Abstract
A 56-year-old man presented to our department with a 2-month history of fever and chills. He had received a mitral valvuloplasty 3 years ago. He had been administered levofloxacin for 2 months. We discontinued levofloxacin and repeated the blood cultures. Bacterial blood cultures were positive and transoesophageal echocardiography revealed vegetation attached to the posterior mitral leaflet. We started the patient on intravenous antibiotic therapy for infectious endocarditis by Streptococcus gallolyticus subspecies pasteurianus. A colonoscopic screening revealed adenomatoid intracellular carcinoma. Previous studies have reported a weak association between colorectal cancer and Streptococcus bovis biotype II/2, which includes S gallolyticus subspecies pasteurianus; however, the rate is notably higher than the rate of colorectal cancer as indicated by positive faecal occult-blood test results. We conclude that colonoscopies should be routine while scanning for colorectal cancer in all patients with S bovis bacteraemia, regardless of the subspecies.
Background
The association between Streptococcus bovis bacteraemia and colorectal cancer is well known. Formerly, the S bovis group had been divided into three biotypes: I, II/1 and II/2.1 In the 1990s, under a new nomenclature system, they were reclassified into four subspecies and renamed; the former biotype I belongs to Streptococcus gallolyticus subspecies gallolyticus, the former biotype II/1 belongs to Streptococcus infantarius subspecies infantarius and S infantarius subspecies coli, the former biotype II/2, belongs to S gallolyticus subspecies pasteurianus.1 2 Here, we report a case of infective endocarditis and colon cancer caused by S gallolyticus subspecies pasteurianus.
Case presentation
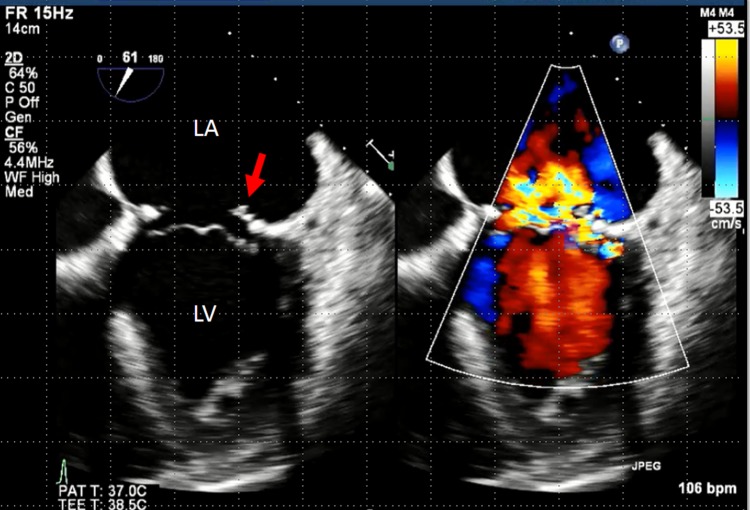
A 56-year-old man presented to our department with a 2-month history of fever and chills. There was no history of significant animal contact, travel or dental procedures. His medical history included a mitral valvuloplasty for the rupture of the chordae tendineae of the mitral valve 3 years ago. Since the surgery, he had received follow-ups in the department of cardiovascular surgery. He was initially administered levofloxacin for the fever whose cause remained unidentified for 2 months. During this period, transthoracic echocardiography and contrast CT of his chest, abdomen and pelvis did not reveal the origin of fever. Four days before admission, he was directed to our department for consultation. He presented with a temperature of 38.8°C, blood pressure 108/61 mm Hg, pulse rate 98 bpm, which was irregular and respiration rate 18 breaths/min. During physical examination, a Levine III/VI pansystolic murmur was best heard at the apex. Examination results were otherwise unremarkable. We strongly suspected infectious endocarditis after valvuloplasty; therefore, we discontinued levofloxacin and repeated the blood cultures. When the blood cultures revealed Gram-positive cocci, the patient was hospitalised. He was empirically treated with 2 g ceftriaxone intravenously every 24 h plus 1 g vancomycin intravenously every 12 h to treat the common pathogens such as Staphylococcus aureus, Streptococcus species (viridans group, S bovis and others), and Enterococcus species. The blood-cultured pathogen was identified as S gallolyticus subspecies pasteurianus, which was susceptible to penicillin and resistant only to levofloxacin. Transthoracic echocardiography suspected vegetation at the mitral valve, and showed a moveable verruca 7 mm in diameter at the posterior mitral leaflet (figure 1). These findings led to a diagnosis of infective endocarditis caused by S gallolyticus subspecies pasteurianus.
Figure 1.
Transoesophageal echocardiogram. A moveable verruca 7 mm in diameter is visible at the posterior mitral leaflet (red arrow).
