Abstract
Hydrogen sulfide (H2S) is a toxic gas now being recognized as an endogenous signaling molecule in multiple organ systems, in particular, the cardiovascular system. H2S is known to regulate cardiac function and protect against ischemic injury. However, little information is available regarding the effect of H2S on cardiac function in insulin resistance. This study was designed to examine the impact of H2S supplementation on cardiac function using an Akt2 knockout model of insulin resistance. Wild-type and Akt2 knockout mice were treated with NaHS (50 μM·kg−1·day−1 ip for 10 days) prior to evaluation of echocardiographic, cardiomyocyte contractile, and intracellular Ca2+ properties, apoptosis, and mitochondrial damage. Our results revealed that Akt2 ablation led to overtly enlarged ventricular end-systolic diameter, reduced myocardial and cardiomyocyte contractile function, and disrupted intracellular Ca2+ homeostasis and apoptosis, the effects of which were ameliorated by H2S. Furthermore, Akt2 knockout displayed upregulated apoptotic protein markers (Bax, caspase-3, caspase-9, and caspace-12) and mitochondrial damage (reduced aconitase activity and NAD+, elevated cytochrome-c release from mitochondria) along with reduced phosphorylation of PTEN, Akt, and GSK3β in the absence of changes in pan protein expression, the effects of which were abolished or significantly ameliorated by H2S treatment. In vitro data revealed that H2S-induced beneficial effect against Akt2 ablation was obliterated by mitochondrial uncoupling. Taken together, our findings suggest the H2S may reconcile Akt2 knockout-induced myocardial contractile defect and intracellular Ca2+ mishandling, possibly via attenuation of mitochondrial injury and apoptosis.
Keywords: hydrogen sulfide, Akt2, insulin resistance, mitochondria, apoptosis, cardiomyocyte
although produced mainly in gastrointestinal and nervous systems, hydrogen sulfide (H2S), a known toxic gas, plays a crucial role as a signaling molecule (34, 49). Recent studies have established a role for H2S as the third endogenous gaseous transmitter in mammals, besides nitric oxide (NO) and carbon monoxide (CO), in the regulation of biological function in multiple organ systems (8, 45, 53, 61). Accumulating evidence has revealed low circulating levels of H2S in diabetic patients and streptozotocin (STZ)-induced experimental diabetes models (24). Not surprisingly, H2S deficiency has been postulated to contribute to the pathogenesis of endothelial dysfunction, nephropathy, and cardiomyopathy in diabetes (29, 50), implicating the potential benefit for H2S in the management of diabetes and diabetic complications. This is supported by various cardiovascular actions of H2S, including the Akt-dependent proangiogenic property (6, 52), antiapoptosis (57), and inhibition of L-type Ca2+ channels (46) in cardiomyocytes.
Insulin resistance is attributed to the obesity pandemic and drastically increases the prevalence of cardiovascular diseases (4). Although a number of scenarios have been postulated for insulin resistance-induced cardiovascular anomalies, including dyslipidemia, inflammation, endoplasmic reticulum, and oxidative stress (21, 26–28), the precise mechanisms behind the cardiac dysfunction induced by insulin resistance still remain controversial, thus making adequate clinical management somewhat challenging. Insulin signaling plays an essential role in the regulation of myocardial oxidative phosphorylation and myocardial contractile function (38). This is supported by the fact that insulin-receptor knockout dampens the oxidative phosphorylation and exacerbates cardiac dysfunction (1, 42). Although insulin signaling is rather complex, involving a large cascade of signaling molecules, the phosphatidylinositol 3-kinase (PI3K)-Akt cascade is deemed perhaps the main player governing the majority of metabolic properties of insulin (14, 30). Akt is a serine/threonine kinase directly downstream of PI3K to mediate the metabolic actions of insulin (14). Interestingly, impaired insulin-stimulated PI3K/Akt has also been implicated in a number of pathological conditions accompanied with insulin resistance, such as obesity, inflammation, cardiovascular and renal complications of diabetes, as well as cancer (41, 55, 58). As a matter of fact, the onset of insulin resistance and later on diabetes mellitus is often linked to changes in Akt phosphorylation. Akt regulates glucose uptake in muscle and adipocytes through stimulating the translocation of GLUT4 glucose transporter to the plasma membrane (20). The identification of a dominant negative Akt2 mutation (R274H), which leads to severe hyperinsulinemia and diabetes in humans, has consolidated a permissive role for Akt2 in metabolic regulation (18). In particular, Akt2 knockout mice exhibit overt global insulin resistance as manifested by decreased glucose uptake into muscle and adipose cells, despite the normal growth and development (9, 17, 39).
Given that cardiac dysfunction is a major complication of insulin resistance where Akt signaling plays a key role in the maintenance of cardiac homeostasis (11, 15, 58), this study was undertaken to examine the effect of Akt2 knockout on myocardial function, and the impact of H2S supplement on Akt2 knockout-induced myocardial anomalies, if any. In an effort to better elucidate the mechanisms involved in Akt2 knockout and H2S supplement-induced myocardial function, mitochondrial integrity, crucial signaling molecules of insulin signaling, such as Akt, phosphatase and tensin homolog on chromosome 10 (PTEN), glycogen synthase kinase 3β (GSK3β), and protein phosphatase, which usually negatively regulates insulin signaling (44), were examined in hearts from wild-type (WT) and Akt2 knockout (KO) mice.
MATERIALS AND METHODS
Experimental animals and H2S treatment.
The animal procedures described in this study were approved by the Institutional Animal Use and Care Committee at the University of Wyoming (Laramie, WY). The Akt2 knockout mice were obtained from Prof. Morris Birnbaum at the University of Pennsylvania (Philadelphia, PA) and were characterized as described previously (9). Wild-type and Akt2KO mice (10 per group) were treated with NaHS (50 μM·kg−1·day−1 ip for 10 days) according to the dosage and duration for NaHS reported previously (6, 40). NaHS-untreated mice (10 mice per group) received equal volume of PBS as the vehicle control. All mice were maintained at 22°C with a 12:12-h light-dark cycle and received lab chow and water ad libitum.
Intraperitoneal glucose tolerance test.
All mice were fasted for 12 h and given an intraperitoneal injection of glucose (2 g/kg body wt ip). Blood samples were drawn from the tail, and glucose levels were determined immediately before glucose challenge, as well as 30, 60, 90, and 120 min thereafter using an Accu-Chek III glucose analyzer (15).
Tissue cystathionine γ-lyase activity.
