Abstract
This study aimed to investigate the effect of bortezomib in the desensitization and treatment of acute antibody mediated rejection (AAMR) in kidney transplantation. Nine patients who received bortezomib therapy for desensitization (DSZ group, n = 3) or treatment of AAMR (AAMR group, n = 6) were included in this study. In the DSZ group, 2 patients required DSZ owing to positive cross match and 1 owing to ABO mismatch with high baseline anti-ABO antibody titer (1:1,024). Bortezomib was used at 1, 3, 8, and 11 days from the start of the treatment. In the AAMR group, 3 patients showed full recovery of allograft function after bortezomib use and decrease in donor specific anti-HLA antibody (HLA-DSA). However, 3 patients did not respond to bortezomib and experienced allograft failure. In the DSZ group, negative conversion of T-CDC (complement-dependent cytotoxicity) was achieved, and HLA-DSA was decreased to lower than a weak level (median fluorescence intensity [MFI] < 5,000) in 2 patients. In the case of ABO mismatch kidney transplantation, the anti-A/B antibody titer decreased to below the target (≤ 1:16) after bortezomib therapy. Therefore, bortezomib could be an alternative therapeutic option for desensitization and treatment of AAMR that is unresponsive to conventional therapies.
Graphical Abstract
Keywords: Kidney Transplantation, Bortezomib, Anti-Humoral Therapy, Desensitization, Antibody Mediated Rejection
INTRODUCTION
The presence of donor-specific anti-human leukocyte antigen antibodies (HLA-DSA) is a critical barrier to successful kidney transplantation (KT). Insufficient reduction or suppression of pre-formed HLA-DSA before KT can result in a hyper-acute or acute antibody mediated rejection (AAMR) (1). In addition, development of HLA-DSA after KT can induce not only acute but also chronic AMR, which could be associated with poor allograft survival (2). For these reasons, research has focused on protocol development to effectively suppress the humoral immune system in kidney transplant recipients (3).
So far, the protocol of plasmapheresis, intravenous immune globulin (PP/IVIG), and rituximab (RTX) has been widely used for desensitization and the treatment of AAMR. This may be because the treatment only depletes B cells or removes circulating antibodies and does not suppress plasma cells that directly produce HLA-DSA (4).
Recently, bortezomib, a proteasome inhibitor, was approved by the Food and Drug Administration for the treatment of multiple myeloma, and has been introduced for use in KT (5). Bortezomib inhibits antibody production from plasma cells, stimulates apoptosis of this cell type, and decreases the number of bone marrow-derived plasma cells (6). Therefore, it is expected that this drug would show stronger suppressive effect for humoral immunity compared with conventional therapies such as rituximab. However, clinical data on the use of bortezomib in KT is currently limited.
Therefore, the aim of this study was to investigate the effect of bortezomib on desensitization before KT and the treatment of AAMR after KT.
MATERIALS AND METHODS
Inclusion criteria and bortezomib protocol
In this study, 9 patients who received bortezomib therapy for desensitization (DSZ group, n = 3) or treatment of AAMR (AAMR group, n = 6) were included. All patients received and did not respond to a conventional treatment composed of RTX and PP/IVIG therapy before use of bortezomib. When the schedules of bortezomib therapy and PP/IVIG put one upon another, bortezomib infused after plasmapheresis.
In the 3 patients of the DSZ group, 2 were highly sensitized to anti-HLA antibody, and 1 was supposed to undergo ABO-incompatible KT and showed extremely high baseline anti-A/B antibody titer (1:1,024). HLA-DSAs were identified using single antigen Luminex bead (Tepnel Lifecodes Corp., Stamford, CT, USA) and reported as MFI. Anti-A/B antibody titer was measured using standard serological techniques (7). Protocol for bortezomib is as follows; at the first day of infusion, we used 1.3 mg/m2 of bortezomib and 375 mg/m2 of RTX. Infusion of bortezomib was repeated at the 4th, 8th, and 11th day from the starting date.
