Abstract
Non‐alcoholic fatty liver disease (NAFLD) describes a spectrum of liver conditions from simple steatosis, steatohepatitis to end‐stage liver disease. The prevalence of NAFLD has been on the rise in many parts of the world, including Asia, and NAFLD is now the liver disease associated with the highest mortality, consequent to the increased risk of cardiovascular diseases and hepatocellular carcinoma. Whereas NAFLD is an independent risk factor for type 2 diabetes, increased hepatic and peripheral insulin resistance contribute to the pathogenesis of both NAFLD and diabetes, which are associated with enhanced cardiovascular risk. Studies in humans and animal models have suggested obesity as the common link of these two diseases, likely mediated by adipose tissue inflammation and dysregulated adipokine production in obesity. In the present review, we discuss recent advances in our understanding of the role of several novel adipokines (adiponectin, adipocyte fatty acid binding protein and fibroblast growth factor‐21) in the pathophysiology of NAFLD and diabetes, as well as their use as potential biomarkers and therapeutic targets for dysglycemia in NAFLD patients.
Keywords: Adipocyte fatty acid binding protein, Adiponectin, Fibroblast growth factor‐21
Introduction
Non‐alcoholic fatty liver disease (NAFLD) was first recognized in 1980. Over the past few decades, it has rapidly become the most common form of liver disease, concomitant with the increasing prevalence of obesity worldwide1. NAFLD describes a spectrum of liver conditions ranging from simple steatosis to severe steatosis with marked inflammation, termed non‐alcoholic steatohepatitis (NASH), which can be complicated by cirrhosis, end‐stage liver failure and hepatocellular carcinoma2. Population screening has estimated the prevalence of NAFLD diagnosed on ultrasound (US‐NAFLD) in the general population in Asian countries to be approximately 15–20%4, akin to that in Western countries6. Its prevalence has doubled in urban Chinese cities in the past two decades8. People with NAFLD are usually asymptomatic at the early stage. However, NAFLD patients have a higher overall mortality than the general population10. In a 21‐year follow up of biopsy‐proven NAFLD, the main causes of death were cardiovascular disease and malignancy12, as opposed to cirrhosis in those with alcoholic liver disease. The pivotal links between NAFLD and cardiovascular disease are metabolic disorders, including diabetes, dyslipidemia and hypertension13.
A strong association exists between NAFLD and type 2 diabetes, with NAFLD found in up to 70% of patients with type 2 diabetes15. In addition, a significant proportion of patients with NAFLD develop impaired glucose tolerance (IGT) or type 2 diabetes, dyslipidemia or hypertension a median of 6 years after diagnosis of NAFLD16. In a 5‐year retrospective review, participants with US‐NAFLD had higher risks of impaired fasting glucose, type 2 diabetes, insulin resistance and hypertriglyceridemia than NALFD‐free controls17. Furthermore, the presence of type 2 diabetes is associated with a more progressive course and higher rate of progression to cirrhosis18. Thus, prediction and early intervention of dysglycemia in NAFLD might have additive benefits in reducing cardiovascular risk and decreasing the rate of NAFLD progression.
Obesity is a major risk factor of both NAFLD and type 2 diabetes, and likely provides the common link through insulin resistance (Figure 1). Specifically, visceral, liver and skeletal fat accumulations each play distinct, but overlapping roles in the development of insulin resistance. It is now recognised that insulin resistance in obesity is largely consequential to adipose tissue inflammation and adipokine dysregulation19.
Figure 1.
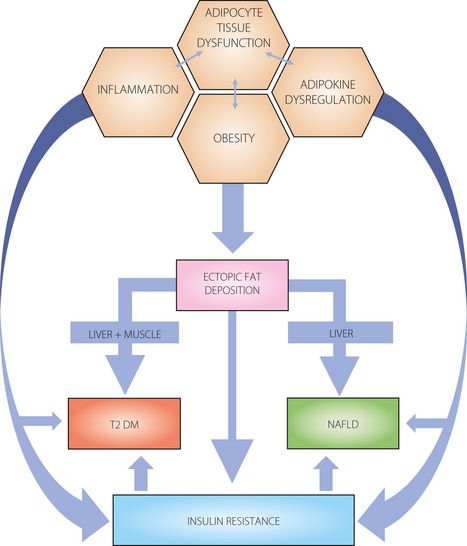
Obesity is a common link between type 2 diabetes (T2DM) and non‐alcoholic fatty liver disease (NAFLD). Adipose tissue dysfunction is characterized by inflammation and adipokine dysregulation, and subsequent ectopic fat deposition in the abdominal viscera and liver, and insulin resistance. It significantly contributes to the development of NAFLD and diabetes mellitus.