Treatment
The patient was treated with 2 g ampicillin sodium intravenously every 4 h, to which the isolated S gallolyticus subspecies pasteurianus were sensitive. Antibiotic administration alleviated the fever, and subsequent blood cultures were negative after 6 weeks of treatment. The vegetation was no longer visible in the second transthoracic echocardiography on the 42nd day after admission.
A colonoscopic screening was performed on the 36th day after admission. The colonoscopy revealed a type Isp polyp at the rectum and endoscopic mucosal resection was performed (figure 2). Pathology confirmed a diagnosis of adenomatous intracellular carcinoma (figure 3).
Figure 2.
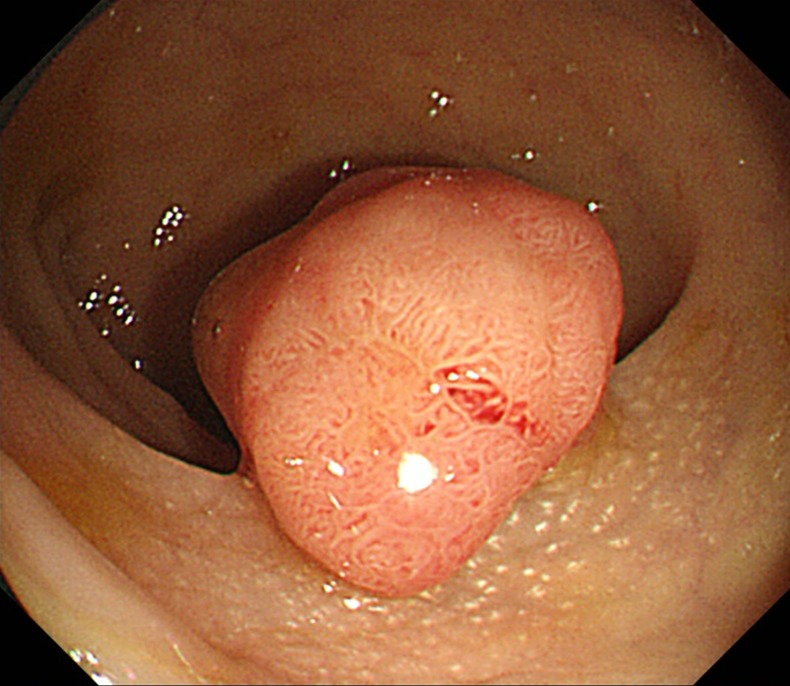
Colonoscopic finding. A type Isp polyp is visible at the rectum.
Figure 3.
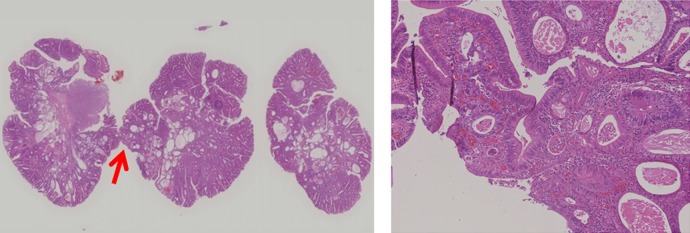
Pathology of a resected endoscopic mucosal polyp. (A) H&E, ×40 image. A red arrow showing the dysplastic lesion. (B) H&E, ×400 image. The dysplastic lesion indicates adenomatous intracellular carcinoma.
Outcome and follow-up
The resolution of infectious endocarditis was confirmed, and complete remission was achieved. The patient also received an earlier diagnosis of colon cancer. Following treatment, he has not relapsed for more than 8 months since his discharge.
Discussion
In this case, although the possibility of infectious endocarditis was initially considered, no blood culture was performed and levofloxacin was prescribed. As a result, the lack of appropriate infectious survey delayed the diagnosis and treatment.
Susceptibility data on S gallolyticus subspecies pasteurianus have not been widely reported, but some have showed them to be fully susceptible to β-lactams, have a high level of resistance to tetracycline and intermediate response to levofloxacin.3 The isolated S gallolyticus subspecies pasteurianus, in this case, was resistant only to levofloxacin. In cases in which patients may have infectious endocarditis, blood cultures should be performed early, and in some cases, they should be repeated. This case confirms that a blind prescription of antibiotics is not acceptable.