Clinical outcome
AAMR is defined by the Banff 2007 classification (update 2005); biopsies consistent with AAMR required 2 of 3 following characteristics: HLA-DSA, histological findings consistent with AAMR (peritubular capillaritis and glomerulitis), or positive C4d staining in the peritubular capillary and other structures (8). The primary outcome of the AAMR group was the recovery of allograft function (measured as a decrease in serum creatinine or condition that did not require renal replacement therapy). In the DSZ group, success was defined as a negative conversion of the cross match test and MFI score of HLA-DSA<5,000.
Statistical analysis
Statistical analysis was performed by using SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA). For continuous variables, means were compared using the Student's t-test.
Ethics statement
The study protocol was approved by the institutional review board of Seoul St. Mary's Hospital (IRB No. KC13TNMI0701) and the need for informed consent from the patients was waived because of the retrospective study design.
RESULTS
Baseline characteristics of AAMR group
The demographic and clinical characteristics of the AAMR group (n=6) are presented in Table 1. There were 2 men and 4 women with a mean age of 41.5 yr (range, 38-46). Two patients underwent deceased and 4 underwent living donor KTs. One was a repeat transplantation. One patient showed a positive cross match test (positive T-CDC anti-human globulin augmented method [AHG] and B-CDC tests) and received desensitization therapy with RTX (375 mg/1.73 m2) and PP/IVIG (9 times) prior to KT. All patients were maintained on immunosuppressive therapy composed of tacrolimus-mycophenolate mofetil-prednisone.
Table 1.
Baseline characteristics of AMR group
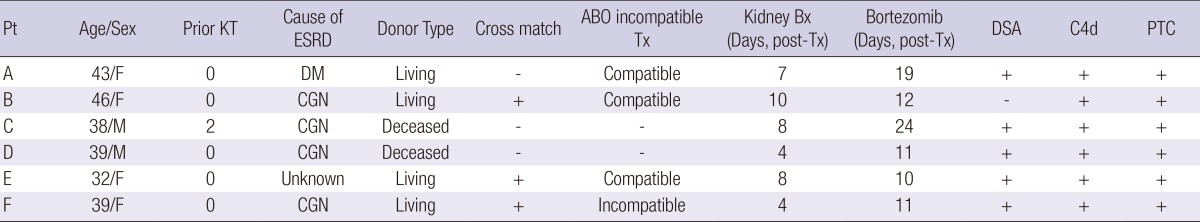
Pt, patient; KT, kidney transplantation; ESRD, end-stage renal disease; Bx, biopsy; Tx, transplantation; DSA, donor specific antibody; PTC, peritubular capillitis; DM, diabetes mellitus; CGN, chronic glomerulonephritis.
Clinical outcome of the AAMR group
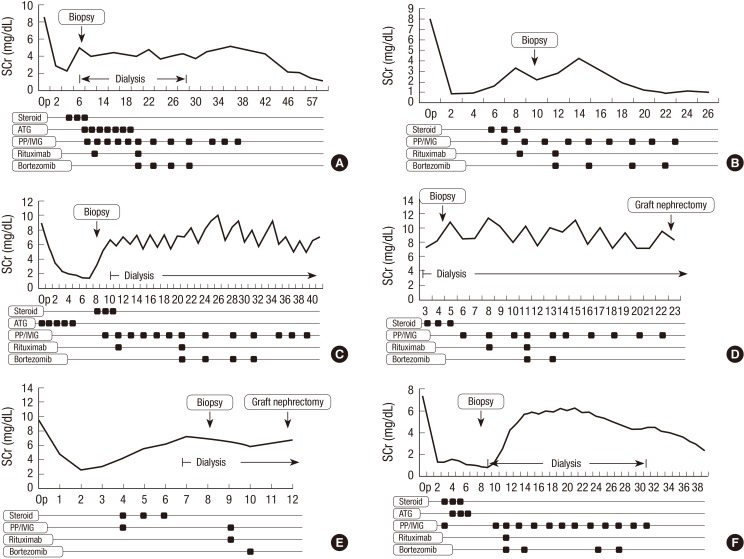
The duration between KT and AAMR was 6.8 days (range, 4-10). In one patient, T-cell mediated rejection was combined with AAMR. Before the use of the bortezomib protocol, PP/IVIG was performed an average of 3.3 times (range, 1-6) and a single dose of rituximab was used. In 4 patients, bortezomib was used 4 times according to the protocol, but in 2 patients (D and E), the treatment schedule could not be completed because thrombocytopenia developed. In 3 of the 6 patients (50%), the serum creatinine level recovered to the baseline value after 4 bortezomib infusions. However, in the remaining 3 patients, the allograft function did not recover in spite of bortezomib use, progressing to allograft failure (Fig. 1).