Relationship Between Liver, Adipose Tissue and Total Insulin Resistance
NAFLD and type 2 diabetes are associated with hepatic and adipose tissue insulin resistance, and reduced whole‐body insulin sensitivity. The ability of insulin to suppress hepatic glucose production was impaired to a similar extent in subjects with NAFLD and in those with type 2 diabetes. Glucose disposal during clamp study, a measure of whole‐body insulin sensitivity, was reduced by nearly 50% in NAFLD subjects, similar to that in type 2 diabetes patients20.
The pathogenesis of NAFLD was originally described by the ‘two‐hit hypothesis’, and subsequently, modified as the ‘multi‐hit hypothesis’, which describes the first hepatic insult as the dysregulation of fatty acid metabolism, leading to steatosis21. Insulin resistance plays a central role in the first insult, contributing to an imbalance between factors that promote hepatic fat accumulation (free fatty acid flux to the liver and de novo lipogenesis) and factors that prevent fatty acid build‐up (fatty acid export and oxidation). This renders hepatocytes susceptible to the secondary insults (‘multiple hits’) of adipokine‐induced liver injury, oxidative and endoplasmic reticulum (ER) stresses, mitochondrial dysfunction, and hepatic apoptosis, which subsequently promote the transition from simple steatosis to NASH22. More recently, lipid partitioning in liver cells, as regulated by stearoyl‐CoA desaturase‐1 (SCD1), the enzyme that converts saturated free fatty acids (SFA) to monounsaturated free fatty acids (MUFA), and the ratio of SFA to MUFA, has been implicated in the progression from simple steatosis to NASH. A higher ratio has been suggested to confer a greater risk of hepatic cell damage by the influx of exogenous free fatty acids (FFA) and apoptosis, inflammation, and fibrosis23.
In addition to hepatic insulin resistance, NAFLD is associated with a defect in insulin‐mediated suppression of lipolysis, in keeping with insulin resistance in adipose tissues24. These findings suggest that insulin resistance might be an intrinsic defect in NAFLD, similar to that in type 2 diabetes, and that blunted insulin responsiveness at the level of the adipocytes might contribute to hepatic steatosis through excess free fatty acid flux to the liver25. Isotope‐tracer studies in obese humans with NAFLD on a low‐fat diet showed that nearly 60% of hepatic triglycerides comes from FFA derived from adipose tissues, 26% from de novo lipogenesis and 15% from diet26. This would suggest that, in the absence of a high‐fat diet, the increased release of fatty acids from adipose tissues is the predominant source of excess hepatic fat accumulation.
Role of Adipokines in the Pathogenesis of NAFLD and Diabetes
As obesity develops, changes in the size of adipocytes and fat deposits result in modifications of paracrine function in the adipose tissues leading to a chronic inflammatory state. In obese adipose tissues, the release of tumor necrosis factor‐alpha (TNF‐α) stimulates adipocytes to secrete monocyte chemoattractant protein‐1 (MCP‐1), leading to macrophage recruitment. Macrophage‐related cytokine signaling promotes lipolysis through a decrease in lipid droplet stabilizing proteins (such as perilipin, fat specific protein 27). Lipolysis and the release of pro‐inflammatory adipokines from adipose tissues; for example, leptin, further promote macrophage activation. The presence of activated macrophages, mediated by adipokine dysregulation, perpetuates a vicious cycle of macrophage recruitment, inflammatory cytokine production, lipolysis and impaired adipocyte function. This state of chronic inflammation stimulates nuclear factor‐κB (NF‐κB) and Jun N‐terminal kinase (JNK) pathways in adipocytes27. We have shown that transgenic mice with selective inactivation of JNK in adipose tissues (aP2‐dn‐JNK mice) are protected against high fat diet (HFD)‐induced obesity, insulin resistance and glucose intolerance. The expression of several pro‐inflammatory cytokines, including TNF‐α, interleukin‐6 and MCP‐1, are decreased in the transgenic mice, compared to wild‐type littermates, whereas that of adiponectin, an anti‐inflammatory adipokine, is increased. The messenger ribonucleic acid (mRNA) levels of hepatic gluconeogenic genes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6‐phosphatase (G6Pase), are also significantly decreased in aP2‐dn‐JNK mice, and the number of lipid‐engorged hepatocytes is reduced, showing that inactivation of JNK attenuates HFD‐induced hepatic steatosis and glucose production28. These findings suggest that interactions between inflammatory and metabolic pathways mediated by macrophages and adipocytes are important in the development of obesity‐related insulin resistance, type 2 diabetes and NAFLD. In particular, the demonstration of a protective role in NAFLD of adiponectin, the most abundant adipokine in the circulation, and its reduction in patients with NAFLD29 has generated extensive research into the role of adipokines in the pathogenesis of NAFLD and its complications.