Cardiac cystathionine γ-lyase (CSE) activity was measured according to the Stipanuk method, as described previously (43, 59). Ventricular tissues and plasma were collected at the end of 10-day H2S treatment and were stored at −80°C. Measurement of H2S was performed 1 wk following sample collection. Briefly, frozen tissues (50 mg) were homogenized in 0.5 ml ice-cold 100 mM potassium phosphate buffer (pH 7.4), then centrifuged at 4°C, 10,000 g for 10 min. The clear supernatant was transferred to an Eppendorf tube before being mixed with cystathionine (2 mM) and pyridoxal-5′-phosphate (0.25 mM) in 100 mM Tris·HCl buffer (pH 8.3) for 60 min at 37°C. Trichloroacetic acid (TCA; 10% vol/vol) was added into the reaction mixture to terminate the reaction. Following centrifugation, supernatants were mixed with 1% ninhydrin reagent and were incubated for 5 min in a boiling-water bath. After heating, the solution was cooled on ice for 2 min, and the color reaction development were assayed for 20 min at 455 nm with a spectrophotometer. CSE activity was assessed by cystathionine consumption, and enzyme activity was expressed as nanomoles of cystathionine consumed per milligram of total protein per hour of incubation.
Plasma H2S levels.
Plasma H2S levels were determined using previously described methods (2, 63). Plasma samples (120 μl) were mixed with 100 μl water and 120 μl TCA (10% vol/vol), reacted for 10 min at room temperature, and then centrifuged at 4°C, 14,000 g for 10 min. The clear supernatant was transferred to an Eppendorf tube containing zinc acetate (1% 60 μl). Subsequently, N, N-dimethylphenylendiamine sulfate 40 μl (20 mM in 7.2 M HCl) and FeCl3 40 μl (30 mM in 1.2 M HCl) were added to the reaction mixture for 20 min at room temperature. The absorbance was measured at a wavelength of 670 nm with a spectrophotometer. The plasma H2S concentration was calculated against the calibration curve of standard H2S solutions, and all samples were assayed in duplicate.
Echocardiographic assessment.
Cardiac geometry and function were evaluated in anesthetized (ketamine 80 mg/kg and xylazine 12 mg/kg ip) mice using the two-dimensional guided M-mode echocardiography (Phillips Sonos 5500) equipped with a 15–16-MHz linear transducer (Phillips Medical Systems, Andover, MD). Left ventricular anterior and posterior wall dimensions during diastole and systole were recorded from three consecutive cycles in M mode using methods adopted by the American Society of Echocardiography. Fractional shortening was calculated from LV end-diastolic (EDD) and end-systolic (ESD) diameters using the equation (EDD − ESD)/EDD. Heart rate was averaged over 10 cardiac cycles (23).
Isolation of cardiomyocytes.
Murine cardiomyocytes were isolated as described by Ceylan-Isik et al. (7). After ketamine/xylazine sedation, hearts were removed and perfused with Ca2+-free Tyrode's solution containing (in mM): 135 NaCl, 4.0 KCl, 1.0 MgCl2, 10 HEPES, 0.33 NaH2PO4, 10 glucose, 10 butanedione monoxime, and the solution was gassed with 5% CO2-95% O2. Hearts were digested with Liberase Blendzyme 4 (Hoffmann-La Roche, Indianapolis, IN) for 20 min. Left ventricles were removed and minced before being filtered. Tissue pieces were gently agitated, and the pellet of cells was resuspended. Extracellular Ca2+ was added incrementally back to 1.20 mM over a period of 30 min. Isolated myocytes were used within 8 h of isolation. Normally, a yield of 50–60% viable rod-shaped cardiomyocytes with clear sarcomere striations was achieved. Only rod-shaped myocytes with clear edges were selected for mechanical study. To discern the role of mitochondria in H2S supplementation's protective effect against Akt2 ablation-induced cardiomyocyte contractile dysfunction, freshly isolated murine cardiomyocytes from wild-type and Akt2KO mice were pretreated with the mitochondrial uncoupler carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; 1 μM) (54) for 1 h prior to the exposure to NaHS (50 μM) (6) for an additional 2 h.
Cell shortening/relengthening.
Mechanical properties of cardiomyocytes were assessed using a SoftEdge MyoCam system (IonOptix, Milton, MA). In brief, cardiomyocytes were placed in a Warner chamber mounted onto the stage of an inverted microscope (Olympus IX-70, Olympus, Tokyo, Japan) and superfused (∼1 ml/min at 25°C) with a buffer containing (in mM) 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES at pH 7.4. The cells were field-stimulated with suprathreshold voltage at a frequency of 0.5 Hz using a pair of platinum wires placed on opposite sides of the chamber connected to a Pulsar 6bp bipolar stimulator (FHC, Brunswick, NJ). The myocyte being studied was displayed on the computer monitor using an IonOptix MyoCam camera. An IonOptix SoftEdge software was used to capture changes in cell length during shortening and relengthening. Cell shortening and relengthening were assessed using the following indices: resting cell length, peak shortening (PS), time-to-PS (TPS), time-to-90% relengthening (TR90), and maximal velocity of shortening/relengthening (± dL/dt) (22).
Intracellular Ca2+ transients.
A cohort of myocytes was loaded with Fura-2 AM (0.5 μM) for 10 min, and fluorescence intensity was recorded with a dual-excitation fluorescence photomultiplier system (IonOptix). Myocytes were placed onto an Olympus IX-70 inverted microscope and imaged through a Fluor 40 oil objective. Cells were exposed to light emitted by a 75-W lamp and passed through either a 360- or a 380-nm filter, while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480 and 520 nm, and qualitative change in Fura-2 AM fluorescence intensity (FFI) was inferred from the FFI ratio at the two wavelengths (360/380). Fluorescence decay time was measured as an indication of the intracellular Ca2+ clearing rate. Single exponential curve fit was used to calculate the intracellular Ca2+ decay constant (22).
Caspase-3 assay.
Caspase-3 is an enzyme activated during induction of apoptosis. In brief, 1 ml of PBS was added to flasks containing mouse cardiomyocytes, and the monolayer was scraped and collected in a microfuge tube. The cells were centrifuged at 10,000 g at 4°C for 10 min, and cell pellets were lysed in 100 μl of ice-cold cell lysis buffer (50 mM HEPES, 0.1% CHAPS, 1 mM dithiothreitol, 0.1 mM EDTA, 0.1% NP40). After cells were lysed, 70 μl of reaction buffer was added to cell lysate (30 μl), followed by an additional 20 μl of caspase-3 colorimetric substrate (Ac-DEVD-pNA) and incubated at 37°C for 1 h, during which time the caspase in the sample was allowed to cleave the chromophore pNA from the substrate molecule. The samples were then read with a microplate reader at 405 nm. Caspase-3 activity was expressed as picomoles of pNA released per microgram of protein per minute (60).
Determination of NAD+.
NAD+ was extracted from frozen ventricular tissues using perchloric acid (10, 16). For these determinations, 30 mg of fresh frozen tissue was powdered in a mortar under liquid nitrogen and thoroughly mixed with 150 μl 0.6 M perchloric acid. The mixture was then homogenized, neutralized with 150 μl 3 M potassium hydroxide, and centrifuged. NAD+ concentrations were determined fluorometrically in dilutions of the supernatant sample using alcohol dehydrogenase (Sigma-Aldrich, St. Louis, MO). Excitation was at 339 nm, and emission wavelength was at 460 nm in a spectrofluorimeter (Spectra MaxGeminiXS, Sunnyvale, CA) (62).
Aconitase activity.