Fig. 1.
The clinical course of AMR group. (A) patient A, (B) patient B, (C) patient C, (D) patient D, (E) patient E, (F) patient F. AMR, antibody mediated rejection; PP, plasmapheresis; IVIG, intravenous immune globulin.
Baseline characteristics of DSZ group
Two highly sensitized patients were scheduled to receive living donor kidney transplant. The baseline characteristics and immunologic data are listed in Table 2. Both patients showed a positive cross match test in CDC-AHG and flow cytometry. For the patient F, this was the third KT. A 35-yr-old male patient also had a high baseline anti-ABO antibody; but panel reactive antibody (PRA) and cross match were negative.
Table 2.
Baseline characteristics of two patients with pre-sensitization to anti-HLA antibody
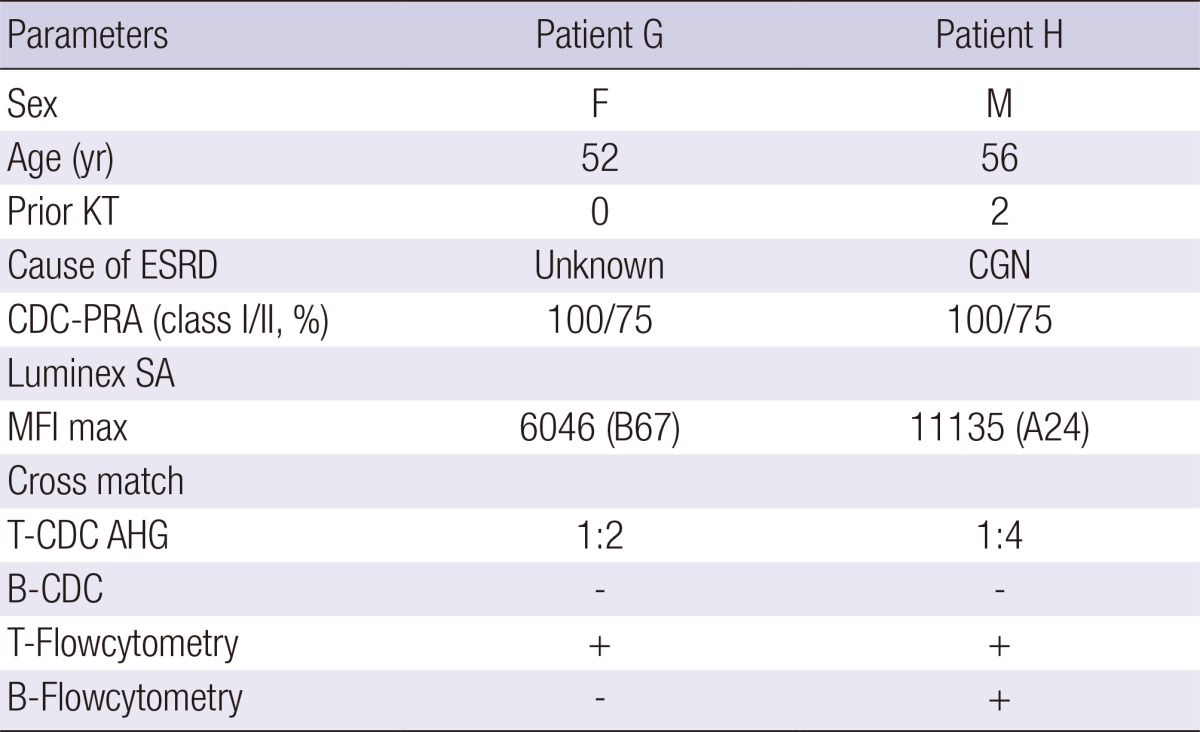
KT, kidney transplantation; ESRD, end-stage renal disease; CGN, chronic glomerulonephritis; CDC, complement-dependent cytotoxicity; PRA, panel reactive antibody; SA, single antigen; MFI, mean fluorescence intensity; AHG, anti-human globulin.
