Abstract
Nesfatin-1 is produced in the periphery and in the brain where it has been demonstrated to regulate appetite, stress hormone secretion, and cardiovascular function. The anorexigenic action of central nesfatin-1 requires recruitment of neurons producing the melanocortins and centrally projecting oxytocin (OT) and corticotropin-releasing hormone (CRH) neurons. We previously have shown that two components of this pathway, the central melanocortin and oxytocin systems, contribute to the hypertensive action of nesfatin-1 as well. We hypothesized that the cardiovascular effect of nesfatin-1 also was dependent on activation of neurons expressing CRH receptors, and that the order of activation of the melanocortin-CRH-oxytocin circuit was preserved for both the anorexigenic and hypertensive actions of the peptide. Pretreatment of male rats with the CRH-2 receptor antagonist astressin2B abrogated nesfatin-1-induced increases in mean arterial pressure (MAP). Furthermore, the hypertensive action of CRH was blocked by pretreatment with an oxytocin receptor antagonist ornithine vasotocin (OVT), indicating that the hypertensive effect of nesfatin-1 may require activation of oxytocinergic (OTergic) neurons in addition to recruitment of CRH neurons. Interestingly, we found that the hypertensive effect of α-melanocyte stimulating hormone (α-MSH) itself was not blocked by either astressin2B or OVT. These data suggest that while α-MSH-producing neurons are part of a core melanocortin-CRH-oxytocin circuit regulating food intake, and a subpopulation of melanocortin neurons activated by nesfatin-1 do mediate the hypertensive action of the peptide, α-MSH can signal independently from this circuit to increase MAP.
Keywords: nesfatin-1, melanocortin, central control of blood pressure, oxytocin, CRH
hypertension, a major comorbidity of obesity, is present in over 50% of overweight and obese patients (19). While the etiology of obesity-associated hypertension is not fully understood, many obese patients exhibit an elevation in sympathetic nervous system (SNS) activity, as measured by microneurography (38) or elevated serum catecholamines (39). It is unclear whether the relationship between obesity and sympathoactivation is causal or correlative; however, several potential mechanisms have been proposed for their co-occurrence. One such hypothesis centers on the role of the adipocyte-derived hormone leptin, which acts centrally to inhibit food intake and increase SNS activity and subsequently mean arterial pressure (MAP). It has been suggested that in the setting of obesity, a state of leptin hypersecretion (32), individuals develop selective resistance to leptin, wherein they are incapable of responding to the anorexigenic effect of the peptide, yet continue to exhibit leptin-induced sympathoactivation (26, 27).
In addition to leptin, adipocytes produce a multitude of paracrine and endocrine factors called adipokines, including the peptide hormone nesfatin-1. Like leptin, nesfatin-1 is found in high circulating levels in obese patients (35, 46) and potently inhibits food intake (20, 43). Nesfatin-1 has been shown to exert its anorexigenic action independently from leptin, since nesfatin-1 inhibited food intake in Zucker fatty rats (20), and nesfatin-1 midsegment significantly reduced food intake in the setting of leptin resistance (31). Single nucleotide polymorphisms in the nesfatin-1 gene have been linked to human obesity (47), and, in addition, nesfatin-1 was shown to elevate MAP (43) and renal sympathetic nerve activity (36). These data indicate that nesfatin-1 may play a role in the development of obesity-associated hypertension.
Interestingly, both leptin and nesfatin-1 must interact with the central melanocortin system to exert their anorexigenic and hypertensive actions (4, 20, 43). The central melanocortin system is an essential neural circuit that integrates nutritional status information from both central and peripheral sources and interacts with downstream effector systems to initiate appropriate behavioral responses (5). Recent evidence suggests that the central melanocortin system contributes to the control of cardiovascular function (5), and hyperstimulation of this system may underlie the development of hypertension in several animal models (7, 30). Thus the selective resistance (anorexigenic but not autonomic) of obese individuals to adipokines, such as leptin and nesfatin-1, may reflect changes at the level at the central melanocortin system, in which select populations of melanocortin neurons, particularly those responsible for regulating appetite, become resistant to peripherally derived energy signals.
The anorexigenic action of nesfatin-1 has been shown to be dependent on central corticotrophin-releasing hormone (CRH) receptors (33) and central oxytocin (OT) receptors (16, 44), in addition to the central melanocortin system (20, 43). We previously have shown that the hypertensive action of nesfatin-1 was also dependent on the central OT system (44), and we sought to determine whether activation of central CRH receptors was essential for this effect of nesfatin-1 as well. In addition, both CRH and OT have been shown to act as downstream mediators of melanocortin action, therefore, we sought to determine whether these systems formed a circuit with the central melanocortin system to regulate acute changes in MAP. Surprisingly, we uncovered two parallel circuits that lie downstream of the central melanocortin system, and differential activation of those circuits may underlie selective resistance to adipokines, such as leptin and nesfatin-1.
