Abstract
Aims/Introduction
The aim of the present study was to determine whether weight reduction is associated with improvement of glycemic control in non‐obese and obese subjects with or without visceral fat accumulation, whose hemoglobin A1c (A1C) is 5.6–6.4%.
Materials and Methods
A total of 798 male subjects whose A1C levels were between 5.6% and 6.4% were divided into subgroups based on body mass index (BMI) and/or estimated visceral fat area (eVFA), and were analyzed with respect to the relationships between 1‐year changes in BMI (ΔBMI) and A1C (ΔA1C).
Results
In both the BMI ≥25 and BMI <25 groups, ΔA1C correlated positively with ΔBMI (BMI ≥25 (n = 321): r = 0.236, P < 0.0001; BMI <25 (n = 477): r = 0.095, P = 0.0387) although the r‐value was very small for the latter group. In addition, for the group with eVFA ≥100 cm2 (n = 436), ΔA1C correlated positively with ΔeVFA (r = 0.150, P = 0.0017), but this correlation was not found for the eVFA <100 cm2 group (n = 339, P = 0.3505). Furthermore, ΔA1C positively correlated with ΔBMI for the groups in BMI ≥25 with eVFA >100 cm2 (n = 293, r = 0.256, P < 0.0001) and BMI <25 with eVFA ≥100 cm2 (n = 145, r = 0.250, P = 0.0024), but not for the groups in BMI ≥25 with eVFA <100 cm2 (n = 28, P = 0.6401) nor BMI <25 with eVFA <100 cm2 (n = 332, P = 0.6605).
Conclusions
These results suggest that the assessment of visceral fat, rather than BMI, might be more important in identifying subjects in whom lifestyle intervention aiming at weight reduction could be effective to prevent diabetes. This trial was registered with University Hospital Medical Information Network Clinical Trials Registry (no. UMIN 000002391).
Keywords: Glycemic control, Visceral fat accumulation, Weight reduction
Introduction
Type 2 diabetes results from both genetic predisposition and environmental risk factors, such as obesity, visceral fat accumulation and physical inactivity; thus, lifestyle interventions aimed at reducing weight and visceral fat are thought to be an effective method of preventing or delaying the onset of this disease. There have been some large‐scale intervention trials in the USA and Europe in which lifestyle intervention to reduce bodyweight could lead to prevention or delay of the onset of type 2 diabetes in subjects with impaired glucose tolerance1, but most subjects included in these studies were obese; therefore, it is unknown whether such an intervention is also effective in individuals who are not obese. In order to address this issue, in the present study, we analyzed whether changes in body mass index (BMI) level correlated with changes in hemoglobin A1c (A1C) in participants whose A1C was 5.6–6.4%, and who were divided into subgroups based on the value of BMI and visceral fat area (VFA), and investigated whether the associations were found in each group similarly or not.
Methods
Participants
The study participants were 2557 male employees of the Amagasaki City Office, Hyogo, Japan, who had completed the annual health check‐up both in 2004 and 2005. Among these participants, 798 males who were not taking any anti‐diabetic medicine, whose A1C level was 5.6–6.4% in 2004 and whose fasting plasma glucose was below 126 mg/dL or postprandial plasma glucose below 200 mg/dL in 2004 were included in the present analysis. A1C 5.6–6.4% is the decision criterion for screening subjects who should receive ‘hokenshido’, which is a kind of health education program, to prevent diabetes according to the guidance recommended by Japan Diabetes Society (JDS). In addition, we excluded from the present study those subjects whose A1C was more than 7.4% in the following year, so that subjects whose bodyweight or visceral fat was reduced not by lifestyle intervention, but by the deterioration of glucose tolerance, should be excluded. In analyzing adiponectin (APN) levels, subjects were also excluded if they were taking any medicines for hypertension and dyslipidemia, as well as diabetes mellitus. After the health check‐up, the medical staff provided risk factor‐oriented health promotion programs to the participants, as reported previously4.
The study was approved by the human ethics committee of Osaka University, and a signed informed consent form was obtained from each participant. This trial was registered with the University Hospital Medical Information Network (no. UMIN 000002391).
Laboratory tests
Plasma glucose levels were determined by the glucose oxidase method (Quick‐auto II GLU‐HKs; Shino‐Test Corporation, Tokyo, Japan). A1C levels were determined by high‐performance liquid chromatography (Rapidia Auto HbA1c‐L; TFB, Tokyo, Japan). The value for A1C (%) was presented using the National Glycohemoglobin Standardization Program (NGSP) value (%). The conversion equation from A1C (JDS) to A1C (NGSP) values was officially certified as follows: NGSP (%) = 1.02 × JDS (%) + 0.25%7.
