Abstract
The reference interval for plasma total homocysteine (tHcy) and serum folate concentrations were estimated. Total of 3,154 reference individuals (1,029 men and 2,125 women) were selected based on stringent exclusion criteria. For plasma tHcy concentration (µM/L), reference values (median [5-95 percentile]) were 7.72 (5.03 to 13.80) and 6.09 (3.95-10.19) in men and women, respectively. For serum folate concentration (nM/L), reference values were 23.71 (11.73-38.44) and 28.95 (15.23-40.44) in men and women, respectively. The tHcy levels of both genders in the present study were lower than those in previous reports from other countries and Korea.
Graphical Abstract
Keywords: Homocysteine, Folic Acid, Reference Intervals, Aged Population
Increased plasma homocysteine (tHcy) concentration and folate deficiency have been recognized as an independent risk factor for a number of pathologic conditions, including cardiovascular disease and cancer (1, 2, 3). Inadequate intake of vitamin co-factors, genetic polymorphisms such as methylenetetrahydrofolate reductase (MTHFR), renal insufficiency and lifestyle have been established as determinants of tHcy concentration (1). Folate has an independent role in homocysteine conversion to methionine as a methyl donor and is negatively associated with tHcy concentration (4). Many previous studies have shown ethnic and gender differences in the concentration of tHcy and folate (5), but they are still inconsistent and have varied with time by lifestyle changes or public intervention programs, such as folic acid fortification (5, 6).
Some previous studies have measured tHcy and folate levels among Koreans, although only limited information for reference values for tHcy and folate concentrations has been available because of their small sample size (7) and issues with the representativeness of study subjects (8). Therefore we determined the age-, gender-, and MTHFR C677T genotype-specific reference intervals for plasma tHcy and serum folate concentrations using a large-sized reference sample group in a middle-aged and older Korean population.
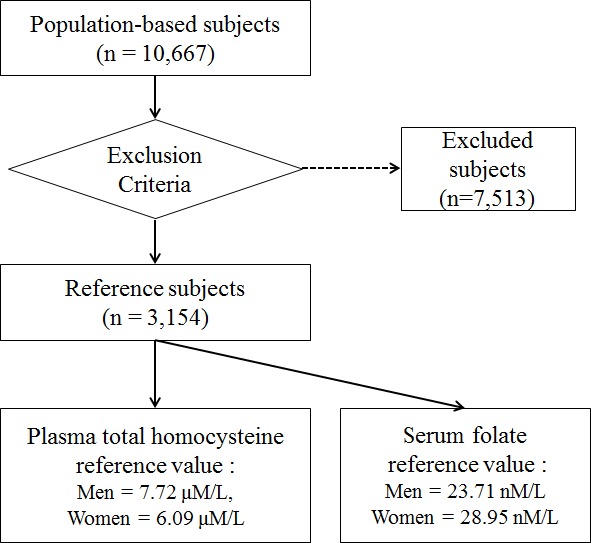
Reference individuals, in this study, were participants in the Namwon Study, an ongoing population-based cohort study that includes 10,667 individuals (4,201 men and 6,466 women), aged from 45 to 74 yr, dwelling in the community of Namwon, Korea. The study design and measurements in the Namwon Study were previously described (9). Among the participants, individuals with missing plasma tHcy, serum folate or MTHFR C677T genotype data (n= 52) and with specific conditions that could potentially alter the plasma tHcy or serum folate concentration were excluded to establish reference sample group. The exclusion criteria were as follows: extreme serum folate or plasma tHcy concentration (< 2.5 percentile or > 97.5 percentile by gender), renal insufficiency (glomerular filtration rate, GFR < 60 mL/min per 1.73 m2), hyperglycemia (fasting glucose ≥ 126 mg/dL), thyroid dysfunction (subclinical/overt-hypothyroidism and -hyperthyroidism), hypertension (systolic blood pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg), hyperlipidemia (total cholesterol ≥ 240 mg/dL), severe anemia (hemoglobin < 8 g/dL for men and < 7 g/dL for women), peripheral arterial disease (ankle brachial index < 0.9), body mass index ≥ 30, known coronary heart disease, stroke, and cancer history, and heavy smoker (≥ 20 pack-years or ≥ 20 cigarettes per day), heavy drinker (14 drinks per week for men and ≥ 7 drinks per week for women), current medication for diabetes, hypertension, and hyperlipidemia, using oral contraceptive or thyroid drug. Finally, 3,154 participants (1,029 men and 2,125 women) were used to determine reference values. The study was approved by the institutional review board of Chonnam National University Hospital (IRB No. 1-2007-07-062). Blood was taken from antecubital vein after overnight fasting, at least 12 hr. For measuring tHcy, whole venous blood was collected into EDTA tubes, immediately packed into crushed ice and protected from light. Within 30 min after collection, the plasma was separated by high-speed cold centrifugation, and stored in aliquots at -70℃ until assayed. Whole blood, which was not treated with anticoagulant, was collected into serum separator tubes and held at room temperature for 30 min before centrifugation. Plasma tHcy concentration was estimated with fluorescence polarization immunoassay and serum folate with ion capture assay, both using Abbott AxSYM analyzer (Abbott Park, IL, USA). Genomic DNA was extracted from peripheral blood and genotyping by real-time polymerase chain reaction (PCR) was performed. Our MTHFR genotyping method has been reported previously (10).