MATERIALS AND METHODS
Animals
All procedures and protocols have been approved by the Saint Louis University Animal Care and Use Committee (protocol no. 2041). Male Sprague-Dawley rats (Harlan, Indianapolis, IN) (230–250 g, 7–8 wk of age) were anesthetized with a mixture of ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA)-xylazine (TranquiVed, Vedco, St. Joseph, MO) intraperitoneally (60 mg ketamin-8 mg Xylazine per ml, 0.1 ml/100 g body wt), and a stainless steel cannula (23 gauge, 17 mm) was inserted into the right lateral cerebroventricle (intracerebroventricular) using a stereotaxic device (stereotaxic coordinates: A, +6.2; H, +7.4; L, −0.9; relative to the interaural line) (23), as previously described (43). A separate group of animals was implanted with a stainless steel cannula into the fourth ventricle (4V, stereotaxic coordinates: A, −2.7; H, +2.5; L, 0.0; relative to the interaural line) (23). Buprenorphine (0.05 mg/kg) was administered subcutaneously on the day of surgery for postoperative pain, and 10 ml sterile saline (0.9% NaCl) was injected subcutaneously to compensate for anticipated postsurgical fluid loss. After a minimum of 5 days of recovery, rats again were anesthetized and an additional cannula (PE-50) was implanted into the left carotid artery (43). The cannula was filled with heparinized saline (200 U/ml in sterile, 0.9% NaCl) and exteriorized between the shoulder blades. Experiments were conducted the day following carotid cannulation.
Blood Pressure Monitoring
One day after implantation of the carotid cannula, rats were habituated to a quiet testing room for at least 2 h. The carotid catheter was connected to a pressure transducer (Digi-Med BPA, Micro-Med, Louisville, KY), and the catheter was flushed with heparinized saline (200 U/ml in sterile, 0.9% NaCl). Baseline MAP was recorded at 1-min intervals for at least 30 min.
Lateral ventricle injections.
Rats bearing intracerebroventricular cannulas were pretreated with either saline vehicle (2 μl), or vehicle containing the OT receptor antagonist ornithine vasotocin (OVT) (10 μg) (44), or the CRH type 2 receptor antagonist astressin2B (30 μg) (33). Ten minutes later, rats received an intracerebroventricular injection of either saline vehicle (2 μl), or vehicle containing 180 pmole nesfatin-1 (43), 0.1, 0.5, or 1 nmole α-melanocyte stimulating hormone (α-MSH) (44), or 20 pg CRH (dose determined in preliminary experiments).
Fourth ventricular injections.
Rats bearing fourth ventricular (4V) cannulas were pretreated with either saline or vehicle containing the melanocortin 3/4 receptor antagonist SHU9119 (300 pmole) (39), 10 min before with either saline vehicle or 30 ng OT (44). MAP was recorded for at least 60 min at 1-min intervals.
Peptides and Antagonists
Nesfatin-1, α-MSH, OT, CRH, OVT, and SHU9119 were purchased from Phoenix Pharmaceuticals (Burlingame, CA). Astressin2B was purchased from Tocris Biosciences (Bristol, UK).
Data Analysis and Statistics
Data are presented as change from preinjection baseline, calculated as the average MAP or heart rate for 5 min before intracerebroventricular injection, to account for the natural variation in resting cardiovascular parameters between animals. Changes in MAP are shown as area under the curve. Data were analyzed using a nonparametric test (Mann-Whitney U) because data were transformed (45).
