Abstract
Insulin sensitivity is impaired in type 1 diabetes (T1D) and may be enhanced by islet transplantation, an effect best explained by improved metabolic control. While the minimal model index of insulin sensitivity, SI, has been used in studies of T1D, it has not before been evaluated against gold-standard measures derived from the euglycemic clamp. We sought to determine how well minimal model SI derived from an insulin-modified frequently sampled intravenous glucose tolerance (FSIGT) test compared with total body and peripheral insulin sensitivity estimates derived from the hyperinsulinemic-euglycemic clamp in subjects with T1D and following islet transplantation. Twenty-one T1D subjects were evaluated, including a subgroup (n = 12) studied again after intrahepatic islet transplantation, with results compared with normal controls (n = 11 for the FSIGT). The transplant recipients received 9,648 ± 666 islet equivalents/kg with reduction in HbA1c from 7.1 ± 0.2 to 5.5 ± 0.1% (P < 0.01) and 10/12 were insulin independent. FSIGT-derived SI was reduced in T1D pre- compared with posttransplant and with normal [1.76 ± 0.45 vs. 4.21 ± 0.34 vs. 4.45 ± 0.81 × 10−4(μU/ml)−1·min−1; P < 0.01 for both]. Similarly, clamp-derived total body, and by the isotopic dilution method with [6,6-2H2]glucose, peripheral insulin sensitivity increased in T1D from pre- to posttransplant (P < 0.05 for both). The predictive power (r2) between volume-corrected SIC and measures of total and peripheral insulin sensitivity was 0.66 and 0.70, respectively (P < 0.00001 for both). That the minimal model SIC is highly correlated to the clamp-derived measures indicates that the FSIGT is an appropriate methodology for the determination of insulin sensitivity in T1D and following islet transplantation.
Keywords: glucose effectiveness, frequently sampled intravenous glucose tolerance test
insulin resistance is an underappreciated feature of type 1 diabetes (T1D) (7, 13, 32, 33), resulting at least in part from the absence of endogenous insulin secretion, frequent periods of sustained hyperglycemia (12, 21, 31), and impaired sensitivity of lipolysis to inhibition by insulin, resulting in elevated free fatty acids (FFA) (24, 33). Islet transplantation restores endogenous insulin secretion that can correct the hyperglycemia of T1D and normalize FFA metabolism (15, 20, 27), effects that have been associated with improved minimal model indexes of insulin sensitivity derived from an insulin-modified frequently sampled intravenous glucose tolerance (FSIGT) test in T1D recipients of islet transplants. More recently, employing the hyperinsulinemic-euglycemic clamp technique with a stable glucose isotope, our group has confirmed this correction of impaired insulin sensitivity in T1D by islet transplantation and shown that the improvement is mediated by effects at the liver and skeletal muscle (18). Extension of these findings to larger populations and to other therapeutic interventions for T1D may be more readily accomplished using the FSIGT test that can be more easily standardized across clinical sites.
Whereas the minimal model index of insulin sensitivity, SI, can be derived in T1D subjects using an insulin-modified FSIGT with good parameter resolution (10, 20, 28), SI has not before been evaluated against measures of insulin sensitivity derived from the gold-standard hyperinsulinemic-euglycemic clamp in T1D as has been performed in subjects with type 2 diabetes (T2D) (22). Because the development of absolute insulin deficiency in T1D is distinct from the relative insulin deficiency present in T2D, a comparative analysis of clamp and minimal model-derived indexes of insulin sensitivity in T1D subjects is critical to the ongoing application of the minimal model approach to the study of insulin sensitivity in this population. The minimal model-derived SI does not distinguish between insulin action to suppress glucose production (primarily from the liver) and that to enhance peripheral glucose disposal (primarily in skeletal muscle), which requires isotope tracer methodology but does provide an estimate of total body insulin sensitivity that is believed to largely represent peripheral insulin action needed for disposal of the injected glucose load. We sought to determine how well the FSIGT minimal model-derived SI compared with total body and peripheral insulin sensitivity estimates derived from the hyperinsulinemic-euglycemic clamp with a stable glucose isotope in subjects with T1D and following islet transplantation.
MATERIALS AND METHODS
T1D subjects included those with long-standing C peptide-negative disease complicated by hypoglycemia unawareness and frequent severe hypoglycemia events who had normal kidney function and were initially considered as potential candidates for islet alone transplantation (n = 21). A subgroup of these subjects received intrahepatic islet transplants (n = 12) as part of the Clinical Islet Transplantation (CIT) Consortium protocols being conducted at the University of Pennsylvania (1). The study protocols were approved by the Institutional Review Board of the University of Pennsylvania, and all subjects gave their written informed consent to participate.
The islet transplant recipients included all 11 subjects participating in the CIT07 protocol from our institution that included thymoglobulin and etanercept for induction immunosuppression (19) and one of two participants who experienced early islet graft failure with the CIT05 protocol consisting of thymoglobulin and rituximab for induction and was later retransplanted under an Edmonton protocol with basiliximab induction. The transplant recipients underwent one or two intraportal infusions of islets to achieve insulin independence. Maintenance immunosuppression consisted of low-dose tacrolimus (12-h blood trough target 3–6 μg/l) and sirolimus (24-h blood trough target 10–15 μg/l for the first 3 mo and 8–12 μg/l thereafter).
Healthy nondiabetic control subjects for the FSIGT (n = 11) were selected for gender, age, and body mass index (BMI) from other studies conducted by our group (23, 25) to match that of the T1D subjects.
Metabolic studies.