S bovis is now one of the prime causes of endocarditis and its incidence is increasing more than that of the Streptococcus viridans group or S aureus in some areas.4 With this background, researchers and physicians should become familiar with the characteristics of this pathogen. There are numerous reports suggesting an association between S bovis bacteria, infectious endocarditis and malignant colorectal cancer. The relationship between S bovis infection and colon cancer has been widely investigated, but the pathogenesis is still not understood. One hypothesis is that the S bovis group is a normal inhabitant of the gastrointestinal tract and can readily enter the bloodstream on mucosal disruption or vascular changes.5 6
However, not all genotypes are equally related to colorectal cancer. S bovis belongs to group D streptococci and is, in general, the predominant species found in the ileum of the human intestine.7 The group was reclassified in the 1990s on the basis of the molecular characteristics: S bovis biotype I became S gallolyticus subspecies gallolyticus, S bovis biotype II/1 became S infantarius subspecies infantarius and S infantarius subspecies coli and S bovis biotype II/2 became S gallolyticus subspecies pasteurianus. Among these subtypes, S gallolyticus subspecies gallolyticus is most often linked to infectious endocarditis concomitant with colon cancer.2 Meta-analysis showed that patients with S bovis biotype I infection had a strongly higher risk of colorectal cancer and infectious endocarditis compared with patients with S bovis biotype II infection.8 Studies have shown that 94% of S bovis bacteraemia cases associated with infectious endocarditis were in fact S bovis biotype I, while only 18% were associated with biotype II.2 Moreover, 71% of cases showed an association between S bovis biotype I bacteraemia and colonic carcinoma, while S bovis biotype II showed only a 17% association. Thus, S gallolyticus subspecies gallolyticus bacteraemia has been found to be less associated with colorectal cancer than other related taxa.
In this case, while the S gallolyticus subspecies pasteurianus pathogen belongs to biotype II/2, and the association between this biotype and colon cancer is not high, we implemented a course of colonoscopy for screening. Colonoscopy revealed Isp polyps at the rectum and pathological examination showed concomitant adenomatous intracellular carcinoma. Colorectal cancer is the third most frequent cancer in the world and early-stage diagnosis is associated with good prognosis.9 The disease develops from benign lesions over about 10 years, making screening and early detection important. One of the most useful tests for the screening of colorectal cancer is faecal occult blood test. Only 2% of patients with a positive test result had cancer.10 In a randomised study, 2% of the colorectal cancer deaths occurred in the screening group that received faecal occult blood testing.11 S bovis biotype II showed a 17% association with colonic cancer, and the association rate is epidemiologically much higher than that suggested by positive faecal occult-blood test results, although it has not yet established that S gallolyticus subspecies pasteurianus is an important causal agent of colorectal cancer and whether the pre-existing polyps or cancer cells make the lumen of microenvironment more suitable to its outgrowth.
We conclude that routine colonoscopies should be used to scan for colorectal cancer in all patients with S bovis bacteraemia, regardless of the subspecies.
Learning points.
If a patient has a history of cardiac disease, especially of the valves, physicians should consider infectious endocarditis as a differential diagnosis, and should perform blood cultures before starting antibiotic treatment.
Streptococcus bovis bacteraemia is associated with colorectal cancer.
Colonoscopies should be routine while scanning for colorectal cancer in all patients with S bovis bacteraemia, regardless of the subspecies.
Acknowledgments
The authors thank Dr Michihiro Saito (MD, Department of Pathology, Jichi Medical University) for supply of pathological images.
Footnotes
Contributors: NT, TK and KM contributed to patient's management. NT, TK, KM and MM contributed to writing and reviewing the report. All authors read and approved the final version of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Schlegel L, Grimont F, Ageron E, et al. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int J Syst Evol Microbiol 2003;53:631–45 [DOI] [PubMed] [Google Scholar]
- 2.Abdulamir AS, Hafidh RR, Abu Bakar F. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res 2011;30:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck M, Frodl R, Funke G. Comprehensive study of strains previously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J Clin Microbiol 2008;46:2966–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corredoira J, Alonso MP, Coira A, et al. Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur J Clin Microbiol Infect Dis 2008;27:285–91 [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Joo SI, Yi J, et al. A case of Streptococcus gallolyticus subsp. gallolyticus infective endocarditis with colon cancer: identification by 16S ribosomal DNA sequencing. Korean J Lab Med 2010;30:160–5 [DOI] [PubMed] [Google Scholar]
- 6.Biarc J, Nguyen IS, Pini A, et al. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S. bovis). Carcinogenesis 2004;25:1477–84 [DOI] [PubMed] [Google Scholar]
- 7.Hensler ME. Streptococcus gallolyticus, infective endocarditis, and colon carcinoma: new light on an intriguing coincidence. J Infect Dis 2011;203:1040–2 [DOI] [PubMed] [Google Scholar]
- 8.Boleij A, Tjalsma H. The itinerary of Streptococcus gallolyticus infection in patients with colonic malignant disease. Lancet Infect Dis 2013;13:719–24 [DOI] [PubMed] [Google Scholar]
- 9.Holme O, Bretthauer M, Fretheim A, et al. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev 2013;9:CD009259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993;328:1365–71 [DOI] [PubMed] [Google Scholar]
- 11.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369:1106–14 [DOI] [PubMed] [Google Scholar]