Abstract
Introduction:
Spinal manipulation therapy (SMT) is characterized by specific kinetic and kinematic parameters that can be modulated. The purpose of this study is to investigate fundamental aspects of SMT dose-physiological response relation in humans by varying SMT impulse duration.
Methods:
Twenty healthy adults were subjected to four different SMT force-time profiles delivered by a servo-controlled linear actuator motor and differing in their impulse duration. EMG responses of the left and right thoracic paraspinal muscles (T6 and T8 levels) and vertebral displacements of T7 and T8 were evaluated for all SMT phases.
Results:
Significant differences in paraspinal EMG were observed during the “Thrust phase” and immediately after (“Post-SMT1”) (all T8 ps < 0.01 and T6 during the thrust ps < 0.05). Sagittal vertebral displacements were similar across all conditions (p > 0.05).
Conclusion:
Decreasing SMT impulse duration leads to a linear increase in EMG response of thoracic paraspinal during and following the SMT thrust.
Keywords: spine, manipulation, dose, impulse duration, chiropractic
Abstract
Introduction :
La manipulation vertébrale (MV) se caractérise par des paramètres cinétiques et cinématiques particuliers qui peuvent être modulés. L’objet de la présente étude est d’examiner des aspects fondamentaux de la relation dose-réponse physiologique de la MV chez des humaines en faisant varier la durée de l’impulsion de la MV.
Méthodologie :
Vingt adultes en santé ont subi quatre différents profils force-temps de MV livrés au moyen d’un actuateur linéaire asservi et ayant des durées d’impulsion différentes. Les réponses EMG des muscles paravertébraux de gauche et de droite (au niveau des vertèbres T6 et T8) et les déplacements des vertèbres T7 et T8 ont été évalués pour toutes les phases de la MV.
Résultats :
Des différences considérables ont été observées dans l’EMG des muscles paravertébraux au cours de la phase de la « poussée » et immédiatement après celle-ci (« post-MV1 ») (T8 : tous les p < 0,01 et T6 lors de la poussée : tous les p < 0,05). Les déplacements sagittaux des vertèbres étaient semblables dans toutes les situations (p < 0,05).
Conclusion :
Une réduction de la durée de l’impulsion de la MV entraîne une augmentation linéaire de la réaction à l’EMG des muscles paravertébraux thoraciques au cours de la poussée de la MV, et après celle-ci.
Keywords: colonne vertébrale, manipulation, dose, durée de l’impulsion, chiropratique
Introduction
Manual therapies are often used in the treatment of spinal conditions; they have been one of the most studied conservative treatment approaches for such conditions.1–3 Recent systematic reviews show that manual therapies such as spinal manipulation and mobilization both have positive, but limited, short-term effects on pain and disability.2,4 Failure to demonstrate larger clinical effects, such as the ones often described by clinicians, may partly be explained by the limited knowledge with regard to the mechanisms of action underlying manual therapies. Many scientists and clinicians have proposed that both spinal manipulation and mobilization exert biologic effects on the nervous system through mechanical deformation of musculoskeletal tissues5–7, but actual data on human subjects remain sparse.
Spinal manipulative therapy (SMT) is usually defined as a dynamic thrust of high-velocity, low-amplitude applied at specific contact points over the spine.7,8 Historically, spinal manipulation, otherwise known in the chiropractic profession as “adjustment”, has been one of the defining elements of the chiropractic therapeutic approach.9 Early conceptualisation of possible SMT biologic effects were based on the premises that biomechanical parameters play a critical role in the nature and amplitude of physiological responses.10
“Attention to the amount of force and speed used, the direction of the thrust, the recording of the places worked upon, all make for a fair amount of predictability that the same procedure followed again can give the same result”.