RESULTS
Hypertensive Action of Nesfatin-1 (Intracerebroventricular) is Reversed by Pretreatment With a CRH Receptor Antagonist
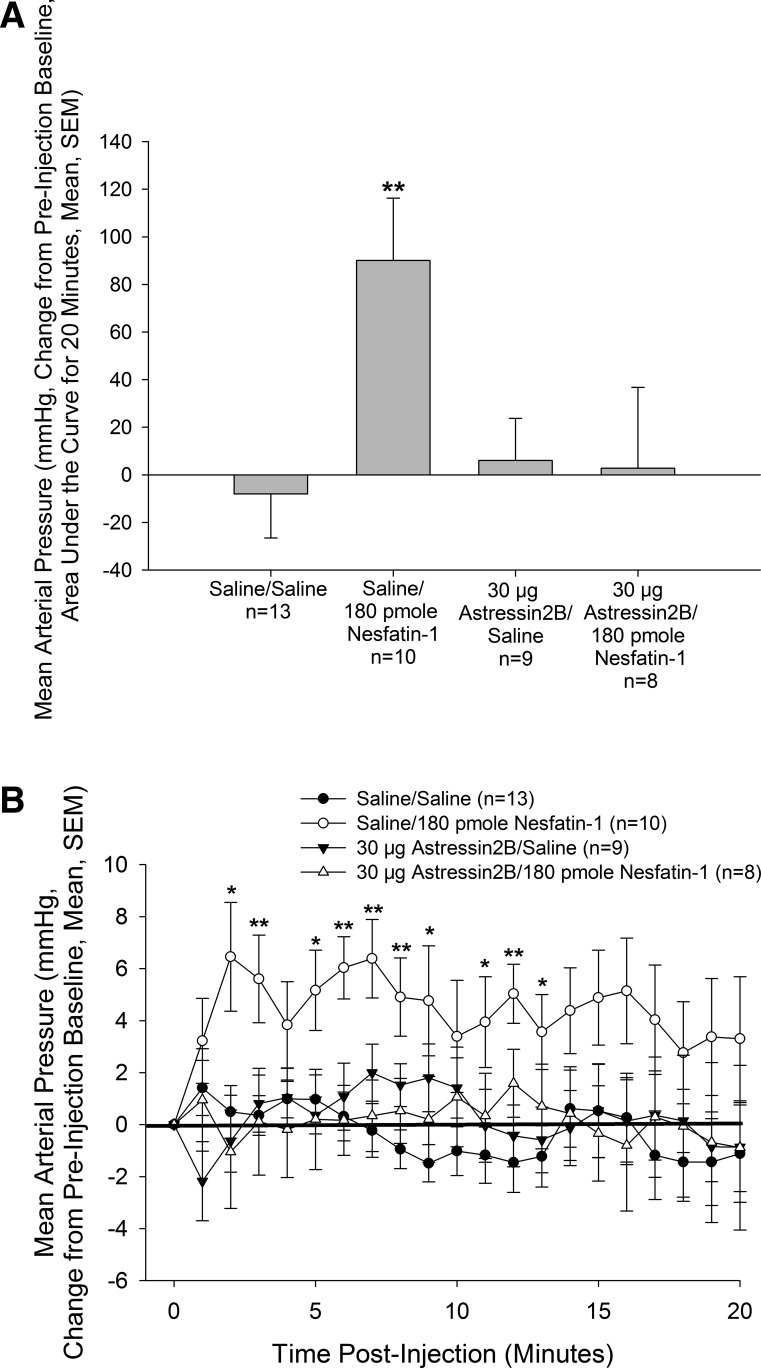
As demonstrated previously (43), treatment with nesfatin-1 elicited a significant increase in MAP (Fig. 1) in animals pretreated with saline vehicle. Although astressin2B did not alter basal MAP, pretreatment with the antagonist completely abrogated the hypertensive action of nesfatin-1 when observed as area under the curve (Fig. 1A) or minute-to-minute traces (Fig. 1B).
Fig. 1.
Hypertensive effect of intracerebroventricular (icv) nesfatin-1 is dependent on corticotropin-releasing hormone (CRH) type 2 receptors. Male rats bearing icv and carotid cannulas were pretreated icv with vehicle or the CRH type 2 receptor antagonist astressin2, before icv treatment with either vehicle or vehicle containing 180 pmole nesfatin-1. Pretreatment with astressin2B completely blocked the effect of nesfatin-1 on mean arterial pressure. Data are presented as changes in mean arterial pressure compared with preinjection baseline, area under the curve (A), or as minute-to-minute traces (B). *P < 0.05, **P < 0.01 vs. Saline/Saline-injected control animals.
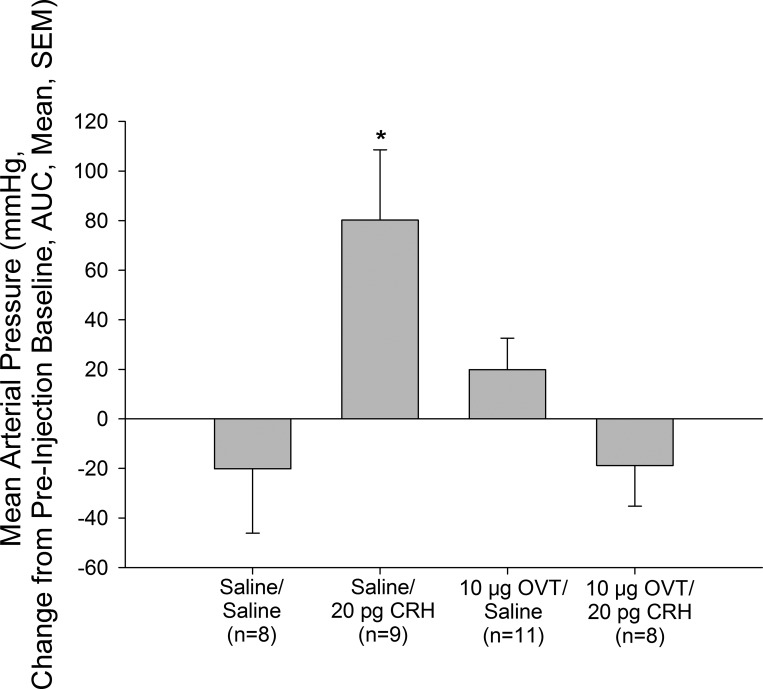
CRH-Induced Increase in MAP is Blocked by Pretreatment With OVT When Injected Intracerebroventricularly
Central injection of CRH resulted in a significant increase in MAP (Fig. 2). Administration of OVT by itself did not significantly alter MAP; however, the central hypertensive effect of CRH was completely abolished by pretreatment with OVT (Fig. 2).