Mitochondrial aconitase, an iron-sulfur enzyme occurring during the citric acid cycle, is readily damaged by oxidative stress via removal of an iron from [4Fe-4S] cluster. Mitochondrial fractions prepared from whole heart homogenate were resuspended in 0.2 mM sodium citrate. Aconitase activity assay (Aconitase activity assay kit, Aconitase-340 assay, OxisResearch, Portland, OR) was performed according to manufacturer instructions with minor modifications. Briefly, mitochondrial sample (50 μl) was mixed in a 96-well plate with 50 μl trisodium citrate (substrate) in Tris·HCl pH 7.4, 50 μl isocitrate dehydrogenase (enzyme) in Tris·HCl, and 50 μl NADP + reagent in Tris·HCl. After incubating for 15 min at 37°C, the absorbance was dynamically recorded at 340 nm every min for 5 min with a spectrophotometer. During the assay, citrate is isomerized by aconitase into isocitrate and eventually α-ketoglutarate. The Aconitase-340 assay measures NADPH formation, a product of the oxidation of isocitrate to α-ketoglutarate. Tris·HCl buffer (pH 7.4) was served as blank (37).
Separation of mitochondrial and cytosolic fractions.
Ventricles were minced and homogenized by Polytron in the ice-cold MES buffer [220 mM mannitol, 70 mM sucrose, 2 mM EGTA, 5 mM 3-(4-morpholino) propane sulfonic acid (MOPS), at pH 7.4, 0.2% BSA] and a protease inhibitor cocktail containing 4-(2-aminoethyl) benzenesulfonyl fluoride, E-64, bestatin, leupeptin, aprotinin, and EDTA obtained from Sigma Chemicals. The homogenates were centrifuged for 10 min at 600 g to remove unbroken tissue and nuclei, and the supernatants were centrifuged for 10 min at 3,000 g to pellet mitochondria. The supernatants were further centrifuged for 30 min at 100,000 g to obtain cytosolic fraction. The mitochondrial pellet was dissolved in the protein lysis buffer and centrifuged at 10,000 g for 30 min at 4°C to make a soluble protein. Fifty micrograms of the mitochondrial or cytosolic protein was separated by 15% SDS-PAGE for Western blot analysis of cytochrome c (13).
Western blot analysis.
Protein samples were prepared as previously described (7). Samples containing an equal amount of proteins were separated on 10% SDS-polyacrylamide gels in a minigel apparatus (Mini-PROTEAN II, Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes. The membranes were blocked with 5% milk in TBS-Tween, and were incubated overnight at 4°C with anti-Akt2, anti-Bax, anti-Bcl-2, anti-cleaved caspase-3, anti-cleaved caspase-9, anti-cleaved caspase-12, anti-PTEN, anti-phospho-PTEN, anti-Akt, anti-phospho-Akt, anti-GSK3β, anti-phospho-GSK3β, and anti-cytochrome c antibodies. After washing blots to remove excessive primary antibody binding, blots were incubated for 1 h with horseradish peroxidase-conjugated secondary antibody (1: 5,000). Antibody binding was detected using enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ), film was scanned, and the intensity of immunoblot bands was detected with a Bio-Rad Calibrated Densitometer (model no. GS-800). All tissue samples were run in duplicate. GAPDH was used as the loading control.
Statistical analysis.
Data are expressed as means ± SE. Statistical significance (P < 0.05) was estimated by a two-way ANOVA followed by a Bonferroni multicomparison analysis when necessary.
RESULTS
General and echocardiographic properties of WT and Akt2KO mice following H2S supplement.
Neither Akt2 knockout nor H2S treatment, or both, significantly affected body, heart, liver, and kidney weights. Echocardiographic assessment revealed comparable heart rate, left ventricular (LV) wall thickness, and LV end-diastolic diameter (LVEDD) among all four mouse groups. However, Akt2 knockout significantly increased LV end-systolic diameter (LVESD) and lessened fractional shortening, the effect of which was abrogated by H2S supplement. H2S itself did not affect any of the echocardiographic indices tested (Table 1).
Table 1.
General characteristics of WT and Akt2KO mice treated with or without NaHS
| WT | WT H2S | Akt2KO | Akt2KO H2S | |
|---|---|---|---|---|
| Body weight, g | 24.8 ± 0.7 | 24.9 ± 0.4 | 24.6 ± 0.6 | 25.0 ± 0.7 |
| Heart weight, mg | 134 ± 5 | 136 ± 6 | 137 ± 6 | 136 ± 6 |
| Liver weight, g | 1.34 ± 0.06 | 1.30 ± 0.03 | 1.36 ± 0.07 | 1.35 ± 0.05 |
| Kidney weight, mg | 330 ± 15 | 324 ± 13 | 328 ± 16 | 328 ± 14 |
| Heart rate, bpm | 497 ± 27 | 485 ± 35 | 489 ± 37 | 476 ± 25 |
| Wall thickness, mm | 0.89 ± 0.02 | 0.95 ± 0.03 | 0.87 ± 0.03 | 0.93 ± 0.02 |
| LVEDD, mm | 2.46 ± 0.13 | 2.43 ± 0.16 | 2.62 ± 0.17 | 2.34 ± 0.11 |
| LVESD, mm | 1.11 ± 0.10 | 1.10 ± 0.08 | 1.63 ± 0.14* | 1.02 ± 0.07# |
| Fractional Shortening, % | 53.7 ± 1.8 | 55.1 ± 2.2 | 38.0 ± 1.9* | 56.2 ± 2.4# |
Values are expressed as means ± SE; n = 7 or 8 mice per group. Dosage for NaHS treatment was 50 μM·kg−1·day−1 ip for 10 days. LV, left ventricular; EDD, end-diastolic diameter; ESD, end-systolic diameter.
P < 0.05 vs. WT group.
P < 0.05 vs. Akt2KO group.
Effect of hydrogen sulfide supplement on Akt2 knockout-induced insulin resistance.