Adiponectin
Adiponectin is an anti‐inflammatory, insulin‐sensitizing hormone secreted from adipocytes, and its circulating levels are inversely proportional to body mass index. Its expression is controlled by peroxisome proliferator‐activated receptor‐gamma (PPAR‐γ), a transcription factor also predominantly expressed in adipose tissue30. Activation of PPAR‐γ by its agonists, such as thiazolidinediones, increases adiponectin and reduces TNF‐α expression31. Adiponectin circulates in the bloodstream as three oligomeric complexes: trimer, hexamer and high molecular weight (HMW) multimer, consisting of 18 or more monomers32. The gene that codes for human adiponectin is located on chromosome 3q27, a locus linked with the susceptibility to diabetes and obesity33. Another gene that is closely linked with NAFLD, the fetuin‐A gene, also resides on chromosome 3q27; its expression is significantly elevated in mice with fatty liver and its plasma concentrations are raised in subjects with high liver fat34. High circulating fetuin‐A levels are found in obesity35 and confers increased risk of diabetes36. This lends further evidence of the interconnections between obesity, diabetes and NAFLD.
The protective effect of adiponectin on hepatic steatosis and liver injury, through its role in lipid homeostasis and anti‐inflammatory action, has been shown in many experimental and clinical studies29. First, adiponectin expression from adipose tissues is markedly reduced in ob/ob mice (a leptin‐deficient model with hyperinsulinemia, insulin resistance and steatosis). Recombinant adiponectin infusion into these obese mice alleviates steatosis, as shown by a significant reduction in hepatic fat content and serum alanine transferase levels29 (Table 1). At a molecular level, the antisteatotic effect of adiponectin is mediated through the activation of 5‐adenosine monophosphate‐activated protein kinase (AMPK)40. AMPK activation phosphorylates acetyl coenzyme A carboxylase (ACC) and attenuates ACC activity, leading to enhanced fatty acid oxidation. Furthermore, AMPK activation downregulates the expression of sterol regulatory element‐binding protein 1c (SREBP 1c), a key transcription factor for lipogenic genes, including ACC and fatty acid synthase (FAS), and glycerol‐3‐phophate acyltransferase (GPAT). Adiponectin administration has been shown to suppress the hepatic mRNA expression of ACC and FAS in alcohol‐induced fatty liver disease in mice29, and the expression of SREBP 1c in cultured hepatocytes and in the liver of +Lepr(db)/+Lepr(db) (db/db) mice41. In addition, adiponectin also stimulates peroxisome proliferator‐activated receptor‐alpha (PPAR‐α), a transcription factor that controls genes encoding fatty acid oxidation enzymes42. In humans, serum adiponectin levels are negatively correlated with alanine aminotransferase (ALT) levels in obese Chinese individuals29. Serum total and HMW adiponectin levels have also been found to be lower in obese subjects with NAFLD compared with non‐obese subjects without NAFLD, in association with increased insulin resistance and elevated hepatic SREBP 1c mRNA expression (real‐time polymerase chain reaction)43. These animal and human studies have shown that adiponectin‐mediated signalling leads to enhanced fatty acid oxidation and reduced lipid synthesis, thus preventing hepatic steatosis.