All 21 T1D subjects underwent FSIGTs and hyperinsulinemic-euglycemic clamps between 2 days and 1 mo apart. When performed the same week, the FSIGT was conducted on the first day, and menstruating women underwent both tests during the follicular phase of their menstrual cycle (26). The 12 subjects who underwent islet transplantation each repeated the FSIGT between 2.5 and 7 mo posttransplant and the euglycemic clamp between 6 and 7 mo posttransplant. The median (interquartile range) of time between the posttransplant FSIGT and euglycemic clamp studies was 3 (2.5–3.5) mo. For both the euglycemic clamp and FSIGT, T1D subjects and insulin-dependent islet transplant recipients were admitted to the University of Pennsylvania Clinical and Translational Research Center (CTRC) the afternoon before study and fasted overnight after 2000 for 12 h before testing. At 2100 subjects were converted from subcutaneous insulin to a low-dose intravenous insulin infusion protocol to target blood glucose at 81–115 mg/dl. Insulin-independent islet transplant recipients and normal controls had the option of fasting overnight either in the CTRC or at home with arrival in the CTRC by 0630 the morning of study. By 0700, one catheter was placed in an antecubital vein for infusions, and one catheter was placed in a hand vein for blood sampling, with the hand placed in a thermoregulated box (∼50°C) or heating pad to promote arterialization of venous blood.
FSIGT.
When used overnight, the insulin infusion was discontinued 20 min before testing. All other medications were withheld until later in the morning. After baseline blood sampling at −10, −5, and −1 min, 0.3 g/kg of 50% glucose was injected over 1 min starting at time (t) = −30 s, and 0.03 U/kg of insulin (1 U/1 ml solution) was injected over 30 s starting at t = 20 min. Additional blood samples were collected at t = 1, 2, 3, 4, 5, 7, 10, 12, 14, 16, 18, 20, 22, 25, 30, 40, 50, 70, 100, 140, and 180 min after the injection of glucose (20, 22, 26). All samples were centrifuged at 4°C, separated, and frozen at −80°C for subsequent analysis. Serum glucose was measured by the glucose hexokinase method using an automated glucose analyzer (Roche Module P; Roche Diagnostics, Indianapolis, IN) and serum insulin by two-site immune-enzymometeric assay using a Tosoh 2000 autoanalyzer (Tosoh Biosciences, San Francisco, CA) at the Northwest Lipid Research Laboratory (University of Washington, Seattle, WA).
Euglycemic clamp.
At t = −120 min a primed (5 mg/kg · fasting plasma glucose in mg/dl ÷ 90 mg/dl over 5 min) continuous (0.05 mg·kg−1·min−1 for 355 min) infusion of the stable glucose isotope tracer [6,6-2H2]glucose (99% enriched; Cambridge Isotopes Laboratories, Andover, MA) was administered to assess endogenous glucose production before and during the induction of hyperinsulinemia (4, 18). When used overnight, the insulin infusion was continued during this period to maintain stable normoglycemia until t = 0. After baseline blood sampling at −20, −10, and −1 min, at t = 0 min a continuous infusion of insulin was initiated at 1 mU·kg−1·min−1 for 240 min to produce hyperinsulinemia. Subsequently, a variable-rate infusion of 20% glucose was administered according to the glycemic clamp technique (8) to maintain the plasma glucose ∼90 mg/dl. To reduce changes in plasma enrichment of [6,6-2H2]glucose during the clamp, the 20% glucose solution was enriched to 2.0% with [6,6-2H2]glucose (4). Morning immunosuppression medications were taken after baseline blood sampling if applicable. Blood samples were taken every 5 min, centrifuged, and measured at bedside with an automated glucose analyzer (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH) to adjust the glucose infusion rate and achieve the desired plasma glucose concentration. Additional blood samples were taken every 20 min for biochemical analysis. All samples were collected on ice in chilled tubes containing EDTA and Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO), centrifuged at 4°C, separated, and frozen at −80°C for subsequent analysis. Plasma glucose was verified in duplicate by the glucose oxidase method using an automated glucose analyzer (YSI 2300; Yellow Springs Instruments), and plasma insulin was measured in duplicate by double-antibody radioimmunoassay (Millipore, Billerica, MA) as previously described (18). Enrichment of [6,6-2H2]glucose was measured by gas chromatography-mass spectrometry at Metabolic Solutions (Nashua, NH) or the Metabolic Tracer Resource at the University of Pennsylvania.
Calculations and statistics.
Basal levels of glucose and insulin were calculated from the mean of the baseline samples preceding t = 0 for the FSIGT. Intravenous glucose tolerance was evaluated from the FSIGT by the glucose disappearance rate Kg = ln[glucose]/min × 100, calculated as the slope of the natural log of glucose values between 10 and 40 min with least-squares linear regression (29) using Origin software (Northampton, MA). The FSIGT parameters for the acute insulin response to the injection of glucose (AIRg), insulin sensitivity (SI), disposition index (DI = AIRg × SI), and glucose effectiveness (SG) were derived from Bergman's minimal model using MINMOD Millennium software (6) as previously described (20, 25, 26).