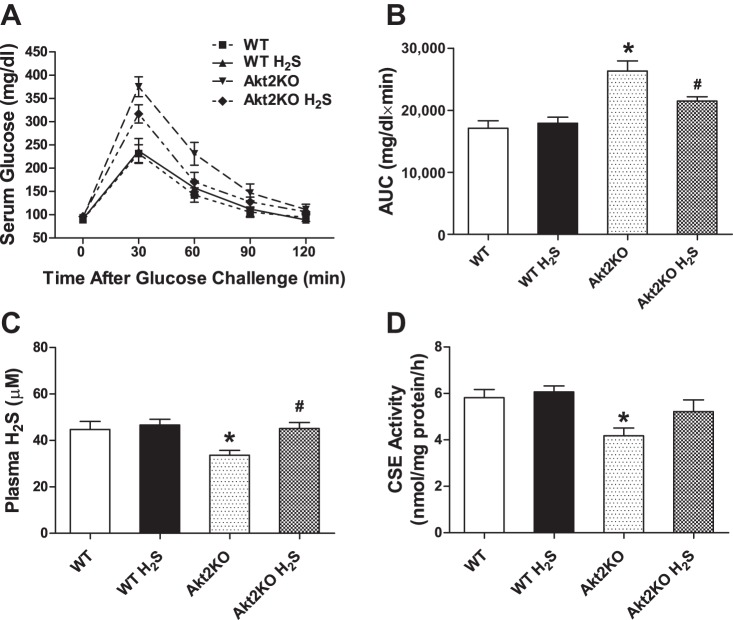
Intraperitoneal glucose tolerance was performed at the end of the 10-day H2S supplement. Following the intraperitoneal glucose challenge (2 g/kg body wt), serum glucose levels started to decline after peaking at 30 min and returned to near baseline levels at 120 min. To the contrary, Akt2 knockout displayed severe glucose intolerance, as evidenced by a much greater area under the curve for IPGTT, although basal glucose levels were comparable to those of WT mice. H2S supplementation failed to affect basal blood glucose levels or glucose disposal rate following glucose challenge in either WT or Akt2 knockout mice. Plasma H2S levels were significantly lower in Akt2KO mice compared with WT mice, the effect of which was abolished by H2S treatment for 10 days. Likewise, cardiac H2S synthase CSE activity, an indicator for tissue H2S synthesis rate, was significantly lower in Akt2KO mice compared with WT mice, the effect of which was removed by the short-term H2S treatment. H2S treatment itself did not overtly affect tissue or plasma H2S levels (Fig. 1).
Fig. 1.
Intraperitoneal glucose tolerance test (IPGTT; 2 g/kg body wt), as well as plasma and tissue hydrogen sulfide (H2S) levels in wild-type (WT) and Akt2 knockout (Akt2KO) mice treated with or without sodium hydrosulfide (NaHS; 50 μM·kg−1·day−1 ip for 10 days). A: IPGTT curve. B: area underneath the curve plotted in panel. C: Plasma H2S level at the end of 10th day of H2S treatment. D: tissue H2S levels assessed by the tissue synthesis rate of the H2S synthase cystathionine γ-lyase (CSE) at 60 min. Values are expressed as means ± SE; n = 7 or 8 mice per group. *P < 0.05 vs. WT group, #P < 0.05 vs. Akt2KO group.
Effect of hydrogen sulfide on cardiomyocyte contractile and intracellular Ca2+ properties.
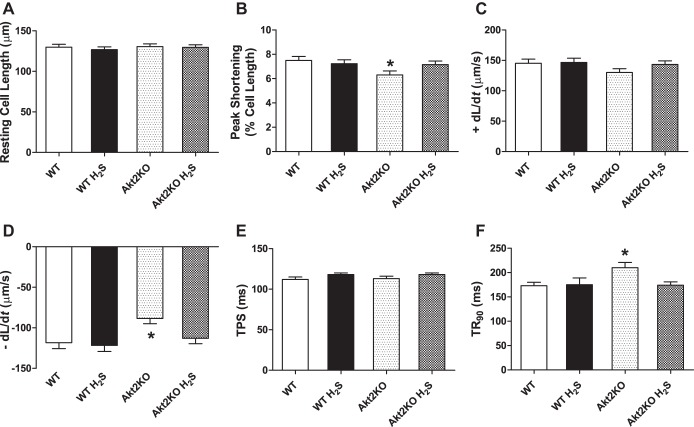
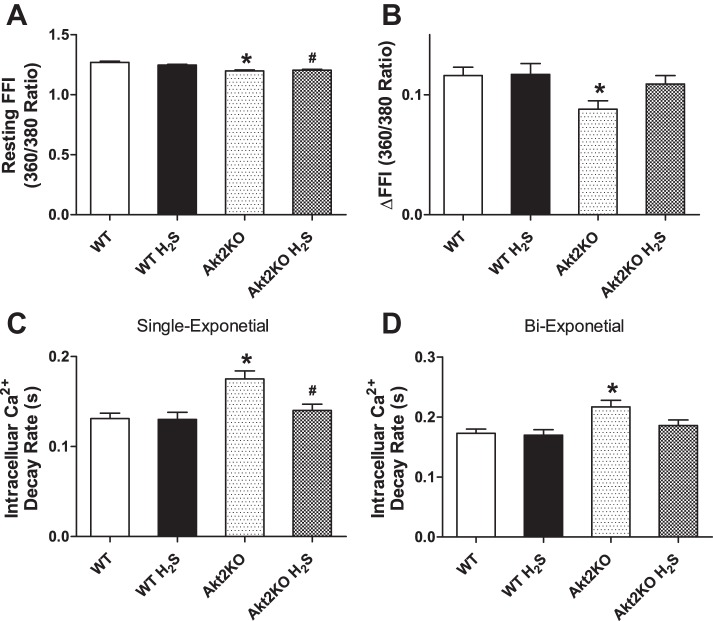
Neither short-term H2S treatment nor Akt2 knockout overtly affected resting cell length in cardiomyocytes. Cardiomyocytes from Akt2 knockout mice displayed significantly depressed PS and ±dL/dt, as well as prolonged TR90 without affecting TPS. Although H2S itself did not affect these mechanical parameters tested, it significantly attenuated Akt2KO-induced mechanical anomalies (Fig. 2). To further understand the possible mechanism of action behind Akt2KO and H2S supplementation-induced cardiac responses, intracellular Ca2+ homeostasis was evaluated in cardiomyocytes using the intracellular Ca2+ fluorescence dye Fura-2 AM. Data presented in Fig. 3 show that Akt2KO significantly elevated the baseline FFI and suppressed electrically stimulated rise in Fura-2 AM fluorescence intensity (ΔFFI), as well as slowed down intracellular Ca2+ decay rate. Although H2S supplementation itself did not affect these intracellular Ca2+ parameters, it abolished or significantly attenuated Akt2KO-induced changes in intracellular Ca2+ handling.
Fig. 2.
Effect of NaHS (50 μM·kg−1·day−1 ip for 10 days) on cardiomyocyte contractile properties in WT and Akt2KO mice. A: resting cell length. B: peak shortening (% of resting cell length). C: maximal velocity of shortening (+dL/dt); D: maximal velocity of relengthening (−dL/dt); E: time-to-peak shortening (TPS). F: time-to-90% relengthening (TR90). Values are expressed as means ± SE; n = 90–100 cells from three mice per group, *P < 0.05 vs. WT group.
Fig. 3.
Effect of NaHS (50 μM·kg−1·day−1 ip for 10 days) on intracellular Ca2+ transients measured using Fura-2 AM in cardiomyocytes from WT and Akt2KO mice. A: baseline Fura-2 fluorescence intensity (FFI). B: change in FFI (ΔFFI) in response to electrical stimuli. C: single exponential intracellular Ca2+ decay rate. D: biexponential intracellular Ca2+ decay rate. Values are expressed as means ± SE; n = 75–80 cells from three mice per group, *P < 0.05 vs. WT group, #P < 0.05 vs. Akt2KO group.
Effect of hydrogen sulfide on caspase-3 activity, cytochrome c release, aconitase activity, and mitochondrial permeation pore opening in Akt2KO mice.