Fig. 2.
CRH acts centrally to increase mean arterial pressure through central oxytocin receptors. Rats were pretreated icv with either vehicle or the oxytocin receptor antagonist ornithine vasotocin (OVT) (10 μg) before central injection with either saline vehicle or a hypertensive dose (20 pg) of CRH. Blockade of oxytocin receptors abrogated the effect of CRH on mean arterial pressure. Data are presented as changes in mean arterial pressure compared with preinjection baseline and area under the curve. *P < 0.05 vs. Saline/Saline-injected control animals.
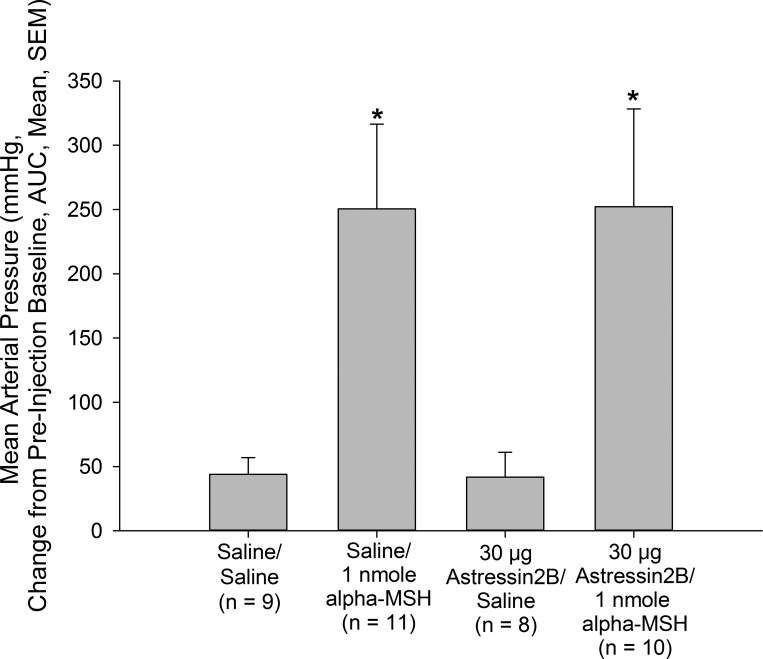
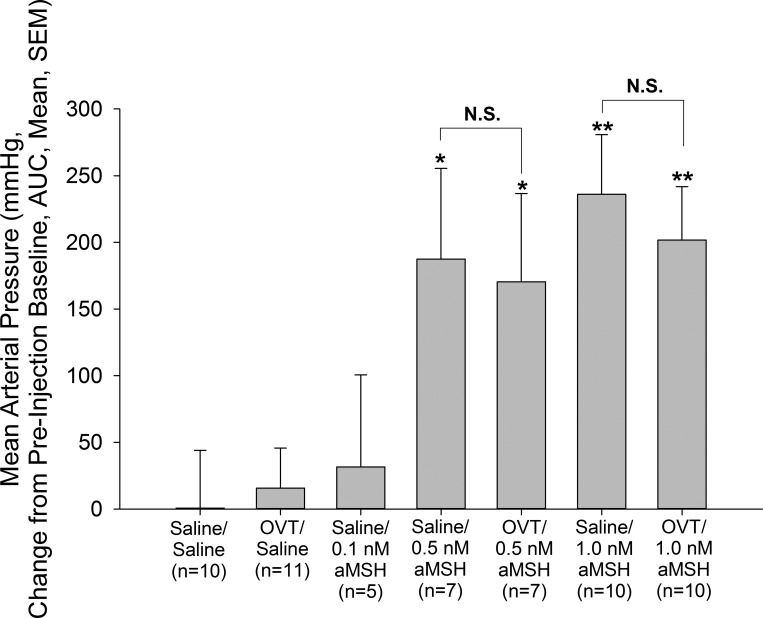
Hypertensive Action of Intracerebroventricular α-MSH is not Reversed by Astressin2B or OVT
Central administration of 0.5 or 1 nmole, but not 0.1 nmole, α-MSH resulted in significant increases in MAP (Figs. 3 and 4) compared with vehicle-pretreated, vehicle-treated control animals. This effect was not blocked by pretreatment with either astressin2B (Fig. 3) or OVT (Fig. 4). As in the previous experiments, neither astressin2B nor OVT altered basal MAP.
Fig. 3.