Verner 1941
SMT is characterized by specific kinetic and kinematic parameters that vary according to the region where it is applied11, the clinician’s experience12,13, and its method of application14. It has been suggested that the clinical effects of SMT are related to the modulation of these parameters, and our research group has undertaken a series of exploratory experiments aimed at evaluating biomechanical and neuromuscular responses to varying dosages of SMT parameters.15 These studies have showed a clear dose-response relationship between forces16 and preload forces (Conference abstract at ACC-RAC 2014) and paraspinal neuromuscular responses. However, impulse duration has not been investigated by our research group. The next section presents the current state of knowledge related to the effects of SMT impulse duration.
Specific effects of SMT impulse duration
Thoracic spine SMT are usually performed within an impulse duration (time-to-peak force) of 130 to 200ms13,17, however, a wide range of impulse durations have been reported when SMT is performed by humans (30–250ms)6. Systematic modulation of biomechanical and physiological responses to varying levels of impulse durations have mostly been investigated in anaesthetised animals. Studies evaluating vertebral displacements or neuro-physiological effects of different impulse durations or velocities on cadavers, anesthetized or healthy humans reported few or no result regarding these parameters.18–21 Studies on anaesthetised animals showed that varying impulse phase durations produces changes in the displacement and acceleration of the contacted and adjacent vertebras.6 Shorter impulse durations produce larger adjacent and fewer contacted vertebral segment motions than longer impulse durations.22 Moreover, recordings of physiological responses in animals showed that changing impulse durations evokes a variety of responses from afferents innervating muscle spindles and Golgi tendon organs. When peak force remains constant, the muscular activity amplitude increases with increasing impulse duration plateauing around 200ms.22 Recent studies revealed that resting muscle spindle discharge is significantly modified by impulse duration in anaesthetized cats, when thrust displacement or thrust force amplitude are unchanged. Muscle spindle responses to increasing speed (shorter impulse duration) are characterized by a curvilinear increase in discharge frequency23 with the steepest increase occurring at an impulse duration of 100 ms or shorter24,25. Overall, these results suggest a possible impulse duration threshold for which spindle responses are specifically and significantly increased under mechanical deformation of the spine. The SMT impulse duration dose-response relationship, however, remains to be investigated in humans.
The purpose of the present study is, therefore, to investigate fundamental aspects of SMT dose-physiological response relation in humans by investigating how different SMT impulse durations could modify biomechanical and neuromuscular responses to spinal manipulation.
Methods
A total of twenty healthy participants aged between 20 and 35 years old were recruited (10 female and 10 male with mean ± standard deviation age and body mass index of 23.75 ± 3.29 years and 23.43 ± 2.58 kg/m2). A general “screening” was performed by an experienced chiropractor in order to rule out any contraindication to SMT. Participants were excluded if they presented thoracic or lumbar pain, previous history of back trauma surgery, severe osteoarthritis, inflammatory arthritis, vascular problems, or any other condition that would limit the usage of SMT. Once included in the study, all participants gave their informed written consent according to the University’s Human Research Ethics Committee certification (No. CER-12-181-06.37).