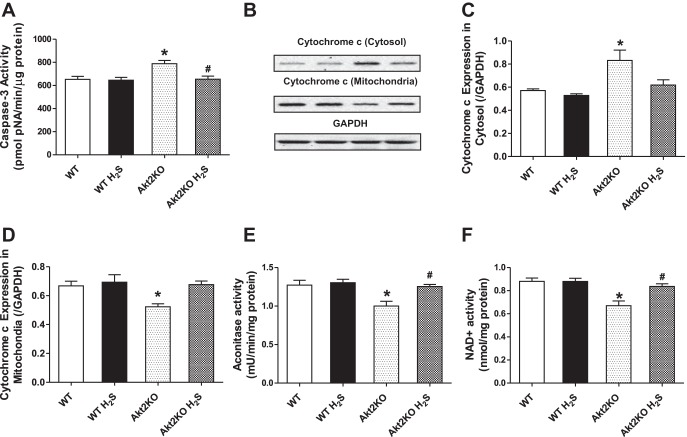
Data shown in Fig. 4 depict that Akt2KO significantly triggers apoptosis (as manifested by elevated caspase-3 activity) and promotes mitochondrial cytochrome c release into cytosol (decreasing mitochondrial content and increasing cytosolic cytochrome c content). Although H2S supplementation did not affect apoptosis and mitochondrial cytochrome c release, it significantly attenuated Akt2KO-induced apoptosis and cytochrome c release. Given that aconitase, an iron sulfur enzyme located in citric acid cycle, is closely associated with oxidative stress and mitochondrial function (37), we further evaluated aconitase activity and NAD+ levels, a marker for mitochondrial permeation pore opening. Our data further revealed significantly decreased aconitase activity and NAD+ levels in Akt2KO mouse hearts, indicating mitochondrial injury. While H2S supplementation itself did not affect aconitase activity and NAD+ levels, it effectively rectified Akt2KO-induced decrease in aconitase activity and mitochondrial permeation pore opening (evidenced by reduced NAD+ levels).
Fig. 4.
Effect of NaHS (50 μM·kg−1·day−1 ip for 10 days) on caspase-3 activity, cytochrome c distribution, aconitase activity, and NAD+ level in WT and Akt2KO mice. Myocardial tissues were separated using differential density centrifugation to yield cytosolic and mitochondrial fractions prior to gel electrophoresis. A: caspase-3 activity. B: representative gel blots depicting level of cytochrome c in cytosol and the mitochondria using respective specific antibodies. GAPDH was used as loading control. C: cytochrome c levels in the cytosol. D: cytochrome c levels in mitochondria. E: aconitase activity. F: NAD+ level depicting mitochondrial permeation pore opening. Values are expressed as means ± SE; n = 4 or 5 mice per group. *P < 0.05 vs. WT group, #P < 0.05 vs. Akt2KO group.
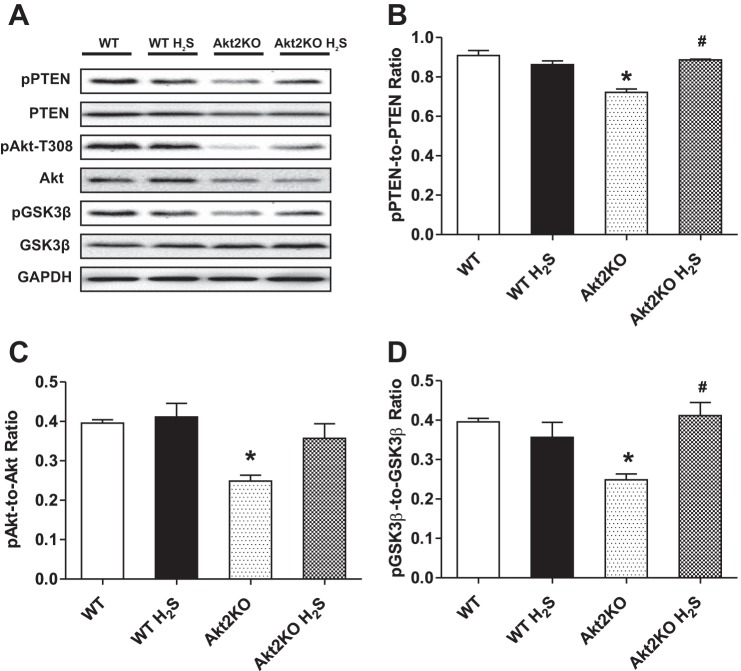
Effect of hydrogen sulfide treatment on pan and phosphorylated Akt, PTEN, and GSK3β in WT and Akt2KO mice.
Our results indicate that although Akt2KO mice had significantly reduced Akt expression, pan protein expression of PTEN and GSK3β was not affected. Moreover, Akt2KO significantly decreased phosphorylation of PTEN, Akt, and GSK3β (absolute or normalized value). Although H2S supplementation failed to alter expression of pan and phosphorylated Akt and GSK3β, it significantly attenuated or ablated Akt2KO-elicited loss in the phosphorylation of Akt, PTEN, and GSK3β (Fig. 5).
Fig. 5.
Effect of NaHS (50 μM·kg−1·day−1 ip for 10 days) on basal and phosphorylated levels of PTEN, Akt, and GSK3β in myocardium from WT and Akt2KO mice. A: representative gel blots depicting expression of pPTEN, PTEN, pAkt, Akt, pGSK3β, and GSK3β using respective specific antibodies. GAPDH was used as loading control. B: pPTEN-to-PTEN ratio. C: pAkt-to-Akt ratio. D: pGSK3β-to-GSK3β ratio. Values are expressed as mean ± SE; n = 4 or 5 mice per group. *P < 0.05 vs. WT group, #P < 0.05 vs. Akt2KO group.
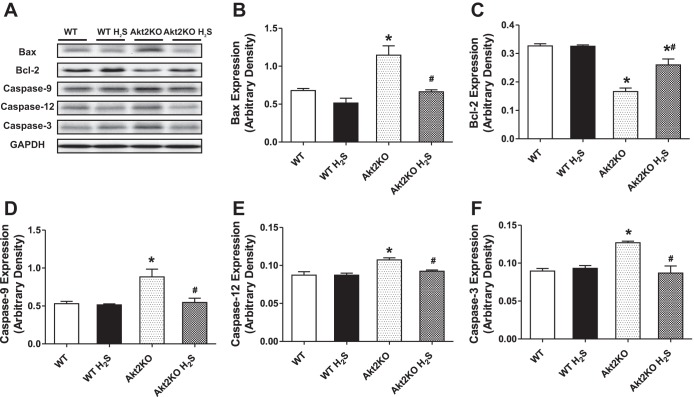
Effect of hydrogen sulfide treatment on apoptotic protein makers in WT and Akt2KO mice.
Our results shown in Fig. 6 indicate that Akt2 knockout led to significantly upregulated expression of the proapoptotic proteins Bax, Caspase-3, Caspase-9, and the ER-specific caspase-12, as well as downregulated expression of the antiapoptotic protein Bcl-2. Although H2S itself failed to affect the expression of these apoptotic proteins, it significantly attenuated or mitigated Akt2KO-elicited responses in Bax, Bcl-2, caspase-3, caspase-9, and caspase-12.