Hypertensive action of α-MSH does not require functional CRH type 2 receptors. Male rats bearing icv and carotid cannulas were pretreated icv with vehicle or the CRH type 2 receptor antagonist astressin2B (30 μg) before icv treatment with either vehicle or vehicle containing 1 nmole α-MSH. Pretreatment with astressin2B did not alter the effect of α-MSH on mean arterial pressure. Data are presented as changes in mean arterial pressure compared with preinjection baseline and area under the curve. *P < 0.05 vs. Saline/Saline-injected control animals. No significant difference was observed between Saline/α-MSH- and atressin2B/α-MSH-treated rats.
Fig. 4.
Blockade of central oxytocin receptors does not affect α-MSH-induced increase in MAP. Rats were pretreated icv with either vehicle or the oxytocin receptor antagonist OVT (10 μg) before central injection with either saline vehicle or a 0.1, 0.5, or 1.0 nmole α-MSH. Blockade of oxytocin receptors did not abrogate the effect of α-MSH on mean arterial pressure. Data are presented as changes in mean arterial pressure compared with preinjection baseline and area under the curve. *P < 0.05, **P < 0.01 vs. Saline/Saline-injected control animals. No significant difference (N.S.) was observed between Saline/α-MSH- and OVT/α-MSH-treated rats.
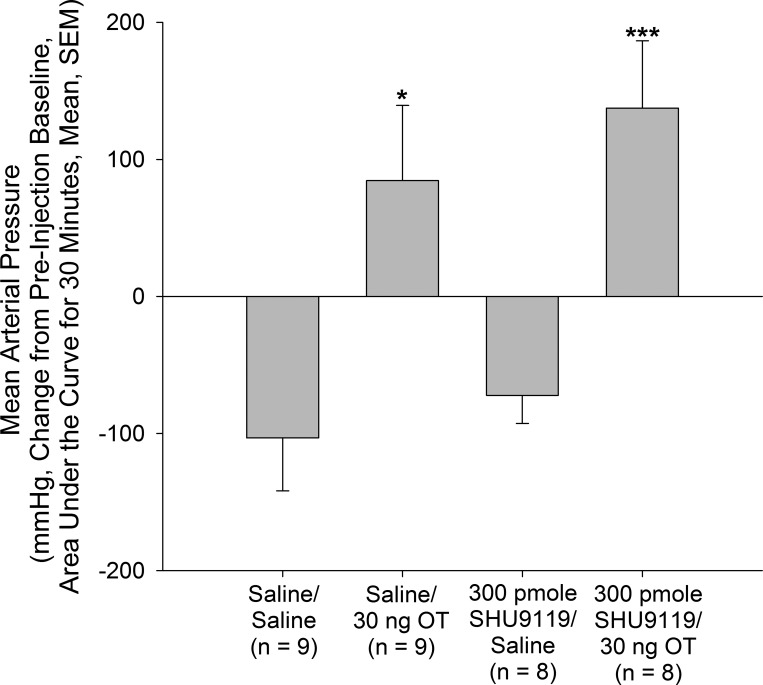
SHU9119 Does Not Inhibit Hypertensive Effect of OT (4V)
The results described above suggested that while the ability of nesfatin-1 to increase MAP depends on activation of a melanocortin-CRH-OT pathway, α-MSH itself can affect autonomic function independent of that circuit. Indeed, it is possible that the circuit activated by nesfatin-1 in hypothalamus communicates via caudally projecting OT neurons to a second population of melanocortin neurons in medulla (12). Third ventricular administration of OT has been shown to inhibit food intake, and this effect was reversed by pretreatment with the melanocortin 3/4 receptor antagonist SHU9119 (16). However, the ability of OT to elevate MAP when administered into the 4V was not prevented in the current experiment by pretreatment with SHU9119 (Fig. 5).
Fig. 5.
Hypertensive action of oxytocin (OT) injected into the fourth ventricle (4V) does not depend on central melanocortin receptors. Rats bearing 4V and carotid cannulas were pretreated via the 4V cannula with either vehicle or the melanocortin 3/4 receptor antagonist SHU9119 (300 pmole) and then injected with vehicle or vehicle containing a hypertensive dose (30 ng) of OT. OT induced a significant increase in mean arterial pressure that was not affected by blockade of central melanocortin receptors by SHU9119. Data are presented as changes in mean arterial pressure compared with preinjection baseline and area under the curve. *P < 0.05, ***P < 0.001 vs. Saline/Saline-injected control animals. No significant difference was observed between Saline/OT- and SHU9119/OT-treated rats.