Experimental protocol:
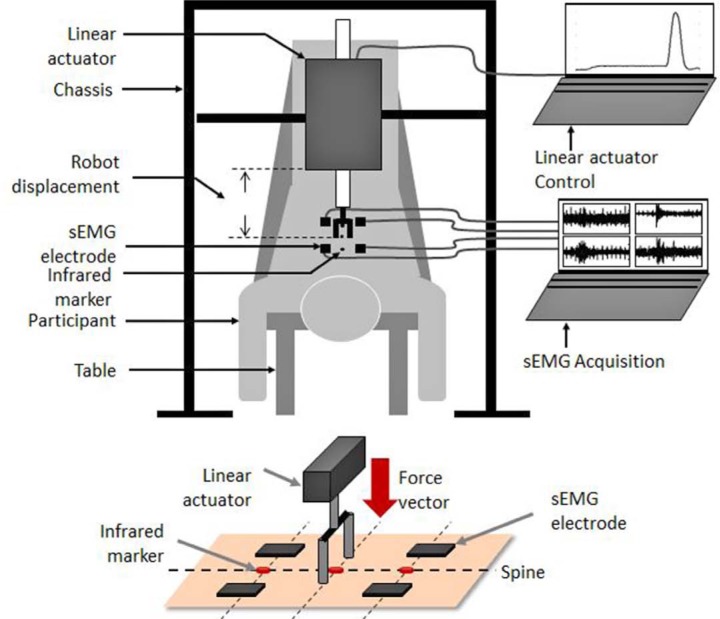
Each participant was first shown a demonstration of a simulated spinal manipulation performed by a servo-controlled linear actuator motor on a rigid body, in order to explain and highlight the basic operating and main security features of the apparatus. Electromyography (EMG) electrodes were applied over the left and right thoracic paraspinal muscles (T6 and T8 levels) following fiber orientation, and kinematic was collected by positioning light-emitting diodes on the spinous processes (T7 and T8 levels). The experimental set up is illustrated in figure 1. Each participant lied down in a prone position on a chiropractic table and was subjected to four different SMT force-time profiles. These four simulated SMT curves consisted of a 20N preload force for 1000ms followed by a “Thrust phase” composed by an “Impulse phase” leading to a peak force of 255N16 and a “Resolution phase”. The four SMT force-time profiles differed in their impulse phase duration respectively set to 125ms, 175ms, 225ms, and 275ms. Resolution phase duration was identical to impulse phase duration. A 20N preload force was chosen to limit the potential physiological responses related to preload forces. A typical SMT force-time profile is illustrated in figure 2. Five minutes of rest were given between each of the four trials, and the various impulse duration conditions were randomized across participants to avoid any sequence effect.
Figure 1:
Illustration of the experimental set up and the main components of the servo-controlled linear actuator motor. Surface EMG (sEMG).
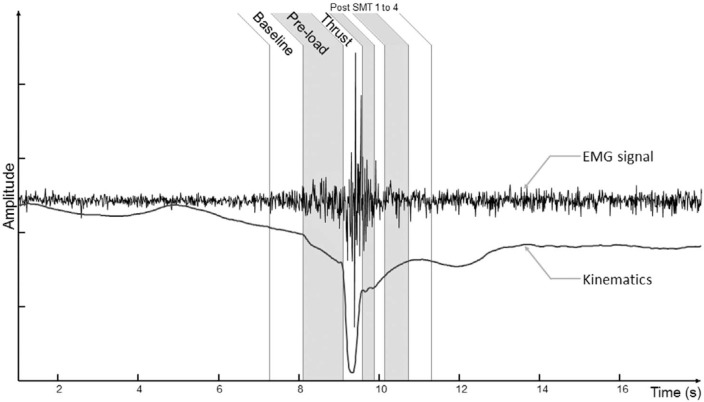
Figure 2:
Typical EMG and kinematic responses throughout the various SMT time-windows defined in the methods section.
Apparatus:
EMG activity was recorded using a Delsys Surface EMG sensor with a common mode rejection ratio of 92dB at 60Hz and input impedance of 1015Ω (Model DE2.1, Delsys Inc., Boston, MA, USA). Interelectrode distance was fixed at 20mm, and electrode diameter was 10mm. Electrodes were applied over the thoracic paraspinal muscles on each side of the spine, approximately 2cm from the T6 and T8 spinous processes. The reference electrode was positioned on the left acromion of each participant. For each electrode, (1) the desired body part (region) was gently shaved, (2) the skin was gently abraded with fine-grade sandpaper (Red Dot Trace Prep, 3 M, St. Paul, MN, USA) and (3) the skin was wiped with alcohol swabs. These three steps were systematically done for each electrode and each participant in order to reduce skin impedance. Data were sampled at 1,000Hz with a 12-bit A/D converter (PCI 6024E, National Instruments, Austin, TX, USA). The data were collected by LabView (National Instruments, Austin, TX, USA) and processed by Matlab (MathWorks, Natick, MA, USA). A motion analysis system (Optotrak Certus; Northern Digital, Waterloo, Ontario, Canada) was used to perform the kinematic data acquisition. Kinematic markers were placed on T7 and T8 spinous processes and data were collected at 100Hz.