All calculations for determining reference values, reference interval (RI) with 90% confidence interval (CI), were based on the guidelines of the Clinical and Laboratory Standards Institute (CLSI) guideline document CA28-A3 (11). Age- and gender-specific percentile curves were constructed by using LMS (lambda, mu, sigma) method with skewness expressed as a Box-Cox transformation. Statistical analyses were done using the GAMLSS package in the statistical program R version 3.0.1 (The R foundation; available from www.r-project.org) and Medcalc Statistical Software version 12.7.3 (Medcalc Software bvba, Ostend, Belgium).
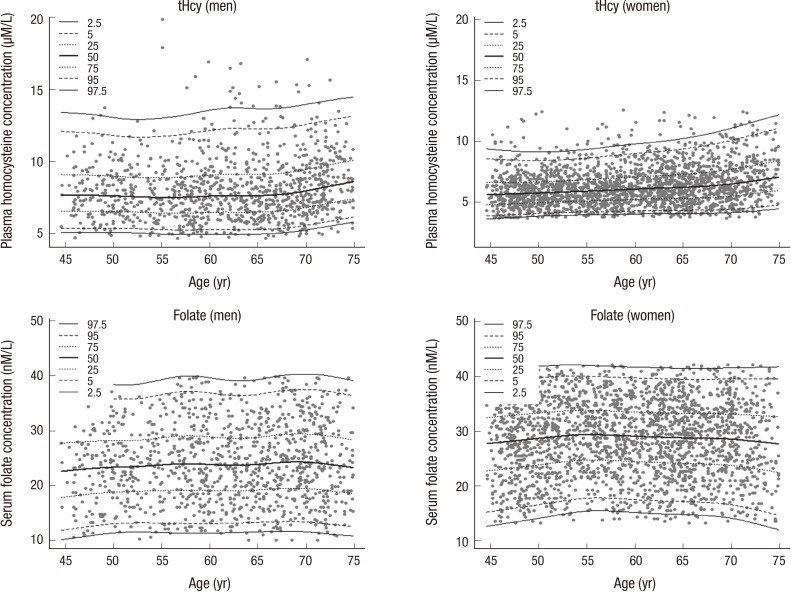
Significant differences of plasma tHcy concentration were observed between the age groups (45-54 yr, 55-64 yr, and 65-74 yr), MTHFR 677 genotypes, and quartiles of serum folate. The RI (90% CI) for plasma tHcy concentration (µM/L) for men (n = 1,029) was 5.03 (4.96-5.12) to 13.80 (13.00-14.85) and for women (n =2,125) was 3.95 (3.90-4.00) to 10.19 (9.87-10.49). No significant difference of serum folate concentration between each age group was found. The RI (90% CI) of serum folate concentration (nM/L) for men was 11.73 (11.48-12.23) to 38.44 (37.69-38.94) and for women was 15.23 (14.98-15.72) to 40.44 (40.19-40.93). MTHFR677 genotype-specific reference values were also significantly different between genotypes in both genders. The highest values for tHcy concentration and the lowest values for folate concentration were observed in the TT genotype (Table 1). Percentile curves of tHcy and folate according to genders and age were presented in Fig. 1.