DISCUSSION
Nesfatin-1 is produced in the brain (3, 10, 11, 20), in particular in the hypothalamus, and nesfatin-1 secreted by peripheral tissues, including stomach, pancreas, and adipose tissue (9, 11, 28, 34), can cross from the circulation into the central nervous system (22, 25). Thus in addition to the well-characterized actions of the peptide on feeding behavior, other potential actions of endogenous nesfatin-1 have been proposed (13, 17, 41). We have demonstrated previously that the action of nesfatin-1 in the brain to elevate MAP was blocked by pretreatment with the melanocortin antagonist SHU9119 (43). Likewise, the anorexigenic action of nesfatin-1 can be blocked by SHU9119 (20, 43). Because a unique receptor for nesfatin-1 has not been identified, we can only infer from our electrophysiology studies (24) that the peptide acts directly to depolarize pro-opiomelanocortin (POMC) neurons in the arcuate nucleus. In those studies we did observe direct, hyperpolarizing actions of nesfatin-1 on neuropeptide Y/agouti-related protein (NPY/AgRP) neurons in the arcuate nucleus, which may have been the basis for the depolarizing actions of the peptide on the neighboring POMC neurons (24). Alternatively, nesfatin-1 could exert direct effects on neurons in the paraventricular or lateral hypothalamic nuclei, since microinjection studies have suggested that nesfatin-1 exerts an anorexigenic effect when injected directly into these sites (16). Recent electrophysiology studies also have identified a potential site of the cardiovascular actions of nesfatin-1 to be the nucleus tractus solitarius (18), where a second population of POMC neurons, that may be responsive to OT, is located (5, 16).
Central administration of nesfatin-1 also activates stress hormone secretion (13, 41), and the anorexigenic action of nesfatin-1 can be blocked by CRH antagonist pretreatment (33). The melanocortin agonist melanotan II (MT II) activated the hypothalamo-pituitary-adrenal axis, and pretreatment with a CRF2 receptor antagonist blocked that activation as well (15), suggesting that the action of nesfatin-1 on stress hormone secretion and food intake might be relayed from POMC neurons to CRH neurons in the PVN, which those authors demonstrated to express melanocortin receptors.
Pretreatment with an OT antagonist also prevented the anorexigenic and autonomic actions of nesfatin-1 (44). A link between POMC and OT neurons in hypothalamus was provided by Sabatier and colleagues (29), and the obese phenotype of Sim-1-deficient mice has been hypothesized to be causally linked to the significant decreases of PVN OT and melanocortin 4 receptor mRNAs in those animals (14, 37). CRH receptors are expressed in OT neurons in the PVN (1, 6). Furthermore, the anorexigenic action of CRH can be blocked by antagonism of central OT receptors (21). Additionally, as noted above, CRH neurons are innervated by POMC neurons, CRH neurons express MC4R receptors (15), and CRH appears to mediate the anorexigenic action of α-MSH (15).
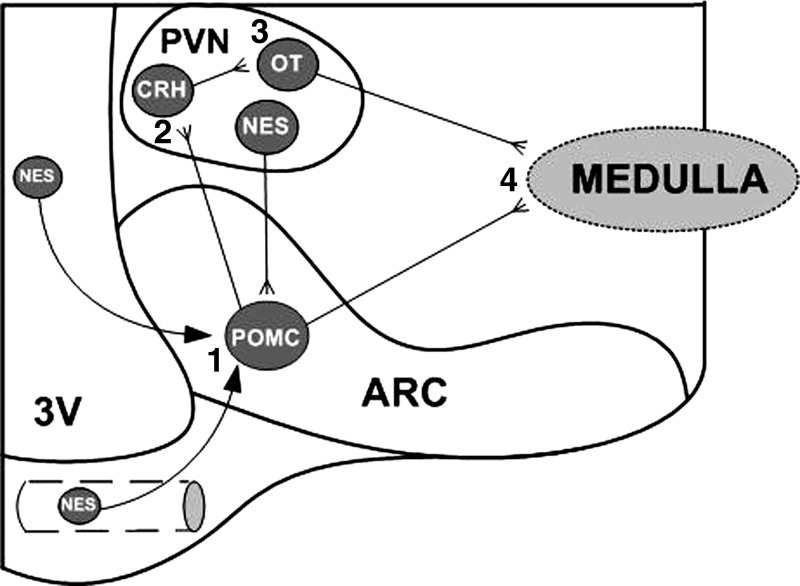
Thus we hypothesized that, like the anorexigenic actions of the peptide, nesfatin-1 exerts its central hypertensive action either by crossing from the circulation into the brain at the level of the arcuate nucleus or via a neural pathway beginning with its action in the arcuate nucleus on POMC neurons (Fig. 6, step 1). Those activated POMC neurons project to and activate CRH neurons in the hypothalamic PVN (Fig. 6, step 2), that relay that message to OT neurons in the same nucleus (Fig. 6, step 3), particularly those that project to brain stem cardiovascular centers (Fig. 6, step 4). If that were the case then antagonism of any step along the circuit should abrogate the hypertensive action of nesfatin-1.
Fig. 6.