A servo-controlled linear actuator motor (Linear Motor Series P01-48x360, LinMot Inc., Zurich, Switzerland) was developed and used to precisely simulate SMT for the four different impulse duration conditions. The linear motor vertically displaced a slider applied directly to the spine. A twin tip padded rod (14mm of diameter and 36mm inter-rod distance), was used as the contact point between the servo-controlled linear actuator motor and transverse processes of T7. A microcontroller accurately controlled the linear motor in order to reproduce a target SMT force-time profile loaded from a computer. A close loop force control constantly provided the needed intensity to maintain the output force as close as possible to the target force-time profile. A complete technical description and details of the safety features are presented in a previous article.15
Data analysis:
EMG data were filtered digitally by a 20 to 450Hz bandpass 4th order Butterworth filter. A band-stop 4th order Butterworth filter was also applied to remove the power supply contribution of 60Hz. Because surface EMG electrodes were positioned in the thoracic spine area, a custom designed digital filter was used to remove ECG artefacts from surface EMG.26
In order to analyse EMG responses according to SMT force events, seven time windows (see figure 2) that spanned across the entire SMT force curve were defined: a “Baseline” of 500ms duration to observe EMG activity before the SMT, a “Preload phase” of 1000ms, a “Thrust phase” and four phases which successively followed the “Thrust phase” with two windows of 250ms and two windows of 500ms (referred as “Post-SMT1” to “Post-SMT4” in figure 2 and 3). Therefore, because the “Thrust phase” duration depended on the imposed impulse phase duration, its possible durations were respectively 250ms, 350ms, 450ms, and 550ms. For each trial, the four EMG recordings were divided in seven normalized root mean square (RMS) values corresponding to each time window. Normalized RMS values were obtained by dividing each RMS value by the RMS value obtained during the “Pre-load phase”. A posterior to anterior force vector was used to perform spinal manipulations, and sagittal plane displacements were calculated. The vertebral displacement from “Preload phase” to peak force was considered for kinematic variable in the study. This value was calculated for the two kinematic markers (T7 and T8).
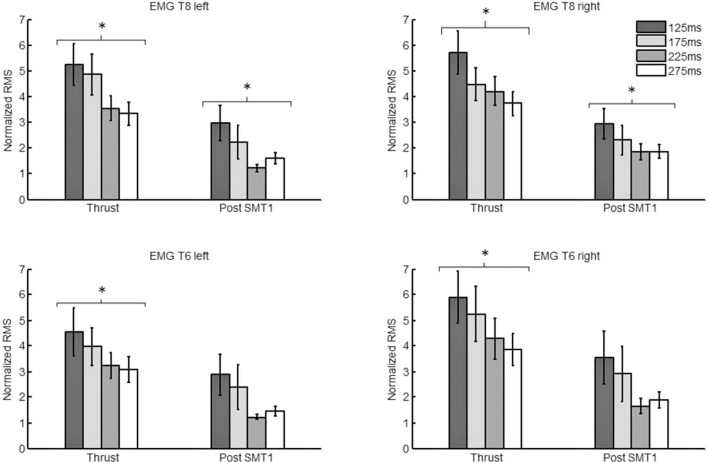
Figure 3:
EMG responses to varying levels of impulse duration during the “Thrust phase” and “Post-SMT1”. Mean (standard error) normalized RMS values (T6 left and right, T8 left and right paraspinal muscles) during the “Thrust phase” and “Post-SMT1” are presented.
Statistical analyses:
All dependent variables were found to be normally distributed and were submitted to 1-way repeated-measures ANOVA (4 different impulse durations). Whenever ANOVA yielded a significant time effect, polynomial contrasts were conducted to test for the linear trend (linear relationship between impulse duration applied and EMG response). Polynomial contrasts provide the opportunity to look at the response curve of the data and determine the nature of the relationship between SMT and EMG responses. The level of statistical significance was set at p<0.05 for all analyses.