The rate of appearance (Ra) of glucose during the euglycemic clamps was calculated using Steele's nonsteady state equation modified for the use of stable isotopes: Ra = {F − V[(C2 + C1)/2][(E2 − E1)/(t2 − t1)]}/[(E2 + E1)/2], where C2 and C1 are the glucose concentrations at the times 2 and 1, respectively (in mg/ml), V is the fractional volume of distribution of glucose (40 ml/kg), F is the tracer infusion rate, and E represents the isotopic enrichment at the respective time points (30). The rate of disposal (Rd) of glucose during the euglycemic clamps was calculated using Steele's non-steady-state equation Rd = Ra − V[(C2 − C1)/(t2 − t1)]. Total body insulin sensitivity was calculated from the final hour of the euglycemic clamp as SI(clamp) = M/(ΔI × G) where M is the glucose infusion rate (GIR), ΔI is the change in insulin concentration from basal to the steady-state condition during the final hour of insulin infusion, and G is the steady-state glucose concentration during the final hour (2). Accounting for space correction (SC), such that M = GIR − SC where SC = (G2 − G1) × 1.9/(t2 − t1) calculates the glucose removed from or added to the glucose space between each time interval where G2 and G1 are the glucose concentrations at the times 2 and 1, respectively (in mg/dl) (8), resulted in nearly identical measures for SI(clamp) (r2 = 0.997; P < 0.00001). Peripheral (primarily skeletal muscle) insulin sensitivity was calculated using the steady-state tracer-derived glucose disposal as SIP(clamp) = ΔRd/(ΔI × G) (3).
Whereas both minimal model (SI) and clamp [SI(clamp) and SIP(clamp)] measures reflect insulin sensitivity that is dominated by extrahepatic effects of insulin (3), they are not directly comparable because they are expressed in different units [(μU/ml)−1 per min and dl·min−1·kg−1 per μU/ml, respectively]. This is due to each parameter being normalized to a different measure of body size, with the volume of distribution of glucose used in the minimal model and body weight in the clamp calculations. To convert them to a common index of insulin sensitivity, SIC, reflecting the unitary change in insulin to cause a given increment in glucose clearance (dl/min per μU/ml), we followed the procedure reported by Bergman's group (3, 22) such that SIC(minmod) = SI × VD where the volume of distribution of glucose is calculated as VD (dl) = dose of glucose injected in milligrams divided by G0 − Gb in mg/dl, where G0 is the peak and Gb the basal glucose derived from the minimal model, SIC(clamp) = SI(clamp) × body weight in kilograms, and SICP(clamp) = SIP(clamp) × body weight in kilograms. Because the conversion factors used for the minimal model and clamp-derived estimates of insulin sensitivity are independent, this procedure excludes bias in the statistical comparison of the two methods for measuring SIC (3).
All data are expressed as means ± SE. Comparison of results between pre- and posttransplant T1D subjects was performed with paired Student's t-tests or Wilcoxon Match Pairs tests for nonparametric data, and comparison of results between each T1D group and controls was performed with unpaired Student's t-tests or the Mann-Whitney U-test as appropriate using Statistica software (StatSoft, Tulsa, OK). Comparison of the minimal model and clamp-derived indexes of insulin sensitivity were performed by least-squares linear regression using Origin software. Significance was considered at P < 0.05 (2-tailed).
RESULTS
Subject characteristics.
The T1D subjects were of comparable gender distribution, age, body weight, and BMI to the control subjects (Table 1). Those T1D subjects who underwent islet transplantation had a decrease in body weight with resulting decrease in BMI (P < 0.01; Table 1), although values of both remained not different from normal. The T1D subjects had ∼30 years of disease duration and an insulin requirement ∼0.5 U·kg−1·day−1 that was substantially reduced (P < 0.01; Table 1) following receipt of 9,648 ± 666 islet equivalents/kg recipient body weight with 10 of the 12 islet recipients insulin independent at the time of posttransplant assessment. HbA1c, which was elevated in T1D, was normalized in the group posttransplant (Table 1), of which seven were insulin-independent following one islet infusion, three were insulin-independent following two islet infusions, one was insulin-dependent while awaiting a second islet infusion, and one remained insulin-dependent after two islet infusions. Subjects maintained appropriate levels of tacrolimus and sirolimus (Table 1) except for one who was converted from sirolimus to mycophenolate mofetil (19).
Table 1.
Subject characteristics at the time of FSIGT testing
| T1D |
||||
|---|---|---|---|---|
| Allevaluated (n = 21) | Pretransplant (n = 12) | Posttransplant (n = 12) | Normal Controls (n = 11) | |
| Sex (M/F) | 9/12 | 5/7 | 5/7 | 6/5 |
| Age, yr | 46 ± 2 | 46 ± 3 | 47 ± 3* | 40 ± 2 |
| Weight, kg | 70 ± 3 | 71 ± 3 | 66 ± 3* | 71 ± 3 |
| BMI, kg/m2 | 25 ± 1 | 25 ± 1 | 23 ± 1* | 24 ± 1 |
| HbA1c, % | 6.8 ± 0.2 | 7.1 ± 0.2 | 5.5 ± 0.1* | ND |
| T1D duration, yr | 31 ± 2 | 29 ± 4 | 31 ± 4* | |
| Insulin use, U·kg−1·day−1 | 0.50 ± 0.03 | 0.48 ± 0.05 | 0.02 ± 0.02* | |
| IE/kg | 9,648 ± 666 | |||
| Tacrolimus, μg/l | 4.8 ± 0.4 | |||
| Sirolimus, μg/l | 9.2 ± 0.7† | |||
Data are means ± SE; n, no. of subjects.
FSIGT, frequently sampled intravenous glucose tolerance; T1D, type 1 diabetes; M, males; F, females; BMI, body mass index; IE/kg, islet equivalents transplanted/kg recipient body wt whereby an IE approximates a standard islet diameter of 150 μm.
P < 0.01 for comparison with T1D pretransplant.