Clinical outcome of the DSZ group
In the patient G, the MFI of HLA-DSA decreased to <5,000 and the T-AHG showed negative conversion after only 2 infusions of bortezomib. Until 8 months after the KT, allograft function showed a stable pattern and no rejection episodes. After 1 cycle of bortezomib therapy (4 infusions) in the patient H, cross matches had converted to negative, but KT was not performed owing to a personal issue. The last patient of the DSZ group had an ABO-mismatch KT with high baseline anti-ABO antibody (≥1:1,024) and negative PRA. In spite of RTX (375 mg/m2) and extensive pre-transplant PP/IVIG (n=7), the anti-A/B antibody titer did not decrease to the target titer (1:16). Therefore, we initiated the bortezomib protocol, and the anti-A/B antibody titer was successfully decreased to less than the target (≤1:16), resulting in successful transplantation.
DISCUSSION
Over the past several years, protocols composed of PP/IVIG and RTX were most widely used in the treatment of AAMR and in pre-transplant desensitization for highly sensitized patients or ABO-mismatch KT. However, this protocol has some limitations: the rate at which reversal of AAMR is achieved is rather gradual than prompt; it costs a great deal; AAMR reversal rates are relatively lower (<80%); chronic rejection may occur after AAMR treatment; and finally, HLA-DSA may persist for a long time after the therapy. These limitations may be due to unsatisfactory effects on mature plasma cells (5).
Our experience with bortezomib was applied in the following situations: treatment of AAMR and desensitization for anti-HLA or anti-ABO antibody before KT. Out of 6 AAMR treatment cases (the patients A-F), 3 patients experienced a full recovery from AAMR after bortezomib treatment. Serum creatinine levels recovered to baseline and HLA-DSA decreased to a weak or negative level. In the patients C, D and E, allograft function did not improve even after 4 bortezomib infusions, and maintenance hemodialysis was required. Additionally, HLA-DSA of those patients did not decrease. The factors associated with response to bortezomib therapy have not been established. In previous reports (5, 9), only 1 study described bortezomib efficiency in early AMR with a relatively preserved renal function and low proteinuria (10). In this study, however, successful response was found in cases of first transplantation from a living donor. However, further investigation on bortezomib therapy in treatment of AAMR, conducted on a larger patient group, is required.
In our study, desensitization in 2 highly sensitized patients (the patient G and H) and 1 ABO mismatch KT with extremely high baseline anti-A/B antibody titer was successful. Until the present, a desensitization protocol using bortezomib has not been established. Previous studies suggested that HLA-DSA reduction can be achieved with bortezomib alone (5), but another recommend to use additional extracorporeal therapy with bortezomib (11). In this study, we decided to include RTX/PP/IVIG in our bortezomib protocol, and desensitization of HLA antibody or ABO antibody was successful in all cases. However, further investigation may be required to disseminate an effective desensitization protocol using bortezomib.
In previous reports, various side effects of bortezomib have been reported. The most common complaints of bortezomib use were fatigue and lethargy (45%) (10). Anemia (20%-26%), thrombocytopenia (25%-35%) and neutropenia (19%-20%) were also relatively common. Other toxicities in KT occurred at similar or lower frequencies to the multiple myeloma population (12). We did not experience any serious complications with bortezomib use (10), and only mild thrombocytopenia and lethargy were reported due to boetrzomib (12). In addition, it is unclear whether the main cause of those side effects was bortezomib or the concomitant therapies (RTX, PP/IVIG, steroid, or ATG). As a result, the use of bortezomib was generally well tolerated in most patients.