Detailed examination
The estimated visceral fat area (eVFA) was determined by the bioelectrical impedance analysis method, as reported previously9. Adiponectin (APN) was measured using the latex particle‐enhanced turbidimetric assay (Otsuka Pharmaceutical, Tokyo, Japan)10. The measurements of APN and eVFA complied with the Guidelines of the Ethical Committees of Osaka University.
Statistical analysis
We investigated the relationships between 1‐year changes in BMI (ΔBMI), eVFA (ΔeVFA), APN (ΔAPN) and A1C (ΔA1C) in the participants using Spearman's linear regression analysis. The significance level was set at P < 0.05.
Results
The clinical characteristics of the study participants are presented in Table 1. In the total participants, ΔA1C positively correlated with ΔBMI (P < 0.0001, r = 0.150). The participants were then divided into subgroups according to BMI (≥25 kg/m2 and <25 kg/m2) and/or eVFA (≥100 cm2 and <100 cm2) based on the measurements made in 2004. A BMI of >25 kg/m2 is the criterion for obesity in Japan. A VFA of >100 cm2 is the criterion for visceral fat accumulation in Japan11, and an excellent correlation was observed in VFA and eVFA, which is the estimation of visceral fat accumulation by abdominal bioelectrical impedance analysis method9. In both the BMI ≥25 and BMI <25 groups, ΔA1C correlated positively with ΔBMI (BMI ≥25 (n = 321): r = 0.236, P < 0.0001; BMI <25 (n = 477): r = 0.095, P =0.0387), although the r‐value was very small for the latter group, suggesting that effect of weight reduction on improvement of glycemic control was relatively small in the non‐obese group (Figure 1a). In addition, for the group with eVFA ≥100 cm2 (n = 438), ΔA1C correlated positively with ΔeVFA (r = 0.150, P = 0.0017) and ΔBMI (r = 0.257, P < 0.0001), but these correlations were not found to be significant for the eVFA <100 cm2 group (n = 339; P = 0.3505 with ΔeVFA, P = 0.5831 with ΔBMI; Figure 1b,c). This shows that the effect of visceral fat decrease on improvement of glycemic control was clearly found only in the visceral fat accumulation group. Next, we also investigated the relationships between ΔA1C and ΔBMI in the four subgroups, divided by lower or higher BMI (<25 kg/m2 or ≥25 kg/m2) and eVFA (<100 cm2 or ≥100 cm2). ΔA1C positively correlated with ΔBMI for the groups of BMI ≥25 with eVFA ≥100 cm2 (n = 293, r = 0.256, P < 0.0001) and BMI <25 with eVFA ≥100 cm2 (n = 145, r = 0.250, P = 0.0024), but not for the groups of BMI ≥25 with eVFA <100 cm2 (n = 28, P = 0.6401) nor BMI <25 with eVFA <100 cm2 (n = 332, P = 0.6605), as shown in Figure 2. These results show that weight reduction is associated with improvement of glycemic control in male subjects whose A1C is 5.6–6.4%, especially with visceral fat accumulation irrespective of BMI, but not without visceral fat accumulation.
Table 1. Clinical characteristics of the subjects in this study.
| n | 798 |
| Age (year) | 51 ± 9 |
| Body weight (BW kg) | 69.1 ± 9.9 |
| Body mass index (BMI) (kg/m2) | 24.4 ± 3.0 |
| Waist circumference (WC) (cm) | 85.1 ± 8.1 |
| Estimated visceral fat area (cm2) | 103.5 ± 40.0 |
| Systolic blood pressure (SBP) (mmHg) | 132.5 ± 18.7 |
| Diastolic blood pressure (DBP) (mmHg) | 82.1 ± 13.0 |
| A1C (%) | 5.85 ± 0.22 |
| Fasting serum glucose (mmol/L) | 5.5 ± 0.5 (120) |
| Postprandial serum glucose (mmol/L) | 5.9 ± 1.2 (678) |
| Total cholesterol (mmol/L) | 5.4 ± 0.9 |
| Triglyceride (mmol/L) | 2.0 ± 1.4 |
| HDL cholesterol (mmol/L) | 1.5 ± 0.4 |
| LDL cholesterol (mmol/L) | 3.0 ± 0.8 |
| Uric acid (UA) (μmol/L) | 355.2 ± 7.7 |
| Adiponectin (μg/mL) | 6.9 ± 2.9 (725) |
Data are mean ± SD.
