Summary
Background
Passenger lymphocyte syndrome (PLS) is most often a result of an ABO minor mismatch between stem cell or solid organ donor and recipient. Relatively few cases of PLS have been reported resulting from non-ABO red cell antibodies.
Case Report
A blood group O/Rh-positive patient received a stem cell transplant from an A/Rh-negative donor who had an identifiable anti-D. Even though the plasma of the stem cell product was reduced and replaced with an electrolyte solution, the recipient developed a positive antibody screen, positive direct antiglobulin test, and significant hemolysis 8 days after transplantation.
Conclusion
PLS can result from non-ABO antibodies and can be associated with a significant degree of hemolysis.
Key Words: Passenger lymphocyte syndrome, PLS, Hemolysis, Transplantation
Introduction
Passenger lymphocyte syndrome (PLS) is a relatively common complication of ABO-incompatible solid organ and stem cell transplantation. Most commonly PLS is associated with minor ABO mismatches between donor and recipient, in which donor B lymphocytes produce antibodies (e.g. anti-A, anti-B) specific for recipient red cell antigens. Because the HLA system is inherited independently of the ABO system, an ABO mismatch is relatively common and has been reported to occur in 30-40% of all cases. Approximately half of these are classified as minor mismatches, but only 10-15% result in immune hemolysis due to alloantibodies produced by passenger lymphocytes [1]. Clinically PLS is characterized by the abrupt onset of hemolysis beginning 5-15 days after stem cell transplantation. The majority of cases of PLS result from the production of anti-A or anti-B isoagglutinins in an ABO-incompatible transplant [2]; however, a small number of cases have been reported in which non-ABO antibodies have been implicated in PLS. We report a case of severe hemolysis due to PLS caused by the presence of anti-D in a stem cell donor.
Case Report
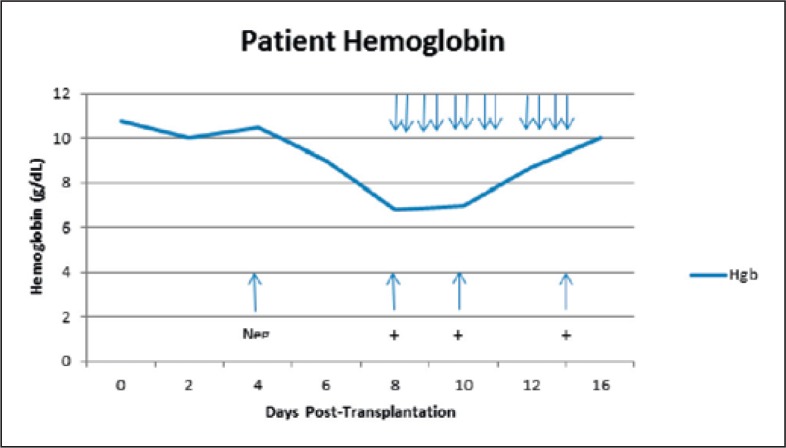
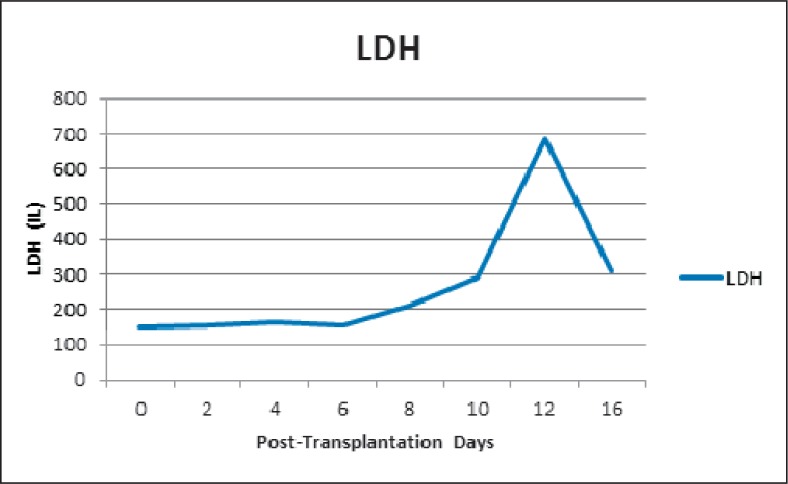
A 58-year-old male reported to the hospital emergency room complaining of progressive weakness and headache. Examination and diagnostic tests revealed that he had chronic myelogenous leukemia in blast crisis. Following initial treatment with imatinib, the patient returned 5 months later for a conditioning regimen using fludarabine/melphalan and a stem cell transplant procedure. The patient was blood group O/Rh(D)-positive, and the sibling donor was a 10-antigen HLA match but was A/Rh(D)-negative. Further, the donor also had an identifiable anti-D antibody as a result of emergency transfusions following a motor vehicle accident several years previously. Due to the presence of anti-D, plasma was removed from the stem cell product and replaced with Plasma-Lyte A® (Baxter Healthcare Corp., Deerfield, IL, USA) and citrate anticoagulant to reduce the risk of hemolysis of the patient's Rh(D)-positive red cells due to anti-D in the donor product. The transplant proceeded without incident, and the patient continued to have a negative antibody screen. However, on day 8 after the transplant the patient was found to have a positive antibody screen and anti-D was identified; the patient also was found to have a positive direct antiglobulin test (DAT) with IgG only; anti-D was eluted from the patient's red cells (table 1). The development of the positive antibody screen (anti-D) and the positive DAT were closely correlated with a significant degree of hemolysis during