Results
Figure 2 illustrates typical kinematic and EMG responses to a given SMT force-time profile. Overall, modulating the impulse duration (125ms, 175ms, 225ms, and 275ms) led to significant differences in paraspinal EMG not only during the “Thrust phase” but also during “Post-SMT1” (all T8 ps < 0.01 and T6 ps < 0.05). Testing for linear trend showed significant linear relationship between impulse duration and EMG responses for T8 (during both the “Thrust phase” and the Post-SMT1) and T6 (“Thrust phase” only) (all ps < 0.05). The linear relationship showed that decreasing the impulse duration led to a significant increase in paraspinal muscle activity. Paraspinal EMG activity was similar across all impulse duration for the remaining time-windows i.e. “Baseline phase”, “Preload phase”, “Post-SMT2”, “Post-SMT3” and “Post-SMT4” time-windows, indicating that changes in impulse duration did not affect muscular activity during these components of SMT (p > 0.05). EMG responses to varying impulse durations during the “Thrust phase” and “Post-SMT1” are presented in figure 3.
Sagittal vertebral displacements from “Preload phase” to peak force were similar across all impulse duration conditions, indicating that spinal displacement during SMT did not change when modulating impulse duration (p > 0.05). Sagittal vertebral displacement ± standard deviation of T7 and T8 are reported in table 1.
Table 1:
Sagittal Vertebral displacement ± standard deviation of T7 and T8 from “Preload phase” to peak force.
| Impulse duration | T7 (mm ± SD) | T8 (mm ± SD) |
|---|---|---|
| 125 ms | 14.52 ± 2.94 | 13.27 ± 3.02 |
| 175 ms | 15.18 ± 2.84 | 13.84 ± 2.95 |
| 225 ms | 15.58 ± 1.83 | 13.78 ± 2.65 |
| 275 ms | 15.51 ± 1.91 | 14.01 ± 2.53 |
Discussion
The main objective of the present study was to investigate the SMT dose-physiological response using systematic modulation of SMT impulse duration. The main findings indicate that EMG responses of thoracic paraspinal muscles increased linearly with decreasing SMT impulse duration. Such dose-response relationship was observed during the SMT “Thrust phase” for both paraspinal muscle levels recorded (T6 and T8), but also in the first 250ms time window following the spinal manipulation impulse for T8 paraspinal muscle level. These muscle activations, however, quickly attenuated in the following time windows (from 250ms to 1.25ms after spinal manipulation impulse).
Neuromuscular responses
Other studies have previously attempted to document the relationship between the impulse duration and para-spinal EMG responses. Using an animal model (Merino sheep), Colloca et al. (2006) reported an increase in the percentage of higher amplitude EMG response to pulse duration of 100ms and 200ms compared to pulse duration of 10ms.22 Comparisons to others studies remain difficult, as the effect of impulse duration variation has not yet been evaluated while controlling (or describing) other parameters such as peak and preload forces in human or cadaveric studies.19,20 Furthermore, studies evaluating instrument assisted SMT were conducted using impulse duration of less than 5ms, which results in spine oscillation for up to 150ms following the application of the force impulse. According to the authors, this oscillation may contribute to impulse-triggered EMG responses and may explain differences between the studies.20,27
The increasing EMG responses observed with shorter SMT impulse duration in the present study seem to be coherent with the results obtained from muscle spindle recordings for which a curvilinear increase in discharge frequency23 was observed when decreasing impulse duration24,25.