Number in parenthesis is the number of available data.
Figure 1.
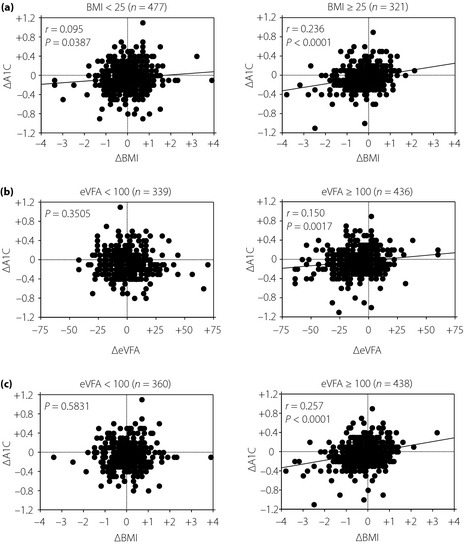
(a) Correlations between 1‐year changes in body mass index (ΔBMI) and hemoglobin A1C (ΔA1C) in male subjects, whose A1C was 5.6–6.4%, in the BMI ≥25 and BMI <25 groups. (b) Correlations between 1‐year changes in estimated visceral fat area (ΔeVFA) and A1C in male participants, whose A1C was 5.6–6.4%, in the eVFA ≥100 cm2 and eVFA <100 cm2 groups. (c) Correlations between ΔBMI and ΔA1C in male participants, whose A1C was 5.6–6.4%, in the eVFA ≥100 cm2 and eVFA <100 cm2 groups.
Figure 2.
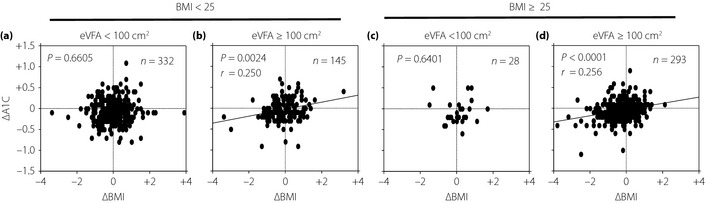
Correlations between 1‐year changes in body mass index (ΔBMI) and hemoglobin A1C (ΔA1C) in male participants, whose A1C was 5.6–6.4%, divided into four groups based on BMI and visceral fat (VFA) are presented. (a) BMI ≤25 with estimated VFA (eVFA) <100 cm2. (b) BMI <25 with eVFA ≥100 cm2. (c) BMI ≥25 with estimated VFA (eVFA) <100 cm2. (d) BMI ≥25 with eVFA ≥100 cm2. ΔA1C positively correlated with ΔBMI for the groups BMI ≥25 with eVFA ≤100 cm2 and BMI <25 with eVFA ≥100 cm2, but not for the groups BMI ≥25 with eVFA <100 cm2 nor BMI <25 with eVFA <100 cm2.
As a negative correlation between 1‐year changes in BMI and APN was found, and an increase of adiponectin level after weight reduction was also identified in the present study, as reported previously, we investigated the association between 1‐year changes in adiponectin level (ΔAPN) and ΔA1C. As APN level was inversely correlated with eVFA and BMI in the present study, and the plasma APN level of 7.0 μg/mL corresponded to eVFA of 100 cm2 in these participants (APN = 9.421–0.024 × eVFA, r = −0.328, P < 0.0001), we set the criterion of hypoadiponectinemia by this value. Divided according to APN (≥7.0 μg/mL and <7.0 μg/mL in 2004), ΔA1C negatively correlated with ΔAPN for the APN <7.0 μg/mL group (n = 252: r = –0.134, P = 0.0337), but not for the APN ≥7.0 μg/mL group (n = 202, P = 0.4829; Figure 3). In addition, ΔA1C positively correlated with ΔBMI for the APN <7.0 μg/mL group (n = 408: r = 0.162, P = 0.0010), but not for the APN ≥7.0 μg/mL group (n = 317, P = 0.0892).
Figure 3.
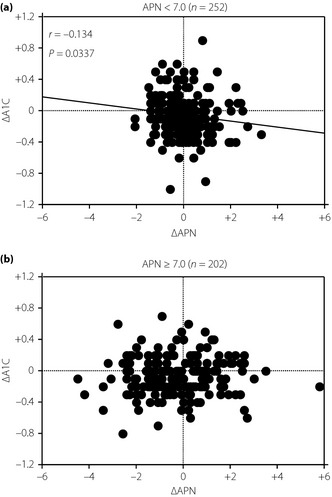
Correlations between 1‐year changes in adiponection level (ΔAPN) and hemoglobin A1C (ΔA1C) in male participants, whose A1C was 5.6–6.4%, in the APN level (a) ≥7.0 μg/mL and (b) <7.0 μg/mL groups.