which the patient's hemoglobin decreased from 10.8 g/dl on the day of transplant to a low of 6.8 g/dl 8 days later (fig. 1). From day 8 to day 15 post-transplant, the patient required the transfusion of 12 units of irradiated O/Rh(D)-negative red cells in order to maintain an adequate hemoglobin level. As further evidence of hemolysis the patient's lactate dehydrogenase (LDH) rose during this period from a low of 148 IU on the day of transplant to a high of 684 IU 12 days later (fig. 2); there was also an increase in total bilirubin over this same time frame, from 0.9 mg/dl on the day of transplant to a high of 5.6 mg/dl 11 days post-transplant. No other cause of hemolysis was identified during the patient's hospitalization. He remained afebrile on all hospital days except 1, and in this case all blood cultures and his chest X-ray were negative. During the post-transplantation period the patient's immunosuppressive therapy included cyclosporine and mycophenolate. He required no transfusions after 15 days post-transplant. However he continued to demonstrate anti-D by tube testing for at least 12 months after stem cell transplantation.
Table 1.
Patient serologic results
| Post-transplant day | ABO Rh(D) | Antibody screen | DAT | Eluate |
|---|---|---|---|---|
| 0 | O/Rh(D)-positive | negative | negative | none |
| 4 | O/Rh(D)-positive | negative | negative | none |
| 8 | O/Rh(D)-positive | positive (IgG only) anti-D | positive | anti-D |
| 10 | O/Rh(D)-positive | positive (IgG only) anti-D | positive | anti-D |
| 15 | O/Rh(D)-negative | positive anti-D | not done | not done |
Fig. 1.
Patient hemoglobin levels. Down-pointing arrows (↓) indicate dates of red blood cell transfusions. Up-pointing arrows (↑) indicate dates and results of antibody screen results for anti-D.
Fig. 2.
Patient LDH levels.
Discussion
Immune hemolysis can occur following ABO-incompatible solid organ or bone marrow transplants. In cases in which there is a ‘minor mismatch’ between donor and recipient, PLS can cause hemolysis due to the presence of donor B lymphocytes in the product (passenger lymphocytes) that retain the ability to actively produce antibodies in the early post-transplant period. The characteristics of PLS were described by Hows et al. [3] and include a positive antibody screen, a positive DAT, and an eluate containing an antibody of the same specificity as that found in the serum that can be detected 1-3 weeks after transplantation. These serologic findings are sometimes accompanied by the abrupt onset of hemolysis 9-16 days after transplantation [3]. In the current case an O/Rh(D)-negative patient received an HLA-matched stem cell transplant from an A/Rh(D)-negative sibling donor who had an anti-D due to previous emergency blood transfusions. In order to minimize the risk of immediate hemolysis due to anti-D, plasma was removed from the stem cell product and replaced with an electrolyte solution and citrate anti-coagulant. Following the transplant, the patient did well and continued to have a negative antibody screen until the 8th day after the transplant when an anti-D was identified in the patient's serum antibody screen and a direct antiglobulin test on the patient's red cells became positive; anti-D was recovered in the eluate of the patient's red cells. The positive antibody screen and positive DAT were associated with hemolysis as demonstrated by a decrease in hemoglobin level (requiring transfusion of 12 units of red cells), an increase in LDH, and an increase in bilirubin.