Proposed model of the circuit underlying the hypertensive action of nesfatin-1 (NES). NES activates α-MSH-producing proopiomelanocortin (POMC) neurons in the arcuate (ARC) nucleus (1), which project to and stimulate CRH neurons in the hypothalamic paraventricular nucleus (PVN) (2). CRH neurons activate OT neurons in the same nucleus (3), which project to brain stem autonomic centers (4). Alternatively, POMC neurons may directly project to brain stem autonomic centers (4). 3V, third ventricle.
As mentioned above, the OT receptor antagonist OVT prevented the ability of nesfatin-1 given into the lateral cerebroventricle to increase MAP in conscious male rats (44). We then moved one step back in the proposed circuit and demonstrated that antagonism of the CRH2 receptors, with astressin2B, also prevented the action of nesfatin-1 to increase MAP. But does that mean that OT neurons necessarily transmit the information conveyed to the CRH neurons by the action of nesfatin-1? We propose that to be the case since antagonism of central OT receptors blocked the increase in MAP observed following CRH alone. Thus we provide here evidence for a neural circuit activated by nesfatin-1 that is initiated by an action on POMC producing neurons in the arcuate nucleus, going through a population of CRH neurons to caudally projecting OT neurons involved in the central control of autonomic function (12). Indeed, injection of OT into the fourth cerebroventricle directly adjacent to those brain stem cardiovascular centers, where OT receptors have been identified (40, 42), resulted in increased MAP.
The anorexigenic action of OT administered into the third cerebroventricle has been reported to be blocked by local SHU9119 administration (16). Could it then be that the proposed circuit that began with the action of nesfatin-1 on melanocortin neurons in arcuate nucleus was finally expressed by an OT-dependent relay to the other population of POMC neurons, those located in the nucleus tractus solitarius? This may not to be the case, since 4V administration of the melanocortin antagonist SHU9119 did not block the hypertensive action of OT administered into the same fluid cavity.
Our experiments also have uncovered evidence for at least two separate neural circuits through which α-MSH can increase MAP. One clearly responds to nesfatin-1 administration recruiting CRH- and OT-producing neurons in the hypothalamus. There must be, however, a second melanocortin pathway that activates autonomic function because the ability of centrally administered α-MSH, unlike that released endogenously in response to nesfatin-1, could not be blocked by antagonism of either CRH2 or OT receptors. Those pathways may originate in POMC-producing neurons in the arcuate nucleus independent of those activated by nesfatin-1 (Fig. 6) or in the second population of central nervous system neurons that produce POMC, in the nucleus tractus solitarius. Regardless, aberrations in melanocortin signaling in the setting of obesity have been demonstrated (8) and thus may contribute to the comorbidities of obesity.
Perspectives and Significance
While it is unclear if that second population of POMC-producing neurons also might be responsive to the major adipokine circulating in plasma, leptin, we would suggest that the melanocortin neurons that mediate the anorexigenic action of leptin, and perhaps nesfatin-1, and which develop resistance to chronically elevated levels of these adipokines, are not the same neurons that mediate the hypertensive actions of leptin and nesfatin-1. Furthermore, we suggest that this is the reason for the maintained sympathostimulatory actions of at least leptin in the obese state (26, 27). Future studies should focus on the identification of those two potentially distinct populations of POMC-producing neurons, whether they are located within the same nucleus as those responsible for the anorexigenic actions of the adipokines or those located caudally that may be more related to central cardiovascular control. Finally our proposed circuit does not identify the downstream target of those OT neurons or even their location. Our intent is to examine both the medullary cardiovascular centers where OT receptors are expressed and temporal lobe structures where OT binding is prominent (40). Additionally, we intend to examine the possible requirement for OT activation of brain-derived neurotrophic factor neurons in the proposed circuit since brain-derived neurotrophic factor has been suggested to be a downstream target of melanocortins in other neural systems (2).
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant HL-066023 and the Midwest Affiliate of the American Heart Association Grant 10GRNT4470043.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.L.C.Y. and W.K.S. conception and design of research; G.L.C.Y. and W.K.S. performed experiments; G.L.C.Y. analyzed data; G.L.C.Y. and W.K.S. interpreted results of experiments; G.L.C.Y. prepared figures; G.L.C.Y. and W.K.S. drafted manuscript; G.L.C.Y. and W.K.S. edited and revised manuscript; G.L.C.Y. and W.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Trini Vargas for his artistic assistance in rendering Fig. 6.
