Kim YJ. Treatment behavior for the eradication of Helicobacter pylori in peptic ulcer. Korean J Aerosp Environ Med. 2002;12:28–31. [Google Scholar]
- 129.Park JS, Hong SS, Lee YS, Lee E, Myung SJ, Jung HY, Yang SK. Eradication rate for Helicobacter pylori in the elderly. J Korean Geriatr Soc. 2000;4:138–147. [Google Scholar]
- 130.Oh MK, Choi WS, Lee YB, Chung HR, Kang GH, Kim JS. The effect of smoking on eradication of Helicobacter pylori. J Korean Acad Fam Med. 1999;20:991–999. [Google Scholar]