One subject was converted from sirolimus to mycophenolate mofetil because of the development of interstitial pneumonia 4 wk posttransplant that subsequently resolved (19).
FSIGT.
Basal glucose was higher in the T1D subjects pre- compared with posttransplant and normal (P < 0.05 for both; Table 2 and Fig. 1A) and was also higher in the posttransplant subjects than in controls (P < 0.05; Table 2 and Fig. 1A), whereas basal insulin was similar in all groups (Table 2). The VD for the injected glucose was higher in the T1D subjects pre- compared with posttransplant and normal (P < 0.05 for both; Table 2), and Kg was slower in the T1D subjects pre- compared with posttransplant and normal (P < 0.01 for both; Table 2). Islet transplantation restored previously absent endogenous insulin secretion with the first-phase AIRg not significantly different from normal (Table 2 and Fig. 1B). SI was reduced in T1D pre- compared with posttransplant and normal (P < 0.01 for both; Table 2). The resulting DI was not significantly different in the islet transplant recipients compared with controls (Table 2). SG was also reduced in T1D pre- compared with posttransplant and with normal (P < 0.01 for both; Table 2).
Table 2.
Metabolic parameters during the FSIGT test and euglycemic clampa
| T1D |
||||
|---|---|---|---|---|
| All evaluated (n = 21) | Pretransplant (n = 12) | Posttransplant (n = 12)b | Normal Controls (n = 11) | |
| Basal glucose, mg/dl | 113 ± 5 | 117 ± 6 | 100 ± 3c | 91 ± 2d,e |
| Basal insulin, μU/ml | 12 ± 2 | 12 ± 4 | 11 ± 3 | 9 ± 1 |
| VD, dl | 170 ± 9 | 162 ± 13 | 113 ± 8d | 129 ± 8c |
| Kg, %/min | 1.05 ± 0.11 | 0.98 ± 0.16 | 2.08 ± 0.16d | 2.78 ± 0.39d |
| AIRg, μU·ml−1·min | 263 ± 58 | 473 ± 161 | ||
| SI × 10−4, (μU/ml)−1min | 2.02 ± 0.31 | 1.76 ± 0.45 | 4.21 ± 0.34d | 4.45 ± 0.81d |
| DI | 994 ± 168 | 1441 ± 247 | ||
| SG, min−1 | 0.010 ± 0.002 | 0.009 ± 0.002 | 0.019 ± 0.002d | 0.021 ± 0.003d |
| Steady-state glucose,a mg/dl | 87 ± 1 | 88 ± 2 | 87 ± 1 | |
| ΔInsulin,a,f μU/ml | 70 ± 7 | 74 ± 9 | 70 ± 7 | |
| Steady-state GIR,a mg·kg−1·min−1 | 6.8 ± 0.4 | 6.7 ± 0.5 | 8.3 ± 0.5c | |
| ΔRd,a,f mg·kg−1·min−1 | 4.8 ± 0.4 | 4.7 ± 0.5 | 6.5 ± 0.6c | |
| SI(clamp) × 102,a dl·min−1·kg−1 per μU/ml | 0.13 ± 0.02 | 0.11 ± 0.01 | 0.15 ± 0.02c | |
| SIP(clamp) × 102,a dl·min−1·kg−1 per μU/ml | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.02c | |
Data are means ± SE; n, no. of subjects. VD, volume of distribution for glucose injected during FSIGT; Kg, glucose disappearance rate; AIRg, acute insulin response to glucose; SI, insulin sensitivity index; DI, disposition index defined as the product AIRg × SI; SG, glucose effectiveness; GIR, glucose infusion rate; Rd, rate of glucose disposal; SI(clamp), total body insulin sensitivity; SIP(clamp), peripheral insulin sensitivity. To convert glucose to mmol/l, multiply by 0.05551; and to convert insulin to pmol/l, multiply by 6.
Data from the euglycemic clamp have been previously published for the 12 T1D subjects who underwent islet transplantation (18).
n = 11 Posttransplant for measures of steady-state glucose, Δinsulin, steady-state GIR, ΔRd, SI(clamp), and SIP(clamp).
P < 0.05 compared with T1D pretransplant.
P < 0.01 compared with T1D pretransplant.
P < 0.05 compared with T1D posttransplant.
Change in concentration from basal to the steady state.
Fig. 1.
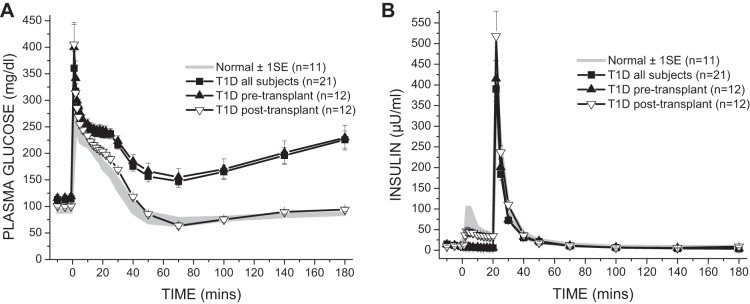
Plasma glucose (A) and insulin (B) levels in response to the frequently sampled intravenous glucose tolerance test. Glucose (0.3 g/kg) is injected over 1 min at time (t) = 0, and insulin (0.03 U/kg) is injected over 30 s at t = 20 min. Intravenous glucose tolerance improved in type 1 diabetes (T1D) from pre- to posttransplant (P < 0.01), with posttransplant glucose disappearance not different from normal. The acute insulin response to the injection of glucose, which was absent in T1D pretransplant, was restored posttransplant to levels comparable to normal.