Kinematic responses
Colloca et al. (2006) reported that a short impulse duration (10ms) produces a smaller movement of the contacted segment as well as a larger adjacent movement than a longer impulse duration (100–200ms).22 Nevertheless, they did not report clear differences between impulse durations of 100 and 200ms, which is consistent with the present results. Interestingly, Lee et al. (1992) reported larger adjacent vertebral segment displacement during shorter impulse duration.18 However, their fast and slow conditions consisted of 500ms and 30sec impulse durations respectively, which are considerably slower durations than those used by Colloca et al.22 and the ones presented in this study. With regard to vertebral displacement, these results suggest an inverted U dose-response relationship where very short impulse (e.g. 10ms) and long duration (30sec) produce less vertebral movement than 100ms and 200ms impulse duration. Studies evaluating a wider range of impulse duration using controlled force and displacements are needed in order to adequately evaluate this relationship.
Practical implications and study limitations
Impulse duration tends to decrease in experienced chiropractic students12, but remains highly variable between clinicians and across repeated SMT28. The results of the current study highlight the possible relationship between SMT impulse duration and neurophysiological responses. Although speed (short impulse duration) is often associated with clinical expertise in the delivery of SMT, its specific contribution to the clinical effects is unknown.
The physiological responses described in this study were obtained from young healthy participants and may not be generalizable to other populations, including patients with spinal pain. Future studies of SMT dose-response relationship in patients with cervical, thoracic and lumbar spine pain are needed. During testing, each kinematic marker were mounted on wooden supports in order to minimise masking of kinematic markers caused by linear actuator motor displacement during SMT. This procedure, in addition to skin motion during SMT may have led to an increased variability in sagittal vertebral displacement, thus minimizing the possibility to identify significant changes in vertebral displacements. An additional floor mounted camera should be added in future studies to allow direct skin positioning of the markers.
Conclusion
The present study objective was to investigate fundamental aspects of the SMT dose-physiological response relation in humans by investigating how different SMT impulse duration can modify biomechanical and neuro-muscular responses to spinal manipulation. The main results indicate that while decreasing SMT impulse duration, EMG response of thoracic paraspinal muscles increased linearly during and following the SMT thrust. Whether or not these differences are of any clinical importance remains to be determined.
Footnotes
Sources of support:
References
- 1.Rubinstein SM, et al. Spinal manipulative therapy for chronic low-back pain: an update of a Cochrane review. Spine (Phila Pa 1976) 2011;36(13):E825–46. doi: 10.1097/BRS.0b013e3182197fe1. [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein SM, et al. Spinal manipulative therapy for acute low back pain: an update of the cochrane review. Spine (Phila Pa 1976) 2013;38(3):E158–77. doi: 10.1097/BRS.0b013e31827dd89d. [DOI] [PubMed] [Google Scholar]
- 3.Furlan AD, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2008;(4):CD001929. doi: 10.1002/14651858.CD001929.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Bronfort G, et al. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J. 2004;4(3):335–56. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Clark BC, et al. The biology of manual therapies. J Am Osteopath Assoc. 2012;112(9):617–29. [PubMed] [Google Scholar]
- 6.Pickar JG, Bolton PS. Spinal manipulative therapy and somatosensory activation. J Electromyogr Kinesiol. 2012;22(5):785–94. doi: 10.1016/j.jelekin.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colloca CJ, Pickar J, Slosberg M. Special focus on spinal manipulation. J Electromyogr Kinesiol. 2012;22(5):629–31. doi: 10.1016/j.jelekin.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Triano JJ, Descarreaux M, Dugas C. Biomechanics – review of approaches for performance training in spinal manipulation. J Electromyogr Kinesiol. 2012;22(5):732–9. doi: 10.1016/j.jelekin.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Haldeman S. Principles and Practice of Chiropractic. Third Edition. McGraw-Hill Education; 2005. [Google Scholar]