Fig. 6.
Effect of NaHS (50 μM·kg−1·day−1 ip for 10 days) on apoptotic proteins Bax, Bcl-2, caspase-9, caspase-12, and caspase-3 in WT and Akt2KO mice. A: representative gel blots depicting expression of Bax, Bcl-2, cleaved caspase-9, caspase-12, and caspase-3 using respective specific antibodies. GAPDH was used as loading control. B: Bax expression. C: Bcl-2 expression. D: cleaved caspase-9 expression. E: cleaved caspase-12 expression. F: cleaved caspase-3 expression. Values are expressed as means ± SE; n = 4 or 5 per group. *P < 0.05 vs. WT group, #P < 0.05 vs. Akt2KO group.
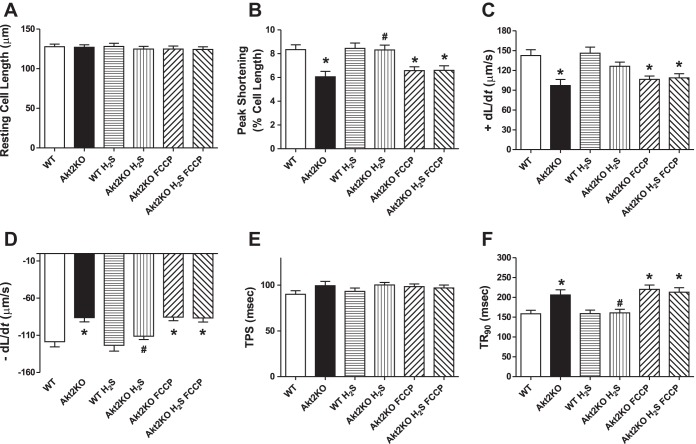
Influence of mitochondrial uncoupling on H2S-induced cardiomyocyte contractile responses.
To further discern the role of mitochondria in H2S supplementation's protective effect against Akt2KO cardiomyocyte anomalies, freshly isolated murine cardiomyocytes from WT and Akt2KO mice were pretreated with the mitochondrial uncoupler FCCP (1 μM) (54) for 1 h prior to the exposure to NaHS (50 μM) (6) for an additional 2 h. Similar to the in vivo observation, short-term coincubation of H2S obliterated or significantly dampened Akt2KO-induced cardiomyocyte contractile function (shown as reduced PS, ±dL/dt and prolonged TR90) without eliciting any effect itself. Interestingly, the beneficial mechanical effects of H2S were nullified by the mitochondrial uncoupler FCCP. Moreover, FCCP did not produce any further effect on Akt2 ablation-induced cardiomyocyte contractile dysfunction (Fig. 7).
Fig. 7.
Effect of NaHS (50 μM) on in vitro cardiomyocyte contractile properties. Cardiomyocytes from WT and Akt2KO mice were pretreated with the mitochondrial uncoupler carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; 1 μM) for 1 h prior to exposing to NaHS (50 μM) for an additional 1 h. Then, mechanical function was evaluated in cardiomyocytes. A: resting cell length. B: peak shortening (normalized to resting cell length). C: maximal velocity of shortening (+dL/dt). D: maximal velocity of relengthening (− dL/dt). E: time-to-peak shortening (TPS). F: time-to-90% relengthening (TR90). Values are expressed as means ± SE; n = 55–60 cells from 3 mice per group. *P < 0.05 vs. WT group, #P < 0.05 vs. Akt2KO group.
DISCUSSION
The salient findings from our present study revealed that Akt2KO exerts a drop in circulating and cardiac tissue H2S levels, cardiac contractile and intracellular Ca2+ derangement (as evidenced by enlarged LVESD and reduced fraction shortening, depressed peak shortening, maximal velocity of shortening/relengthening, prolonged duration of relengthening, increased basal intracellular Ca2+ levels, and reduced intracellular Ca2+ release in response to electrical stimuli). The compromised cardiac function and intracellular Ca2+ handling in Akt2KO mice were accompanied with upregulated proapoptotic protein markers (Bax, caspase-3, caspase-9, and caspase-12), downregulated anti-apoptotic protein marker Bcl-2, elevated caspase-3 activity, and overt mitochondrial injury (reduced aconitase activity and NAD+ and elevated cytochrome c release from mitochondria). Evaluation of cell signaling mechanism revealed that Akt2KO-induced insulin resistance suppressed phosphorylation of PTEN, Akt, and GSK-3β in the myocardium, the effect of which was attenuated or reversed by H2S supplementation. These data implicate a possible role of Akt-GSK3β signaling in H2S-offered cardioprotection. These findings have indicated a favorable effect of H2S supplementation against myocardial anomalies in Akt2KO-induced insulin resistance.
Our findings suggest that knockout of Akt2 elicits overt glucose intolerance, as manifested by increased area under the curve for IPGTT, which is associated with unchanged baseline blood glucose levels (except for the presence of outright diabetes), validating the murine model of insulin resistance (9). Our data further revealed that knockout of Akt2 promoted myocardial contractile anomalies, including enlarged LVESD; reduced fractional shortening, peak shortening, and maximal velocity of shortening/relengthening (±dL/dt), and prolonged duration of relengthening duration (TR90), which is associated with the unchanged LV wall thickness, LVEDD, cardiomyocyte cell length, and duration of shortening (TPS). Our data revealed impaired intracellular Ca2+ handling, which was manifested as elevated baseline intracellular Ca2+ levels, reduced intracellular Ca2+ rise (ΔFFI), and prolonged intracellular Ca2+ clearance in cardiomyocytes from Akt2KO mice, indicating an essential role of intracellular Ca2+ mishandling in Akt2KO-induced myocardial mechanical defects. These findings are somewhat consistent with our earlier findings using a high-sucrose or high-fat diet-induced insulin resistance model (11, 12, 15, 31) and confirm a pivotal role of Akt in the regulation of glucose metabolism and myocardial function (25, 41, 58).
In our hands, Akt2KO-induced insulin resistance is associated with a significant reduction in both plasma and tissue H2S levels, consistent with the findings from both genetically predisposed (nonobese diabetic) (5) and chemically induced (STZ) (24) experimental diabetes models. After the 10-day NaHS treatment, Akt2KO-induced decrease in plasma and tissue H2S levels (or synthesis rate) was significantly attenuated or ablated, in a manner somewhat consistent with the finding reported in experimental diabetes (32).