Euglycemic clamp.
The steady-state glucose concentration and change in insulin concentration were similar in all groups during the final hour of the clamp, with one posttransplant subject not completing the clamp because of intravenous line failure (Table 2) (18). The GIR was less pre- than posttransplant (P < 0.05; Table 2), with similar results for the change in Rd (P < 0.05; Table 2). As a result, both total body, SI(clamp), and peripheral, SIP(clamp), insulin sensitivity were lower in T1D pre- compared with posttransplant (P < 0.05 for both; Table 2).
When the data were adjusted for BMI using a linear mixed model, the change in BMI did not have a significant effect on the change in FSIGT or euglycemic clamp-derived insulin sensitivity measures.
Comparison of FSIGT to euglycemic clamp-derived insulin sensitivity.
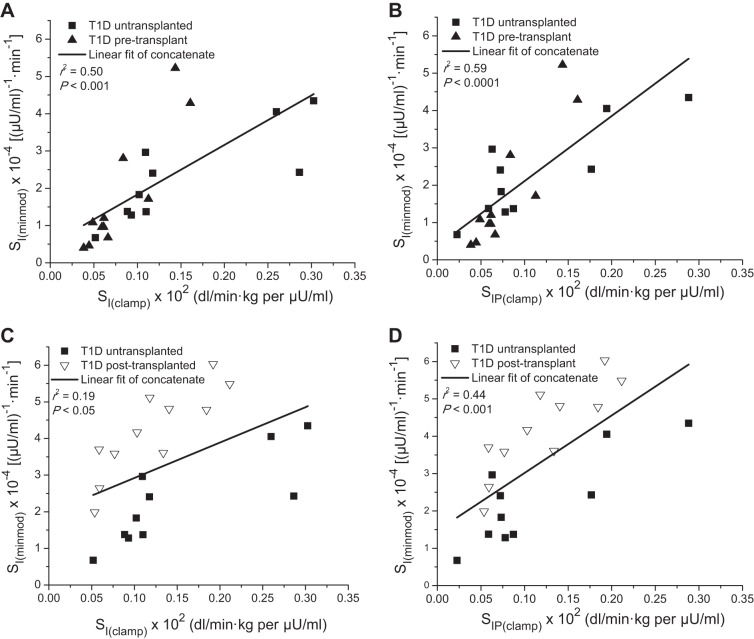
Before correction, SI(minmod) was reasonably predictive of SI(clamp) (r2 = 0.50; P < 0.001; Fig. 2A) and SIP(clamp) (r2 = 0.59; P < 0.0001; Fig. 2B) for all 21 T1D subjects, including those not transplanted (n = 9) and those pretransplant (n = 12). When considering the untransplanted subjects (n = 9) and those posttransplant (n = 11), the predictive value of SI(minmod) was weaker for SI(clamp) (r2 = 0.19; P < 0.05; Fig. 2C) and SIP(clamp) (r2 = 0.44; P < 0.001; Fig. 2D). This is explained by distinct relationships appearing between the untransplanted and posttransplant subjects such that, when each group is considered separately, SI(minmod) was more predictive of SI(clamp) (r2 = 0.63 and 0.62, respectively; P < 0.01; Fig. 2C) and SIP(clamp) (r2 = 0.69 and 0.66, respectively; P < 0.01; Fig. 2D) than when considering the untransplanted and posttransplant subjects together.
Fig. 2.
Correlations between total body insulin sensitivity estimated by the minimal model [SI(minmod)], total body insulin sensitivity based on glucose infusion rate, M, from the euglycemic clamp [SI(clamp); on the left], and peripheral insulin sensitivity based on glucose disposal rate, Rd, from the euglycemic clamp [SIP(clamp); on the right]. The predictive relationships of SI(minmod) to SI(clamp) and SIP(clamp) were stronger when considering all 21 T1D subjects (A and B) than when considering those not transplanted and those posttransplant (C and D).
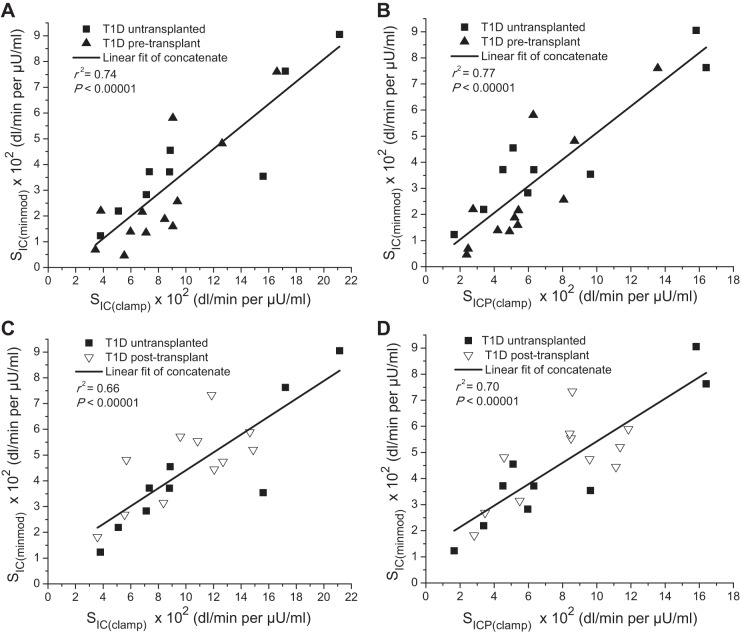
Following correction to common indexes of insulin sensitivity, the common insulin sensitivity index derived from the minimal model, SIC(minmod), was highly predictive of the common measures SIC(clamp) (r2 = 0.74; P < 0.00001; Fig. 3A) and SICP(clamp) (r2 = 0.77; P < 0.00001; Fig. 3B) derived from the euglycemic clamp when examining all 21 T1D subjects, those not transplanted (n = 9), and those pretransplant (n = 12). This was also the case when considering individual T1D subjects who were not transplanted (n = 9) and those posttransplant (n = 11) for comparison of SIC(minmod) with both SIC(clamp) (r2 = 0.66; P < 0.00001; Fig. 3C) and SICP(clamp) (r2 = 0.70; P < 0.00001; Fig. 3D).