There are at least 2 possible explanations for the hemolysis seen in this case. One possibility is the simple passive transfer of antibody (anti-D) from the donor stem cell product to the patient. However, this is unlikely in that the product was ‘plasma-reduced’ to minimize the amount of antibody transfused to the patient, and the patient's antibody screen remained negative for the first 7 days following the transplant. A more probable explanation is that this represents an example of PLS in which passenger B lymphocytes in the stem cell product retained the ability to produce anti-D when stimulated by the Rh-positive red cells of the patient. The time frame for the abrupt-onset hemolysis is consistent with that reported for PLS, and the development of an anti-D in the patient's serum and the positive DAT probably represent the production of this antibody by transfused B lymphocytes.
This case is interesting for several reasons. PLS is far more commonly associated with ABO-incompatible stem cell transplants than with other, non-ABO, red cell alloantibodies. Notably, Hows et al. [3] described several cases of anti-D causing hemolysis in bone marrow transplant patients, and Leo et al. [2] described a patient in whom an anti-Jka caused hemolysis following an allogeneic peripheral blood progenitor cell transplant. It is also interesting in this regard that Hows et al. [3] note that while donor-derived antibodies disappear rather quickly (approximately 3 months after transplant) in ABO-associated PLS, anti-D antibodies tend to remain in the patient for a much longer period of time (up to 1 year). This is consistent with the finding in our patient in that the anti-D is still present at least 12 months after transplantation. This case of PLS was also associated with a significant amount of hemolysis. As noted by Worel et al. [1], only 10-15% of marrow transplant recipients who received a minor ABO- or D-mismatched transplant experienced significant hemolysis. The current patient clearly experienced a significant hemolytic episode as demonstrated not only by a decrease in hemoglobin from 10.5 g/dl to 6.8 g/dl over a 5-day period, but also by an increase in LDH and bilirubin. Whether PLS is associated with hemolysis, as well as the severity of that hemolysis, may be dependent upon several factors such as amount of lymphoid tissue transplanted, level of antibody before transplant, a rapid rise in antibody titer in the recipient after transplant, and the rapidity of engraftment [4,5].
This case illustrates several important points that must be considered in managing a blood transfusion at institutions where stem cell (or solid organ) transplantation takes place: i) Minor ABO incompatibility between solid organ or stem cell donor and the intended recipient can result in PLS; ii) PLS can result not only from ABO incompatibility, but other, non-ABO antibodies can also cause PLS; iii) The serologic abnormalities (positive antibody screen; positive DAT) in PLS generally occur 5-15 days after transplantation; and iv) PLS can, in a minority of cases, cause severe hemolysis, often requiring transfusion support.
Disclosure Statement
The author has no conflict of interest to declare.
References
- 1.Worel N, Greinix HT, Keil F, Mitterbauer M, Lechner K, Fischer G, Mayr W, Hocker P, Kalhs P. Severe immune hemolysis after minor ABO-mismatched allogeneic peripheral blood progenitor cell transplantation occurs more frequently after nonmyeloablative than myeloablative conditioning. Transfusion. 2002;42:1293–1301. doi: 10.1046/j.1537-2995.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 2.Leo A, Mytilineos J, Voso MT, Weber-Nordt R, Liebisch P, Lensing C, Schraven B. Passenger lymphocyte syndrome with severe hemolytic anemia due to anti-Jka after allogeneic PBPC transplantation. Transfusion. 2000;40:632–636. doi: 10.1046/j.1537-2995.2000.40060632.x. [DOI] [PubMed] [Google Scholar]
- 3.Hows J, Baddow K, Gordon-Smith E, Branch DR, Spruce W, Sniecinski I, Krance RA, Petz LD. Donor-derived red cell antibodies and immune hemol ysis after allogeneic bone marrow transplantation. Blood. 1986;67:177–181. [PubMed] [Google Scholar]
- 4.Sokol RJ, Stamps R, Booker DJ, Scott FM, Laidlaw ST, Vandenberghe EA, Barker HF. Posttransplant immune-mediated hemolysis. Transfusion. 2002;42:198–204. doi: 10.1046/j.1537-2995.2002.00026.x. [DOI] [PubMed] [Google Scholar]
- 5.Reed M, Yearsley M, Krugh D, Kennedy MS. Severe hemolysis due to passenger lymphocyte syndrome after hematopoietic stem cell transplantation from an HLA-matched related donor. Arch Pathol Lab Med. 2003;127:1366–1368. doi: 10.5858/2003-127-1366-SHDTPL. [DOI] [PubMed] [Google Scholar]