Table 1.
Reference values for plasma tHcy and serum folate concentration by gender, age-groups, MTHFR 677 genotypes, and other lifestyle factors in middle-aged and older Korean population
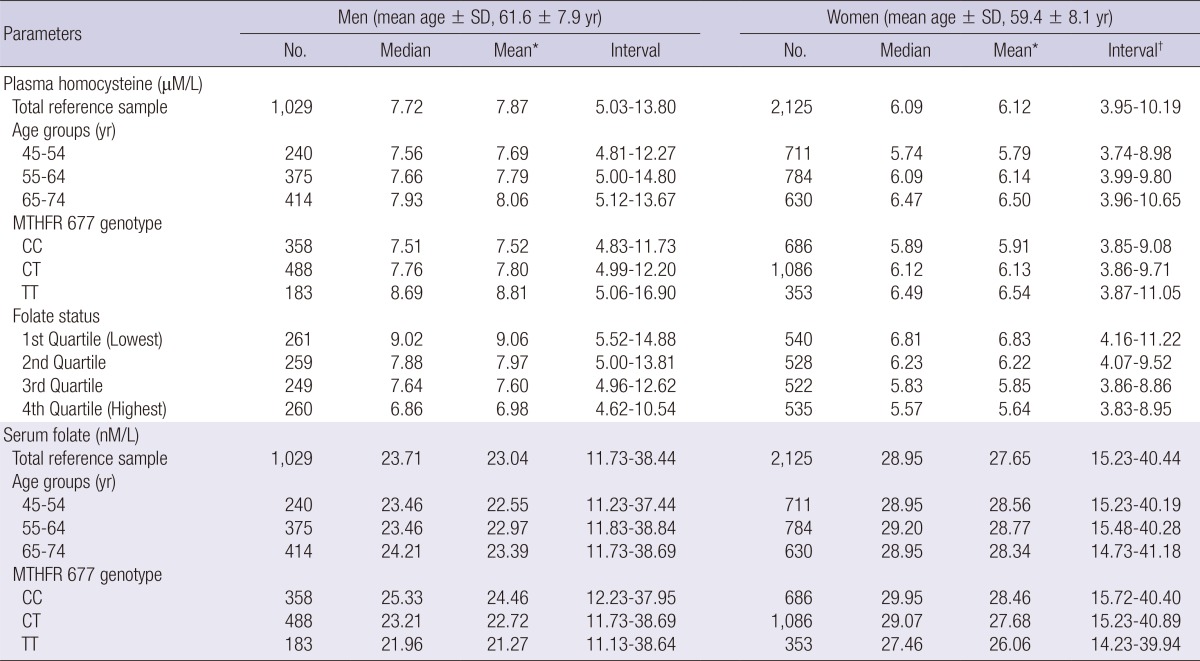
*Geometric mean; †5 percentile-95 percentile. MTHFR, methylenetetrahydrofolate reductase; SD, standard deviation.
Fig. 1.
Percentile curves of plasma homocysteine and serum folate concentration by genders (n= 3,154).
The present study reported reference values for plasma tHcy and serum folate in middle aged and older Koreans using a large-sized population sample. Age-specific, gender-specific, and MTHFR C677T genotype-specific reference values were also provided. We believe that the present reference values of plasma tHcy and serum folate concentrations were estimated accurately, based on the conservative strategies in sample selection that effectively prevented pre-analytic variation, and the standard guidelines of data analysis. Wide-ranging exclusion criteria were applied to establish the reference sample.