This study has some limitations. It includes a small number of patients with less than 1 yr of follow up in desensitization. Further investigation conducted on a larger patient group with a longer follow-up time may be required to elucidate its safety and efficacy as an anti-humoral therapy.
In conclusion, bortezomib could be considered as an alternative therapeutic option for desensitization and treatment of AAMR in cases that do not respond to conventional therapies such as RTX/PP/IVIG.
Footnotes
The authors have declared that no conflict of interest exists.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Korea (NRF-2013R1A1A1009718).
References
- 1.Yoon HE, Hyoung BJ, Hwang HS, Lee SY, Jeon YJ, Song JC, Oh EJ, Park SC, Choi BS, Moon IS, et al. Successful renal transplantation with desensitization in highly sensitized patients: a single center experience. J Korean Med Sci. 2009;24:S148–S155. doi: 10.3346/jkms.2009.24.S1.S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everly MJ, Rebellato LM, Ozawa M, Briley KP, Catrou PG, Haisch CE, Terasaki PI. Beyond histology: lowering human leukocyte antigen antibody to improve renal allograft survival in acute rejection. Transplantation. 2010;89:962–967. doi: 10.1097/TP.0b013e3181cbac02. [DOI] [PubMed] [Google Scholar]
- 3.Marfo K, Lu A, Ling M, Akalin E. Desensitization protocols and their outcome. Clin J Am Soc Nephrol. 2011;6:922–936. doi: 10.2215/CJN.08140910. [DOI] [PubMed] [Google Scholar]
- 4.Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007;7:402–407. doi: 10.1111/j.1600-6143.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 5.Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, Roy-Chaudhury P, Govil A, Mogilishetty G, Rike AH, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 6.Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, Stegall MD. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201–209. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 7.Chung BH, Lee JY, Kang SH, Sun IO, Choi SR, Park HS, Kim JI, Moon IS, Choi BS, Park CW, et al. Comparison of clinical outcome between high and low baseline anti-ABO antibody titers in ABO-incompatible kidney transplantation. Ren Fail. 2011;33:150–158. doi: 10.3109/0886022X.2011.552149. [DOI] [PubMed] [Google Scholar]
- 8.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigos JG, Arora S, Nath P, Hussain SM, Marcus RJ, Ko TY, Sureshkumar KK. Treatment of antibody-mediated rejection in kidney transplant recipients: a single-center experience with a bortezomib-based regimen. Exp Clin Transplant. 2012;10:609–613. doi: 10.6002/ect.2012.0131. [DOI] [PubMed] [Google Scholar]
- 10.Flechner SM, Fatica R, Askar M, Stephany BR, Poggio E, Koo A, Banning S, Chiesa-Vottero A, Srinivas T. The role of proteasome inhibition with bortezomib in the treatment of antibody-mediated rejection after kidney-only or kidney-combined organ transplantation. Transplantation. 2010;90:1486–1492. doi: 10.1097/TP.0b013e3181fdd9b0. [DOI] [PubMed] [Google Scholar]
- 11.Wahrmann M, Haidinger M, Körmöczi GF, Weichhart T, Säemann MD, Geyeregger R, Kikić Z, Prikoszovich T, Drach J, Böhmig GA. Effect of the proteasome inhibitor bortezomib on humoral immunity in two presensitized renal transplant candidates. Transplantation. 2010;89:1385–1390. doi: 10.1097/TP.0b013e3181d9e1c0. [DOI] [PubMed] [Google Scholar]
- 12.Sadaka B, Alloway RR, Woodle ES. Clinical and investigational use of proteasome inhibitors for transplant rejection. Expert Opin Investig Drugs. 2011;20:1535–1542. doi: 10.1517/13543784.2011.618494. [DOI] [PubMed] [Google Scholar]