Fig. 3.
Correlations between corrected measures of SIC(minmod) and SIC(clamp) (on the left), and SICP(clamp) (on the right). The minimal model and clamp-derived measures were each independently converted to a common index of insulin sensitivity, SIC, reflecting the unitary change in insulin to cause a given increment in glucose clearance and expressed ×102 in dl/min per μU/ml. The resulting predictive relationships of SIC(minmod) to SIC(clamp) and SICP(clamp) were robust both when considering all 21 T1D subjects (A and B), and when considering those not transplanted and those posttransplant (C and D).
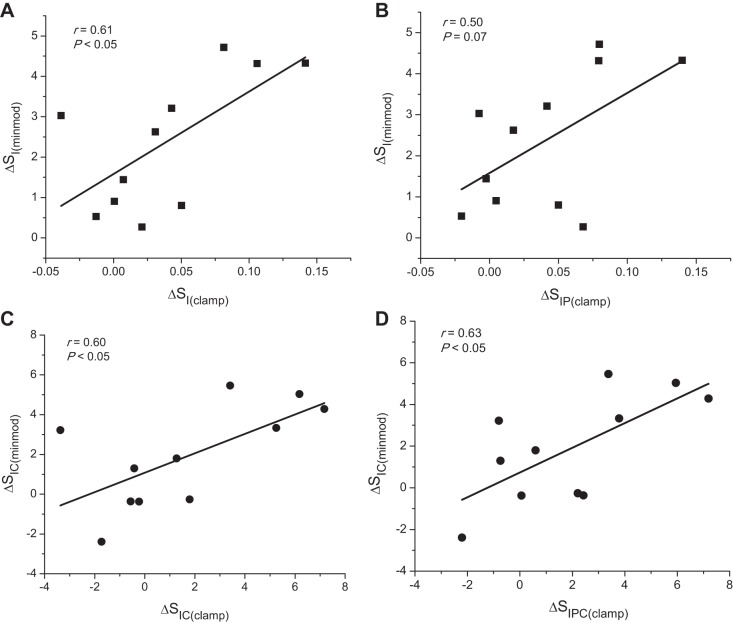
When examining the change in uncorrected insulin sensitivity measures in the transplanted subjects (n = 11), ΔSI(minmod) correlated with ΔSI(clamp) (r = 0.61; P < 0.05; Fig. 4A) and more weakly with ΔSIP(clamp) (r = 0.50; P = 0.07; Fig. 4B). For the change in corrected insulin sensitivity measures from pre- to posttransplant (n = 11), ΔSIC(minmod) correlated with both ΔSIC(clamp) (r = 0.60; P < 0.05; Fig. 4C) and with ΔSICP(clamp) (r = 0.63; P < 0.05; Fig. 4D). Similar to the results with SI(minmod) (Table 2), after adjusting for VD of the injected glucose, insulin sensitivity measured as SIC(minmod) was lower in T1D pre- compared with posttransplant and with normal (2.71 ± 0.63 vs. 4.72 ± 0.44 vs. 5.36 ± 0.88 dl/min per μU/ml; P < 0.05 for both).
Fig. 4.
A and B: correlations between the changes from pre- to posttransplant in uncorrected measures of ΔSI(minmod) and ΔSI(clamp) (A), and ΔSIP(clamp) (B). C and D: correlations between the changes from pre- to posttransplant in the corrected measures of ΔSIC(minmod) and ΔSIC(clamp) (C) and ΔSICP(clamp) (D).
DISCUSSION
The present study is the first to demonstrate a high correlation of the minimal model-derived SIC to euglycemic clamp-derived measures of insulin sensitivity in T1D and islet transplant recipients. These results confirm prior reports of improved insulin sensitivity following islet transplantation for T1D that used minimal model-derived estimates from the FSIGT (15, 20) and validate the minimal model-derived SI as a measure of both total body and peripheral insulin sensitivity in T1D. In fact, the correlations shown here were slightly stronger with clamp-derived peripheral insulin sensitivity, SIP(clamp), based on tracer-calculated glucose disposal, Rd, than clamp-derived total body insulin sensitivity, SI(clamp), based on glucose infusion rate. Importantly, the relationships between the minimal model and clamp-derived estimates were stronger when considering the volume-corrected SIC, particularly when evaluating untransplanted and posttransplant subject together, an effect explained by the different VD for the injected glucose between these groups. Indeed, the SIC measure is more appropriate than the standard SI when comparing T1D subjects with varying VD, since similar relationships were present using SIC whether considering only T1D subjects or including those postislet transplantation.