The findings of relative higher concentrations of tHcy and lower concentrations of folate in men, older group and MTHFR 677 TT genotypes are consistent with most previous studies. The median plasma tHcy concentrations (µM/L) in our reference sample (7.7 in men and 6.1 in women) were lower than those of previous reports from Korea (7, 8), other Asian countries (12, 13), European countries (14), the USA and Canada in both of pre- and post-folic acid fortification era (6, 15), even than those of Mexican Americans, an ethnicity with the lowest tHcy level in National Health and Nutrition Examination Survey (6). These differences of tHcy concentrations can be explained by the higher level of serum folate in our population. The median serum folate concentrations in our reference sample (23.7 nM/L in men and 29.0 nM/L in women) were about 2-fold higher than those of the non-fortified populations (5) and similar to those of the fortified USA population (6). Dietary patterns with high intake of vegetables, fruits, and fibers, as well as low meat consumption, have been associated with decreased tHcy and increased folate concentrations (14). In previous reports from nationwide nutrition surveys, fruit and vegetable consumption of Koreans was higher than those of western countries (16, 17). Within the same ethnicity, differences between geographic areas and their characteristics, such as agricultural production, are believed to play a role in maintaining the tHcy concentration. A Chinese study reported that plasma homocysteine concentration in rural areas is lower than in urban areas (12). There have still been inconsistent findings in regional variations (12, 18), although these variations of tHcy concentration are highly suggestive that B vitamin status or intake patterns differ between urban and rural areas (16). All participants of the Namwon study are living in a rural area at a high altitude in Korea. Considering the characteristics of the study design, the impact of the seasonal and geographic variations cannot be ruled out as potential explanations of low tHcy and high folate concentrations in our sample.
In addition, tHcy is released from erythrocytes in a time- and temperature-dependent manner, about 10% increase per hour at room temperature (1). In our study, blood was drawn into EDTA and then immediately stored in an ice bucket. Separation was performed using a 4℃ cold centrifuge within 30 min after the blood draw. It was believed that any artificial increase by tHcy export from blood cell is prevented effectively. Over the past decades, extensive evidence that genetic factors are important determinants for tHcy level has accumulated. Individuals with the MTHFR 677TT genotype, the most common genetic determinant, usually have higher tHcy (up to 2.5 µM/L) than those with the 677CC genotype (1). In our study, plasma tHcy concentrations were significantly higher and serum folate concentrations were significantly lower in the subjects with the TT genotype than in those with the CC or CT genotype (P value< 0.05 by Tukey's post hoc test in both sexes). In addition, the 677TT genotype is associated with high tHcy only in individuals with low folate status (19), however, no folate deficiency (< 7.5 nM/L) was found among our reference individuals, and tHcy concentrations in each MTHFR C677T genotype in our sample were lower than those of other populations with the same genotype (20). Our study has some limitations. First, cobalamin status, an important determinant for tHcy level, was not considered. Second, measurements were not cross-validated with the values being measured by HPLC or stable-isotope dilution method. Third, most participants were recruited in a rural area during the winter season, and impact of these variation has not been fully figured out.
In summary, the reference values of tHcy and folate were lower and higher than those in most previous reports, respectively. These findings were maintained across genders, age groups, MTHFR677 genotypes, and other lifestyle factors.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 2.Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc Nutr Soc. 2012;71:75–83. doi: 10.1017/S0029665111003302. [DOI] [PubMed] [Google Scholar]