- 10.Verner JR. In: The Science and Logic of Chiropractic. Verner I J Robinson., editor. New Jersey: 1941. p. 314. [Google Scholar]
- 11.Herzog W, et al. Forces exerted during spinal manipulative therapy. Spine (Phila Pa 1976) 1993;18(9):1206–12. doi: 10.1097/00007632-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Triano JJ, et al. Maturation in rate of high-velocity, low-amplitude force development. J Manipulative Physiol Ther. 2011;34(3):173–80. doi: 10.1016/j.jmpt.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Descarreaux M, et al. Kinetic analysis of expertise in spinal manipulative therapy using an instrumented manikin. J Chiropr Med. 2005;4(2):53–60. doi: 10.1016/S0899-3467(07)60114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cambridge ED, et al. Comparison of force development strategies of spinal manipulation used for thoracic pain. Man Ther. 2012;17(3):241–5. doi: 10.1016/j.math.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Descarreaux M, Nougarou F, Dugas C. Standardization of spinal manipulation therapy in humans: development of a novel device designed to measure dose-response. J Manipulative Physiol Ther. 2013;36(2):78–83. doi: 10.1016/j.jmpt.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Nougarou F, et al. Physiological responses to spinal manipulation therapy: Investigation of the relationship between electromyographic responses and peak force. J Manipulative Physiol Ther. 2013;36(9):557–63. doi: 10.1016/j.jmpt.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Herzog W, Kats M, Symons B. The effective forces transmitted by high-speed, low-amplitude thoracic manipulation. Spine (Phila Pa 1976) 2001;26(19):2105–10. doi: 10.1097/00007632-200110010-00012. discussion 2110–1. [DOI] [PubMed] [Google Scholar]
- 18.Lee R, Evans J. Load-displacement-time characteristics of the spine under posteroanterior mobilization. Austr J Physio. 1992;38:115–123. doi: 10.1016/S0004-9514(14)60556-0. [DOI] [PubMed] [Google Scholar]
- 19.Gal J, et al. Movements of vertebrae during manipulative thrusts to unembalmed human cadavers. J Manipulative Physiol Ther. 1997;20(1):30–40. [PubMed] [Google Scholar]
- 20.Colloca CJ, Keller TS, Gunzburg R. Neuromechanical characterization of in vivo lumbar spinal manipulation. Part II. Neurophysiological response. J Manipulative Physiol Ther. 2003;26(9):579–91. doi: 10.1016/j.jmpt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Keller TS, Colloca CJ, Gunzburg R. Neuromechanical characterization of in vivo lumbar spinal manipulation. Part I. Vertebral motion. J Manipulative Physiol Ther. 2003;26(9):567–78. doi: 10.1016/j.jmpt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Colloca CJ, et al. Spinal manipulation force and duration affect vertebral movement and neuromuscular responses. Clin Biomech. 2006;21(3):254–62. doi: 10.1016/j.clinbiomech.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Pickar JG, et al. Response of lumbar paraspinal muscles spindles is greater to spinal manipulative loading compared with slower loading under length control. Spine J. 2007;7(5):583–95. doi: 10.1016/j.spinee.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed WR, et al. Relationship between biomechanical characteristics of spinal manipulation and neural responses in an animal model: effect of linear control of thrust displacement versus force, thrust amplitude, thrust duration, and thrust rate. Evidence-based complementary and alternative medicine. 2013;2013:492039. doi: 10.1155/2013/492039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao DY, et al. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. J Manip Physio Thera. 2013;36(2):68–77. doi: 10.1016/j.jmpt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nougarou F, Massicotte D, Descarreaux M. Efficient combination of DWT and ICA to localize and remove ECG from surface electromyography measurement. 18th IEEE International Conference on digital signal processing (DSP2013); Greece: IEEE; 2013. [Google Scholar]
- 27.Keller TS, et al. Three-dimensional vertebral motions produced by mechanical force spinal manipulation. J Manip Physiol Ther. 2006;29(6):425–36. doi: 10.1016/j.jmpt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Kawchuk GN, et al. Variability of force magnitude and force duration in manual and instrument-based manipulation techniques. J Manip Physiol Ther. 2006;29(8):611–8. doi: 10.1016/j.jmpt.2006.08.013. [DOI] [PubMed] [Google Scholar]