Perhaps the most intriguing finding from our study is that H2S supplementation effectively ameliorated Akt2KO-induced glucose intolerance and myocardial anomalies. Treatment of H2S for 10 days was effective in reversing myocardial contractile and intracellular Ca2+ defects in Akt2KO mice. Our study also depicted that H2S supplementation attenuated Akt2KO-induced insulin resistance, myocardial apoptosis, and mitochondrial injury. These findings denote a possible beneficial role for H2S supplementation for cardiac anomalies under insulin resistance and possibly Type 2 diabetes mellitus. These results are in line with the earlier report that H2S may facilitate glucose uptake, insulin receptor sensitivity, and phosphorylation of PI3K/Akt in muscles (56). Beneficial properties of chronic H2S supplementation are also present in vasculatures, including promoted migration and tube formation in vascular endothelial cells (6). Given the multiple protective roles for H2S in the cardiovascular system (8, 45, 53, 61), it is plausible to speculate that reduced circulating and cardiac tissue H2S levels may contribute to the development of insulin resistance-induced myocardial anomalies. Nonetheless, further study is warranted to elucidate the mechanism of action behind H2S deficiency-induced cardiac pathology.
Although the precise mechanism(s) of action behind H2S supplement ion's protective action remains somewhat elusive, several possible scenarios may be considered. Our findings exhibited that H2S is capable of alleviating Akt2KO-induced mitochondrial injury. Emerging evidence has indicated a unique role for mitochondrial integrity in the pathogenesis and management of cardiac dysfunction in insulin resistance (38). Mitochondria exerts a key role in the control of energy metabolism, cell survival, and myocardial function (38). Our observation of reduced aconitase levels and elevated release of cytochrome c, an essential component of the electron transport chain in mitochondria (51), in hearts from Akt2KO mice supports a role of mitochondrial injury in insulin resistance-triggered cardiac anomalies. Adequate Akt signaling is indispensable in the regulation of mitochondrial function in the heart. The Akt downstream signaling molecules hexokinase and Pim-1 kinase are known to preserve mitochondrial function (47). Moreover, Akt suppresses mitochondrial permeation pore (mPTP) opening, thus protecting mitochondrial integrity via phosphorylation of GSK-3β (60). In our study, NAD+ was measured to indirectly assess mPTP opening. Di Lisa et al. (10) reported that cyclosporin A, a potent mPTP inhibitor, prevents NAD+ depletion, considering that NAD+ loss can be directly attributed to mPTP opening. In our hands, knockout of Akt2 lowered phosphorylation of PTEN, which would lead to a higher kinase activity of PTEN, a negative regulator of Akt. This effect, in conjunction with dampened Akt phosphorylation, contributes to the Akt2KO-induced GSK-3β dephosphorylation, en route to mitochondrial injury. H2S supplementation reversed or partially restored Akt2KO-induced loss of phosphorylation in PTEN, Akt, and GSK-3β to exert its beneficial effect on preservation of mitochondrial integrity in the heart. Our in vitro finding that mitochondrial uncoupler FCCP mitigated H2S-induced beneficial effects further strengthened a cause-effect relationship of mitochondrial integrity in H2S-offered protection.
Our results also demonstrated that H2S exerts protective effects against insulin resistance-induced apoptosis of cardiomyocytes, in a manner similar to mechanical and intracellular Ca2+ responses, favoring a role of lessened apoptosis in H2S-offered beneficial effect in Akt2KO mice. In addition, data from cytochrome c release further supported H2S-induced antiapoptotic responses. As an intermediate in apoptosis and a controlled form of cell death in the process of development or in response to infection or DNA damage (33), cytochrome c is released from the mitochondria in response to proapoptotic stimuli (3). This release of cytochrome c, in turn, activates caspase-9, and subsequently caspase-3, leading to ultimate cell death (19). Our finding of elevated levels of caspase-3, caspase-9, and caspase-12 along with caspase-3 activity supports a likely role for apoptosis in Akt2KO-induced cardiac mechanical anomalies. Along the same line, H2S was found to protect against myocardial injury in diabetes through alleviating apoptosis and oxidative stress (36, 48). Finally, H2S is considered a classic cytochrome c oxidase inhibitor and an in vitro oxidase substrate (35). Similar to other low-molecular-weight messengers and oxidase inhibitors, including NO and CO, H2S may bind to oxidase sites prior to oxidization to generate persulfide species. Thus, sulfide may rapidly inhibit mitochondrial cytochrome c oxidase (35). It is possible that persulfidation of phosphatases and/or inhibition of mitochondrial cytochrome c oxidase may contribute to H2S-induced beneficial effects, although this is beyond the scope of this study.
Perspectives and Significance
Insulin resistance is a major independent risk factor for diabetes and cardiovascular diseases. Our article reveals that H2S may provide rescue from glucose intolerance and cardiac contractile and intracellular Ca2+ anomalies in Akt2KO-induced insulin resistance, depicting a favorable role for H2S in insulin resistance and associated cardiac complications. Ample data have revealed low levels of H2S in insulin resistance and diabetes, which may contribute to cardiac complications in these comorbidities (36). Given the impaired PI3K/Akt signaling in insulin resistance and diabetes mellitus, the jury is still out as to whether decreased H2S levels are directly responsible for compromised insulin sensitivity and ultimately insulin resistance. This study, using Akt2KO model, provides the first evidence that H2S supplementation is sufficient to reverse insulin resistance-induced cardiomyopathy. Our results support the notion that H2S benefits cardiac function in insulin resistance through alleviation of PTEN-Akt-GSK3β-mediated mitochondrial injury and apoptosis. Since evidence from human subjects is still lacking with regard to the interplay between Akt2 gene polymorphism and cardiac function, caution must be taken when extrapolating the H2S findings obtained using the Akt2 ablation murine model to the more broadly defined “insulin resistance” model.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: N.H. conception and design of research; N.H. and M.D. performed experiments; N.H. and J.R. prepared figures; N.H. and J.R. edited and revised manuscript; N.H. and J.R. approved final version of manuscript; M.D. and J.R. analyzed data.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants INBRE P20-RR-16474 and P20-GM-103432, as well as the National Natural Science Foundation of China (Grant 81372055).