- 3.Kang JY, Park IK, Lee JY, Sung SH, Chang YK, Park YK, Choi TI. Use of serum homocysteine to predict cardiovascular disease in Korean men with or without metabolic syndrome. J Korean Med Sci. 2012;27:500–505. doi: 10.3346/jkms.2012.27.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96:239–246. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 5.Ganji V, Kafai MR. Population reference values for plasma total homocysteine concentrations in US adults after the fortification of cereals with folic acid. Am J Clin Nutr. 2006;84:989–994. doi: 10.1093/ajcn/84.5.989. [DOI] [PubMed] [Google Scholar]
- 6.Pfeiffer CM, Osterloh JD, Kennedy-Stephenson J, Picciano MF, Yetley EA, Rader JI, Johnson CL. Trends in circulating concentrations of total homocysteine among US adolescents and adults: findings from the 1991-1994 and 1999-2004 National Health and Nutrition Examination Surveys. Clin Chem. 2008;54:801–813. doi: 10.1373/clinchem.2007.100214. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Kim MK, Kim JU, Ha HY, Choi BY. Major Determinants of serum homocysteine concentrations in a Korean population. J Korean Med Sci. 2010;25:509–516. doi: 10.3346/jkms.2010.25.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon HW, Whang DH, Ko YJ, Joo SY, Yun YM, Hur M, Kim JQ. Reference interval and determinants of the serum homocysteine level in a Korean population. J Clin Lab Anal. 2011;25:317–323. doi: 10.1002/jcla.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kweon SS, Shin MH, Jeong SK, Nam HS, Lee YH, Park KS, Ryu SY, Choi SW, Kim BH, Rhee JA, et al. Cohort profile: the Namwon Study and the Dong-gu Study. Int J Epidemiol. 2013 doi: 10.1093/ije/dys244. doi: 10.1093/ije/dys244. [DOI] [PubMed] [Google Scholar]
- 10.Shin MH, Choi JS, Rhee JA, Lee YH, Nam HS, Jeong SK, Park KS, Kim HY, Ryu SY, Choi SW, et al. Association between methylenetetrahydrofolate reductase C677T polymorphism and bone mineral density: the Dong-gu Study and the Namwon Study. J Korean Med Sci. 2013;28:965–968. doi: 10.3346/jkms.2013.28.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Defining, establishing, and verifying reference intervals in the clinical laboratory: approved guideline. CLSI C28-A3. 3rd ed. Wayne: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 12.Hao L, Ma J, Zhu J, Stampfer MJ, Tian Y, Willett WC, Li Z. High prevalence of hyperhomocysteinemia in Chinese adults is associated with low folate, vitamin B-12, and vitamin B-6 status. J Nutr. 2007;137:407–413. doi: 10.1093/jn/137.2.407. [DOI] [PubMed] [Google Scholar]
- 13.Moriyama Y, Okamura T, Kajinami K, Iso H, Inazu A, Kawashiri M, Mizuno M, Takeda Y, Sakamoto Y, Kimura H, et al. Effects of serum B vitamins on elevated plasma homocysteine levels associated with the mutation of methylenetetrahydrofolate reductase gene in Japanese. Atherosclerosis. 2002;164:321–328. doi: 10.1016/s0021-9150(02)00105-3. [DOI] [PubMed] [Google Scholar]
- 14.Konstantinova SV, Vollset SE, Berstad P, Ueland PM, Drevon CA, Refsum H, Tell GS. Dietary predictors of plasma total homocysteine in the Hordaland Homocysteine Study. Br J Nutr. 2007;98:201–210. doi: 10.1017/S0007114507691788. [DOI] [PubMed] [Google Scholar]
- 15.Garcia AA, Day AG, Zanibbi K, Zunzunegui MV. Long-term effects of folic acid fortification and B-vitamin supplementation on total folate, homocysteine, methylmalonic acid and cobalamin in older adults. Can J Public Health. 2008;99:428–433. doi: 10.1007/BF03405255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong SA, Kim K, Kim MK. Educational attainment and differences in fruit and vegetable consumption among middle-aged adults in the Korean National Health and Nutrition Examination Survey IV. Nutr Res Pract. 2012;6:263–269. doi: 10.4162/nrp.2012.6.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamers SL, Agurs-Collins T, Dodd KW, Nebeling L. US and France adult fruit and vegetable consumption patterns: an international comparison. Eur J Clin Nutr. 2009;63:11–17. doi: 10.1038/ejcn.2008.2. [DOI] [PubMed] [Google Scholar]
- 18.Kim MK, Ordovas JM, Selhub J, Campos H. B vitamins and plasma homocysteine concentrations in an urban and rural area of Costa Rica. J Am Coll Nutr. 2003;22:224–231. doi: 10.1080/07315724.2003.10719297. [DOI] [PubMed] [Google Scholar]
- 19.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Bailey L, Clarke R, Flanders WD, Liu T, Yesupriya A, Khoury MJ, Friedman JM. Prospective study of methylenetetrahydrofolate reductase (MTHFR) variant C677T and risk of all-cause and cardiovascular disease mortality among 6000 US adults. Am J Clin Nutr. 2012;95:1245–1253. doi: 10.3945/ajcn.111.022384. [DOI] [PubMed] [Google Scholar]