Prior reports from our group and others have established that the minimal model can adequately resolve the parameter SI in T1D (10, 20, 28), despite a late rise in glucose during the FSIGT as seen in our study (Fig. 1). Other studies involving T2D have encountered estimates of SI equal to zero, a problem avoided in the present work we believe by the use of an overnight insulin infusion targeting near-normal glycemia that was present at the start of testing. It is important to note that the minimal model SI underestimates insulin sensitivity as measured by the clamp when the values were normalized to identical units with the slope for correlations of SIC(minmod) against SIC(clamp) and SICP(clamp) both being around 0.5 (Fig. 2). A similar underestimation was also reported for the analysis comparing the minimal model and clamp-derived measures of insulin sensitivity in subjects that ranged from normal with those with impaired glucose tolerance, including overt T2D (22). This underestimation is likely explained by the difference in the effect on glucose disposal of an insulin bolus, as given with the FSIGT, compared with a continuous infusion of insulin during the euglycemic clamp since insulin-stimulated glucose disposal increases with time (9). This same difference in insulin administration may also explain the greater magnitude of increase in insulin sensitivity seen with the minimal model than with the euglycemic clamp measures and suggests that the FSIGT may be a more sensitive methodology for identifying changes in insulin sensitivity in T1D. Given these differences in performance characteristics, the minimal model SI should still be regarded as an index of insulin sensitivity and not be directly substituted for direct measures obtained from euglycemic clamp studies.
This is the first study to show that the impaired SG seen in T1D (10, 28) can be normalized following islet transplantation. The low SG derived from the FSIGT in T1D may in part be a consequence of absent insulin secretory function, since greater AIRg in normal subjects may lead to an overestimation of SG by the minimal model approach (11). In our prior study of islet transplant recipients where SG was less than normal, the AIRg was significantly reduced (20). In the present study, AIRg was not different from normal, although individual subjects did have reduced responses, and so demonstrates that, in the presence of near-normal β-cell function, there is no impairment of SG in islet transplant recipients. Glucose effectiveness is comprised of peripheral and hepatic components, with glucose autoregulation of glucose production by the liver contributing importantly to overall endogenous glucose production (5). Because glucose effectiveness describes the capacity for glucose to mediate its own disposal independent from insulin, the normalization of SG reported here provides additional evidence to the already reported improvements in hepatic and peripheral insulin sensitivity (18) against a detrimental effect of the intrahepatic site of transplantation or immunosuppression regimen on the maintenance of glucose homeostasis in islet recipients.
In conclusion, the minimal model-derived index of total body insulin sensitivity, SIC, is highly predictive of clamp-derived measures of both total and peripheral insulin sensitivity in T1D and following islet transplantation. When employing the FSIGT to generate the minimal model SI, simultaneous generation of SG is possible, with both indexes impaired in T1D and capable of correction as shown here with islet transplantation. The FSIGT parameter AIRg also provides a measure of islet graft β-cell function that is predictive of insulin requirements (14) and so may also be of interest during postislet transplant metabolic assessment. Thus, our data indicate the usefulness of the minimal model for longitudinal or cross-sectional studies in T1D populations when performance of the euglycemic clamp is not feasible for economic or practical reasons. Finally, the FSIGT may be the desired methodological approach in T1D for additional consideration of potential therapeutic effects on glucose effectiveness and β-cell function.
GRANTS
This work was performed as a project of the Clinical Islet Transplantation Consortium, a collaborative clinical research program headquartered at the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases and supported by National Institutes of Health Research Grants U01-DK-070430 (to A. Naji), R01-DK-091331 (to M. R. Rickels), UL1-TR-00003 (Penn Clinical & Translational Research Center), and P30-DK-19525 (Penn Diabetes Research Center).
DISCLOSURES
No potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
Author contributions: M.R.R., K.L.T., and A.N. conception and design of research; M.R.R., C.F., and C.D.-B. performed experiments; M.R.R., S.M.K., J.F.F., M.P.R., K.L.T., and A.N. analyzed data; M.R.R., S.M.K., C.F., J.F.F., M.P.R., K.L.T., and A.N. interpreted results of experiments; M.R.R. and S.M.K. prepared figures; M.R.R. drafted manuscript; M.R.R., S.M.K., C.F., C.D.-B., J.F.F., M.P.R., K.L.T., and A.N. edited and revised manuscript; M.R.R., S.M.K., C.F., C.D.-B., J.F.F., M.P.R., K.L.T., and A.N. approved final version of manuscript.
ACKNOWLEDGEMENTS
We thank the T1D subjects for participation, Eileen Markmann and Maral Palangian for coordinating the clinical care of the T1D subjects, the nursing staff of the Clinical & Translational Research Center for subject care and technical assistance, Dr. Heather Collins of the Penn Diabetes Research Center for performance of the radioimmunoassays, Dr. John Millar of the Penn Metabolic Tracer Resource in the Institute for Diabetes, Obesity & Metabolism for performance of the gas chromatography-mass spectrometry, and Huong-Lan Nguyen from the Monell Chemical Senses Center for laboratory assistance.
Current address for K. L. Teff: Div. of Diabetes, Endocrinology & Metabolic Diseases, National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.2011 CIT Website. http://www.isletstudy.org/ [1 May 2012].