We also analyzed the relationships between A1C and various parameters (age, BMI, systolic blood pressure, total cholesterol, creatinine, uric acid, and γ‐glutamyl transpeptidase) by stepwise multiple regression in a cross‐sectional and 1‐year longitudinal (except age) study, and identified age and BMI as significant determinants of A1C in the cross‐sectional study, and also identified only ΔBMI and ΔTC as significant determinants of ΔA1C in the longitudinal study. In addition, we analyzed the participants divided into three groups based on age in order to clarify whether age affects the association of ΔBMI with ΔAlC in those with visceral fat accumulation, and found a significant association in all three groups (age <52 years (n = 149): r = 0.222, P = 0.0064; age 52–57 years (n = 141): r = 0.269, P = 0.0012; age ≥57 years (n = 148): r = 0.296, P = 0.0003), suggesting the association does not depend on age. However, judging from the r‐value in each group, the association of ΔBMI with ΔAlC might be a little stronger in elderly men.
Discussion
The Diabetes Prevention Program, a randomized clinical trial to prevent diabetes, showed that intensive lifestyle intervention to reduce bodyweight could lead to prevention of type 2 diabetes in subjects with impaired glucose tolerance3. In this trial, diabetes incidence was reduced by 58% in the 2.8 years with an average weight reduction of 5.8 kg in the lifestyle intervention group. However, the mean bodyweight and BMI of the participants at baseline in the trial were more than 90 kg and 31, respectively, and were very different from those in the Japanese population. In fact, the mean bodyweight and BMI of the present study participants were approximately 69 kg and 24, respectively, and many non‐obese (BMI <25) participants were included. To consider an effective intervention for the Japanese population, it is important to clarify whether weight reduction is also effective in individuals who are not obese.
In the present study, we first found out that ΔA1C correlated positively with ΔBMI with both obese and non‐obese, but the r‐value was very small for non‐obese participants. This suggests that weight reduction would also be effective in individuals who are not obese, but the degree of its effect on improving glycemic control might be relatively small in non‐obese subjects. Next, we observed that ΔA1C correlated positively with ΔeVFA and ΔBMI in participants with visceral fat accumulation, but not without visceral fat. It has already been reported that the decrease of visceral fat is strongly correlated with the improvement of plasma glucose in visceral fat obesity12, but it was unclear whether this is also true in subjects without visceral fat. No correlation between ΔA1C and ΔeVFA or ΔBMI in participants without visceral fat in the present study indicates that the effects of visceral fat on glycemic control are relatively small in these subjects; and other factors, such as insulin secretion capacity, would be more dominant.
Furthermore, we showed that ΔA1C correlated positively well with ΔBMI in participants with visceral fat accumulation, irrespective of BMI (<25 or ≥25), but not without visceral fat. We previously reported that subjects with visceral fat accumulation had a cluster of metabolic risk factors irrespective of BMI, and that assessment of visceral fat accumulation is useful for identifying high‐risk groups for atherosclerotic cardiovascular diseases5. The aforementioned results show that assessment of visceral fat accumulation rather than BMI might also be useful for identifying subjects in which weight reduction should be effective for improving glycemic control.
ΔA1C negatively correlated with ΔAPN and positively with ΔBMI in participants with hypoadiponectinemia, but not without hypoadiponectinemia. These results suggest the intervention to increase serum adiponectin level could improve glycemic control in hypoadiponectinemia, and assessment of adiponectin level would be also useful for identifying subjects in which weight reduction should be carried out for preventing diabetes.
We enrolled participants in the present study whose A1C level was 5.6–6.4%, and whose fasting plasma glucose was below 126 mg/dL or postprandial plasma glucose was below 200 mg/dL. According to this criterion, we could not completely exclude diabetic patients from our subjects, because we did not check fasting and postprandial plasma glucose in all the participants. Thus, we could not regard all our subjects as prediabetes. However, in practice, the subjects whose A1C is 5.6–6.4%, if they have no data of their fasting and postprandial plasma glucose, should receive ‘hokenshido’, so it is very important to find out what kind of lifestyle intervention should be carried out to improve glycemic control for these subjects.