REFERENCES
- 1.Amorim PA, Nguyen TD, Shingu Y, Schwarzer M, Mohr FW, Schrepper A, Doenst T. Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression. J Thorac Cardiovasc Surg 140: 1160–1167, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Ang SF, Moochhala SM, Bhatia M. Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit Care Med 38: 619–628, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol 159: 923–929, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 51: 1775–1783, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Brancaleone V, Roviezzo F, Vellecco V, De Gruttola L, Bucci M, Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br J Pharmacol 155: 673–680, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ceylan-Isik AF, Zhao P, Zhang B, Xiao X, Su G, Ren J. Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J Mol Cell Cardiol 48: 367–378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 34: 573–585, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292: 1728–1731, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem 276: 2571–2575, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Dong F, Fang CX, Yang X, Zhang X, Lopez FL, Ren J. Cardiac overexpression of catalase rescues cardiac contractile dysfunction induced by insulin resistance: Role of oxidative stress, protein carbonyl formation and insulin sensitivity. Diabetologia 49: 1421–1433, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Dong F, Li Q, Sreejayan N, Nunn JM, Ren J. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: role of peroxisome proliferator activated receptor gamma coactivator 1α and mitochondrial biogenesis. Diabetes 56: 2201–2212, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Dong F, Zhang X, Culver B, Chew HG, Jr, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond) 109: 277–286, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans 35: 231–235, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Fang CX, Dong F, Ren BH, Epstein PN, Ren J. Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B, PPARγ and c-Jun. Diabetologia 48: 2412–2421, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3β. Anesthesiology 103: 987–995, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Friedrichsen M, Birk JB, Richter EA, Ribel-Madsen R, Pehmoller C, Hansen BF, Beck-Nielsen H, Hirshman MF, Goodyear LJ, Vaag A, Poulsen P, Wojtaszewski JF. Akt2 influences glycogen synthase activity in human skeletal muscle through regulation of NH2-terminal (sites 2 + 2a) phosphorylation. Am J Physiol Endocrinol Metab 304: E631–E639, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, Dunger DB, Barford D, Umpleby AM, Wareham NJ, Davies HA, Schafer AJ, Stoffel M, O'Rahilly S, Barroso I. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304: 1325–1328, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal 23: 1515–1527, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu N, Guo R, Han X, Zhu B, Ren J. Cardiac-specific overexpression of metallothionein rescues nicotine-induced cardiac contractile dysfunction and interstitial fibrosis. Toxicol Lett 202: 8–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu N, Han X, Lane EK, Gao F, Zhang Y, Ren J. Cardiac-specific overexpression of metallothionein rescues against cigarette smoking exposure-induced myocardial contractile and mitochondrial damage. PloS One 8: e57151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal 12: 1333–1337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji L, Fu F, Zhang L, Liu W, Cai X, Zheng Q, Zhang H, Gao F. Insulin attenuates myocardial ischemia/reperfusion injury via reducing oxidative/nitrative stress. Am J Physiol Endocrinol Metab 298: E871–E880, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Kao DP, Witteles RM, Quon A, Wu JC, Gambhir SS, Fowler MB. Rosiglitazone increases myocardial glucose metabolism in insulin-resistant cardiomyopathy. J Am Coll Cardiol 55: 926–927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh KK, Oh PC, Quon MJ. Does reversal of oxidative stress and inflammation provide vascular protection? Cardiovasc Res 81: 649–659, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Lastra G, Manrique C. The expanding role of oxidative stress, renin angiotensin system, and beta-cell dysfunction in the cardiometabolic syndrome and Type 2 diabetes mellitus. Antioxid Redox Signal 9: 943–954, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lefer DJ. Potential importance of alterations in hydrogen sulphide (H2S) bioavailability in diabetes. Br J Pharmacol 155: 617–619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal 8: 1765–1774, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Li L, Hua Y, Ren J. Short-chain fatty acid propionate alleviates Akt2 knockout-induced myocardial contractile dysfunction. Exp Diabetes Res 2012: 851717, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Liu F, Chen DD, Sun X, Xie HH, Yuan H, Jia W, Chen AF. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Lowicka E, Beltowski J. Hydrogen sulfide (H2S)—the third gas of interest for pharmacologists. Pharmacol Rep 59: 4–24, 2007 [PubMed] [Google Scholar]
- 35.Nicholls P, Marshall DC, Cooper CE, Wilson MT. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem Soc Trans 41: 1312–1316, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Peake BF, Nicholson CK, Lambert JP, Hood RL, Amin H, Amin S, Calvert JW. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am J Physiol Heart Circ Physiol 304: H1215–H1224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Relling DP, Esberg LB, Fang CX, Johnson WT, Murphy EJ, Carlson EC, Saari JT, Ren J. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J Hypertens 24: 549–561, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med 88: 993–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds THt Merrell E, Cinquino N, Gaugler M, Ng L. Disassociation of insulin action and Akt/FOXO signaling in skeletal muscle of older Akt-deficient mice. Am J Physiol Regul Integr Comp Physiol 303: R1186–R1194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saga Y, Hashimoto H, Yachiku S, Iwata T, Tokumitsu M. Reversal of acquired cisplatin resistance by modulation of metallothionein in transplanted murine tumors. Int J Urol 11: 407–415, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Semple D, Smith K, Bhandari S, Seymour AM. Uremic cardiomyopathy and insulin resistance: a critical role for Akt? J Am Soc Nephrol 22: 207–215, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Sharma N, Arias EB, Sajan MP, MacKrell JG, Bhat AD, Farese RV, Cartee GD. Insulin resistance for glucose uptake and Akt2 phosphorylation in the soleus, but not epitrochlearis, muscles of old vs. adult rats. J Appl Physiol 108: 1631–1640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen X, Carlstrom M, Borniquel S, Jadert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen YH, Zhang L, Gan Y, Wang X, Wang J, LeMaire SA, Coselli JS, Wang XL. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem 281: 7727–7736, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R, Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock 31: 267–274, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Sussman MA. Mitochondrial integrity: preservation through Akt/Pim-1 kinase signaling in the cardiomyocyte. Expert Rev Cardiovasc Ther 7: 929–938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci USA 108: 13829–13834, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 17: 68–80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tafani M, Karpinich NO, Hurster KA, Pastorino JG, Schneider T, Russo MA, Farber JL. Cytochrome c release upon Fas receptor activation depends on translocation of full-length bid and the induction of the mitochondrial permeability transition. J Biol Chem 277: 10073–10082, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 12: 1065–1077, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Zhang Y, Guo J, Jin K, Li J, Guo X, Scott GI, Zheng Q, Ren J. Inhibition of protein kinase C βII isoform rescues glucose toxicity-induced cardiomyocyte contractile dysfunction: role of mitochondria. Life Sci 93: 116–124, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstadt H, Tavare JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue R, Hao DD, Sun JP, Li WW, Zhao MM, Li XH, Chen Y, Zhu JH, Ding YJ, Liu J, Zhu YC. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid Redox Signal 19: 5–23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao LL, Huang XW, Wang YG, Cao YX, Zhang CC, Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3β-dependent opening of mPTP. Am J Physiol Heart Circ Physiol 298: H1310–H1319, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Yu Q, Gao F, Ma XL. Insulin says NO to cardiovascular disease. Cardiovasc Res 89: 516–524, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Zavaczki E, Jeney V, Agarwal A, Zarjou A, Oros M, Katko M, Varga Z, Balla G, Balla J. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int 80: 731–739, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Xia Z, La Cour KH, Ren J. Activation of Akt rescues endoplasmic reticulum stress-impaired murine cardiac contractile function via glycogen synthase kinase-3β-mediated suppression of mitochondrial permeation pore opening. Antioxid Redox Signal 15: 2407–2424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can J Physiol Pharmacol 85: 1248–1253, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Zhu J, Rebecchi MJ, Tan M, Glass PS, Brink PR, Liu L. Age-associated differences in activation of Akt/GSK-3β signaling pathways and inhibition of mitochondrial permeability transition pore opening in the rat heart. J Gerontol Ser A, Biol Sci Med Sci 65: 611–619, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Zhuo Y, Chen PF, Zhang AZ, Zhong H, Chen CQ, Zhu YZ. Cardioprotective effect of hydrogen sulfide in ischemic reperfusion experimental rats and its influence on expression of survivin gene. Biol Pharmaceut Bull 32: 1406–1410, 2009 [DOI] [PubMed] [Google Scholar]