- 2.Beard JC, Bergman RN, Ward WK, Porte D. The insulin sensitivity index in nondiabetic man- correlation between clamp-derived and IVGTT-derived values. Diabetes 35: 362–369, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 79: 790–800, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernroider E, Brehm A, Krssak M, Anderwald C, Trajanoski Z, Cline G, Shulman GI, Roden M. The role of intramyocellular lipids during hypoglycemia in patients with intensively treated type 1 diabetes. J Clin Endocrinol Metab 90: 5559–5565, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 19: 1018–1030, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5: 1003–1015, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Defronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 31: 795–801, 1982 [DOI] [PubMed] [Google Scholar]
- 8.Defronzo RA, Tobin JD, Andres R. Glucose clamp technique: Method for quantifying insulin-secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Doberne L, Greenfield MS, Schulz B, Reaven GM. Enhanced glucose utilization during prolonged glucose clamp studies. Diabetes 30: 829–835, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Finegood DT, Hramiak IM, Dupre J. A modified protocol for estimation of insulin sensitivity with the minimal model of glucose kinetics in patients with insulin-dependent diabetes. J Clin Endocrinol Metab 70: 1538–1549, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Finegood DT, Tzur D. Reduced glucose effectiveness associated with reduced insulin release: an artifact of the minimal-model method. Am J Physiol Endocrinol Metab 271: E485–E495, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Fowelin J, Attvall S, Vonschenck H, Bengtsson BA, Smith U, Lager I. Effect of prolonged hyperglycemia on growth-hormone levels and insulin sensitivity in insulin-dependent diabetes-mellitus. Metabolism 42: 387–394, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Greenbaum CJ. Insulin resistance in type 1 diabetes. Diabetes Metab Res Rev 18: 192–200, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch D, Odorico J, Danobeitia JS, Alejandro R, Rickels MR, Hanson M, Radke N, Baidal D, Hullett D, Naji A, Ricordi C, Kaufman D, Fernandez L. Early metabolic markers that anticipate loss of insulin independence in type 1 diabetic islet allograft recipients. Am J Transplant 12: 1275–1289, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch D, Odorico J, Radke N, Hanson M, Danobeitia JS, Hullett D, Alejandro R, Ricordi C, Fernandez LA. Correction of insulin sensitivity and glucose disposal after pancreatic islet transplantation: preliminary results. Diabetes Obes Metab 12: 994–1003, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen JL, Bennett RG, Burkman T, Ramirez AL, Yamamoto S, Gulizia J, Radio S, Hamel FG. Tacrolimus and sirolimus cause insulin resistance in normal Sprague Dawley rats. Transplantation 82: 466–470, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Talavera JC, Garcia-Ocana A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology 145: 467–474, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Rickels MR, Kong SM, Fuller C, Dalton-Bakes C, Ferguson JF, Reilly MP, Teff KL, Naji A. Improvement in insulin sensitivity after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 98: E1780–E1785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, Min Z, Markmann E, Palangian M, Dalton-Bakes C, Fuller C, Chiou AJ, Barker CF, Luning Prak ET, Naji A. Improvement in beta-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 62: 2890–2897, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rickels MR, Naji A, Teff KL. Insulin sensitivity, glucose effectiveness, and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 91: 2138–2144, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rossetti L, Smith D, Shulman GI, Papachristou D, Defronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79: 1510–1515, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saad MF, Anderson RL, Laws A, Watanabe RM, Kades WW, Chen YDI, Sands RE, Pei D, Savage PJ, Bergman RN. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose-tolerance. Diabetes 43: 1114–1121, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Shah RY, Ferguson JF, Shah R, Rickels MR, Mehta NN, Reilly MP. Acute endotoxemia reduces insulin sensitivity and glucose effectiveness in healthy Caucasians, but not in African Americans (Abstract). Diabetes 61, Suppl 1: A-11, 2012 [Google Scholar]
- 24.Trevisan R, Nosadini R, Avogaro A, Lippe G, Duner E, Fioretto P, Deana R, Tessari P, Tiengo A, Velussi M, Cernigoi A, Delprato S, Crepaldi G. Type-I diabetes is characterized by insulin resistance not only with regard to glucose, but also to lipid and amino-acid-metabolism. J Clin Endocrinol Metab 62: 1155–1162, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Trout KK, Basel-Brown L, Rickels MR, Schutta MH, Petrova M, Freeman EW, Tkacs NC, Teff KL. Insulin sensitivity, food intake, and cravings with premenstrual syndrome: a pilot study. J Womens Health 17: 657–665, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trout KK, Rickels MR, Schutta MH, Petrova M, Freeman EW, Tkacs NC, Teff KL. Menstrual cycle effects on insulin sensitivity in women with type 1 diabetes: a pilot study. Diabetes Technol Ther 9: 176–182, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Vethakkan SR, Walters JM, Gooley JL, Boston RC, Kay TW, Goodman DJ, Jenkins AJ, Ward GM. Normalized NEFA dynamics during an OGTT after islet transplantation. Transplantation 94: e49–e51, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Ward GM, Weber KM, Walters IM, Aitken PM, Lee B, Best JD, Boston RC, Alford FP. A modified minimal model analysis of insulin sensitivity and glucose-mediated glucose disposal in insulin-dependent diabetes. Metabolism 40: 4–9, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Ward WK, Beard JC, Porte D., Jr Islet B-cell function in human subjects. In: Methods in Diabetes Research: Clinical Methods, edited by Clarke WL, Larner J, Pohl SL. New York, NY: Wiley, 1986 [Google Scholar]
- 30.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley, 2005 [Google Scholar]
- 31.Ykijarvinen H, Helve E, Koivisto VA. Hyperglycemia decreases glucose-uptake in type-I diabetes. Diabetes 36: 892–896, 1987 [DOI] [PubMed] [Google Scholar]
- 32.Ykijarvinen H, Koivisto VA. Natural course of insulin resistance in type-I diabetes. N Engl J Med 315: 224–230, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Zunigaguajardo S, Zinman B. The metabolic response to the eugylcemic insulin clamp in type-I diabetes and normal humans. Metabolism 34: 926–930, 1985 [DOI] [PubMed] [Google Scholar]