REFERENCES
- 1.Arima H, Aguilera G. Vasopressin and oxytocin neurons of hypothalamic supraoptic and paraventricular nuclei co-express mRNA for type-1 and type-2 corticotropin-releasing hormone receptors. J Neuroendocrinol 12: 833–842, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Bariohay B, Roux J, Tardivel C, Trouslard J, Jean A, Lebrun B. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology 150: 2646–2653, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1 distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148: 5088–5094, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138: 4489–4492, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotropin-releasing factor and oxytocin systems in the hypothalamus and the bed bucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocinology 36: 1312–1326, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Silva AA, do Carmo JM, Hall JE. Role of leptin and central nervous system melanocortins in obesity hypertension. Curr Opin Nephrol Hypertens 2: 135–140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemons L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Foo KS, Brauner H, Ostenson CG, Broberger C. Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state. J Endocrinol 204: 255–263, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156: 563–579, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Goebel-Stengel M, Wang L, Stengel A, Tache Y. Localization of nesfatin-1 neurons in mouse brain and functional implication. Brain Res 1396: 20–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jhamandas JH, MacTavish D. Central administration of Neuropeptide FF causes activation of oxytocin paraventricular hypothalamic neurons that project to brainstem. J Neuroendocrinol 15: 24–32, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Konczol K, Bodnar I, Zelena Z, Pinter O, Papp RS, Palkovits M, Najy GM, Toth ZE. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem Int 57: 189–197, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol 22: 1723–1734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 23: 7863–7872, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maejima Y, Sedbazar U, Suyama S, Kohno D, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh -IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10: 355–365, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-behavior in the rat. Psychopharmacology 201: 115–123, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Mimee A, Smith PM, Ferguson AV. Nesfatin-1 influences the excitability of neurons in the nucleus of the solitary tract and regulates cardiovascular function. Am J Physiol Regul Integr Comp Physiol 302: R1297–R1304, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg 207: 928–934, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709–712, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am J Physiol Regul Integr Comp Physiol 260: R448–R452, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides 28: 2223–2228, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (Compact) (3rd ed). San Diego, CA. Academic, 1997 [Google Scholar]
- 24.Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res 1230: 99–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides 28: 2372–2381, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Rahmouni K, Haynes WG. Leptin and the cardiovascular system. Recent Prog Horm Res 59: 225–244, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Ramanjaneya M, Chen J, Brown JE, Tripathi G, Hallschmid M, Patel S, Kern W, Hillhouse EW, Lehnert H, Tan BK, Randeva HS. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology 151: 3169–3180, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Sabatier N, Caqineau C, Dayaithi G, Bull P, Douglas AJ, Guan XMM, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci 23: 10351–10358, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal-Lieberman G, Rosenthal T. Animal models in obesity and hypertension. Curr Hypertens Rep 15: 190–193, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Shimizu H, Oh -IS, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, Okada S, Yamada M, Yada T, Mori M. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology 150: 662–671, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Sinha MK, Opentanova I, Ohannesian JP, Kolaczynski JW, Heiman ML, Hale J, Becker GW, Bowsher RR, Stephens TW, Caro JF. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J Clin Invest 98: 1277–1282, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Moennikes H, Lambrecht NWG, Tache Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying: differential role of corticotropin-releasing-factor2 receptor. Endocrinology 150: 4911–4919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Tache Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 150: 232–238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan BK, Hallschmid M, Kern W, Lehnert H, Randeva HS. Decreased cerebrospinal fluid/plasma ratio of the novel satiety molecule, nesfatin-1/NUCB-2, in obese human: evidence of nesfatin-1/NUCB-2 resistance and implications for obesity treatment. J Clin Endocrinol Metab 96: E669–E673, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Tanida M, Mori M. Nesfatin-1 stimulates renal sympathetic nerve activity in rats. Neuroreport 22: 309–312, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci 30: 3803–3812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trombetta IC, Batalha LT, Urbana M, Rondon PB, Laterza MC, Kuniyoshi FHS, Gowdak MMG, Barretto ACP, Halpern A, Villares SMF, Negrao CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol 285: H974–H982, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Tuck ML. Obesity, the sympathetic nervous system, and essential hypertension. Hypertension 19, Suppl 1: 167–177, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messaneger ribonucleic acids in brain. Endocrinology 139: 5014–5033, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Yoshida N, Maejima Y, Yamada M, Osaki A, Oh -IS, Ariyama Y, Takahashi H, Okada S, Hashimoto K, Kurashina T, Onaka T, Dezaki K, Nakata M, Mori M, Yada T. Stressor-responsive central nesfatin-1 activates corticotropin releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamo-pituiatry-adrenal axis. Aging 2: 775–784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptors expressed in serotonergic neurons in mice. J Neurosci 29: 2259–2271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yosten GLC, Samson WK. Nesfatin-1 exerts cardiovascular effects in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol 297: R330–R336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yosten GLC, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 298: R1642–R1647, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zar JH. Biostatistical Analysis (2nd ed.). Englewood Cliffs, NJ. Prentice Hall, 1984 [Google Scholar]
- 46.Zhang Z, Li L, Yang M, Liu H, Boden G, Yang G. Increased plasma levels of nesfatin-1 in patients with newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 120: 91–95, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Zegers D, Beckers S, Mertens IL, Van Gaal LF, Van Hul W. Association between polymorphisms of the Nesfatin gene, NUCB2, and obesity in men. Mol Genet Metab 103: 282–286, 2011 [DOI] [PubMed] [Google Scholar]