Our result that no correlation between ΔA1C and ΔBMI was observed in the BMI ≥25 with eVFA <100 cm2 group suggests that weight reduction might not be so effective in obese subjects without visceral fat accumulation. It might be due to the rich constituents, such as muscle and/or subcutaneous fat, in the body of these subjects. However, it should be further elucidated in a larger population whether it is really true, as the population in the present study was very small (n = 28). In addition, it should also be clarified in the future as to what kind of intervention would be more effective for improving glycemic control in subjects without visceral fat. For example, increase of dietary fibre intake delaying carbohydrate absorption might contribute to improving glycemic control and prevention of diabetes in non‐visceral fat subjects, as alpha‐glucosidase inhibitor has been reported to delay the onset of diabetes effectively in impaired glucose tolerance without obesity13.
In the present study, we did not present the data on female subjects, because we had only 148 subjects, including premenopausal women. When we analyzed the female subjects similarly to the males, we did not find an association between ΔBMI and ΔAlC in the total subjects (n = 148), as well as those with obese (n = 24) or visceral fat accumulation (n = 13). Further studies are necessary to clarify this issue of analyzing female subjects, including a large number of postmenopausal females with visceral fat, because adiposity is not supposed to matter so much in premenopausal women from the aspect of obesity‐related cardiovascular risk factors, including glucose tolerance14.
In conclusion, we showed that weight reduction was closely associated with improvement of glycemic control in male subjects whose A1C was 5.6–6.4% with visceral fat accumulation, irrespective of BMI, but not without visceral fat accumulation. The assessment of visceral fat, rather than BMI, might be more important in identifying subjects in whom lifestyle intervention aiming at weight reduction should be carried out to prevent diabetes.
Acknowledgements
This research was supported in part by grants from the Japan Heart Foundation & Astellas/Pfizer Grant for Research on Atherosclerosis Update (to K Kishida) and from the Manpei Suzuki Diabetes Foundation (to T Nakamura). No other potential conflicts of interest relevant to this article were reported. We thank Tomoko Ogawa, Sayaka Kobayashi, Hitoshi Nishizawa, Miwa Ryo and Shigeo Takahashi for their helpful comments.
(J Diabetes Invest, doi: 10.1111/jdi.12084, 2013)
References
- 1.Eriksson KF, Lindgärde F. Prevention of type 2 (non‐insulin‐dependent) diabetes mellitus by diet and physical exercise. The 6‐year malmö feasibility study. Diabetologia 1991; 34: 891–898 [DOI] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindström J, Eriksson JG, et al Finnish diabetes prevention study group: prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350 [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett‐Connor E, Fowler SE, et al Diabetes prevention program research group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okauchi Y, Nishizawa H, Funahashi T, et al Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care 2007; 30: 2392–2394 [DOI] [PubMed] [Google Scholar]
- 5.Okauchi Y, Kishida K, Funahashi T, et al 4‐year follow‐up of cardiovascular events and changes in visceral fat accumulation after health promotion program in the Amagasaki visceral fat study. Atherosclerosis 2010; 212: 698–700 [DOI] [PubMed] [Google Scholar]
- 6.Ryo M, Nakamura T, Funahashi T, et al Health education “Hokenshido” program reduced metabolic syndrome in the Amagasaki visceral fat study. Three‐year follow‐up study of 3,174 Japanese employees. Intern Med 2011; 50: 1643–1648 [DOI] [PubMed] [Google Scholar]
- 7.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan diabetes society to national glycohemoglobin standardization program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryo M, Maeda K, Onda T, et al A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care 2005; 28: 451–453 [DOI] [PubMed] [Google Scholar]
- 10.Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle‐enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta 2006; 371: 163–168 [DOI] [PubMed] [Google Scholar]
- 11.Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity . New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66: 987–992 [DOI] [PubMed] [Google Scholar]
- 12.Fujioka S, Matsuzawa Y, Tokunaga K, et al Improvement of glucose and lipid metabolism associated with selective reduction of intra‐abdominal visceral fat in premenopausal women with visceral fat obesity. Int J Obes 1991; 15: 853–859 [PubMed] [Google Scholar]
- 13.Kawamori R, Tajima N, Iwamoto Y, et al Voglibose for prevention of type 2 diabetes mellitus: a randomized, double‐blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009; 373: 1607–1614 [DOI] [PubMed] [Google Scholar]
- 14.Okauchi Y, Kishida K, Funahashi T, et al Absolute value of bioelectrical impedance analysis‐measured visceral fat area with obesity‐related cardiovascular risk factors in Japanese workers. J Atheroscler Thromb 2010; 17: 1237–1245 [DOI] [PubMed] [Google Scholar]