Table 1. Adipokines in animal studies for non‐alcoholic fatty liver disease and diabetes.
| Animal models/cell types | Adipokine | Effects of interventions | Reference |
|---|---|---|---|
| Adiponectin | Treatment | ||
| ob/ob mice | Reduced expression in adipose tissue |
• Alleviates hepatic steatosis by reducing hepatic fat content and ALT levels • Reduces TNF‐α production |
29 |
| db/db mice | • Suppresses hepatic SREBP‐1 expression | 41 | |
| db/db mice |
• Alleviates hyperglycemia, hypertriglyceridemia, insulin resistance • Alleviates hepatic steatosis |
32 | |
| A‐FABP | |||
|
Obese mice lacking A‐FABP |
• A‐FABP deficiency protects against hepatic steatosis, insulin resistance, hyperinsulinemia and hyperglycemia; and reduces liver stearoyl‐CoA desaturase‐1, a rate‐limiting enzyme that promotes hepatic fat accumulation | 64 | |
| Diet‐induced obese mice with NASH | Elevated hepatic expression in Kupffer cells | • A‐FABP inhibition alleviates hepatic steatohepatitis | 67 |
| ob/ob mice | • A‐FABP inhibition alleviates diabetes | 75 | |
| FGF21 | Treatment | ||
| Diet‐induced obese mice | • Alleviates hepatic steatosis | 83 | |
| Diet‐induced obese mice |
• Reduces triglyceride levels • Reverses fatty liver disease via the inhibition of SREBP‐1 |
84 | |
|
ob/ob mice db/db mice |
• Reduces blood glucose and triglyceride levels | 81 |
A‐FABP, adipocyte‐fatty acid binding protein; ALT, alanine transaminase; FGF21, fibroblast growth factor‐21; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; SREBP‐1, sterol regulatory element‐binding protein; TNF‐α, tumor necrosis factor‐alpha.
Second, adiponectin exerts an anti‐inflammatory effect, thus protecting against secondary liver insults (in the ‘multi‐hit model’), largely by suppressing TNF‐α function through inhibition of its expression and opposition to its actions29. Adiponectin treatment suppresses the augmented production of TNF‐α in ob/ob mice29. In humans, decreased serum adiponectin levels and increased TNF‐α and soluble TNF‐α receptor 2 (TNFR2) levels correlate with the presence of NASH. Serum adiponectin levels are also inversely correlated with necro‐inflammation in NASH38. However, the relationship between adiponectin and fibrosis is more controversial, with some authors reporting raised adiponectin levels found in cirrhosis45, whereas others46 have shown a negative correlation between adiponectin and advanced hepatic fibrosis. Supportive of the antifibrotic effect of adiponectin, obese and diabetic mice with increased fibrosis lack physiological upregulation in adiponectin levels47.
Mitochondrial dysfunction contributes to the increased susceptibility to secondary liver injuries induced by obesity. Adiponectin has been shown to decrease hepatic mitochondrial dysfunction through induction of uncoupling protein 2 (UCP2), a mitochondrial inner membrane transporter. The protein and mRNA levels of UCP2 are decreased in liver tissues of adiponectin knockout mice and are upregulated by adiponectin treatment44. Adiponectin or UCP2 replenishment restores mitochondrial function and depletes lipid accumulation by reducing fatty acyl coenzyme A accumulation in livers of adiponectin knockout mice48.
Hypoadiponectinemia is also implicated in the pathogenesis of type 2 diabetes in obese subjects and in individuals with impaired hepatic glucose production. The HMW oligomer of adiponectin has been shown to be the major active form responsible for its insulin‐sensitizing effect in hepatocytes49. Similar to its antisteatotic effect, its glucose‐lowering effect is also partly mediated through AMPK, which in turn inhibits hepatic glucose production by decreasing the expression of key gluconeogenic genes, such as phosphoenolpyruvate carboxykinase and G6Pase49. We have shown that the magnitude of AMPK phosphorylation in liver tissue and the metabolic effects of adiponectin in db/db mice correlate with the expression of HMW adiponectin oligomers32. Similar to its involvement in NAFLD, adiponectin deficiency is also implicated in mitochondrial dysfunction and glucose homeostasis in adipocytes51. It has been shown in vitro that both mRNA expression and secreted levels of adiponectin are decreased in adipocytes with mitochondrial dysfunction induced by oligomycin A, and the reduced levels of adiponectin and insulin sensitivity in mature adipocytes reflect a decrease in mitochondrial respiratory function52.
As adiponectin plays such important causal roles in NAFLD and type 2 diabetes, linked by obesity‐related insulin resistance, it has been recognised to be a potential biomarker for the detection and prediction of NAFLD and type 2 diabetes, or both. In NAFLD, a score combining serum adiponectin, homeostasis model assessment‐insulin resistance (HOMA‐IR) index (cut‐off value ≥3.0) and serum type IV collagen 7S (cut‐off value ≥5.0 ng/mL) predicted approximately 90% of patients with early‐stage NASH, with a sensitivity of 94% and a specificity of 74%53. In another study, subjects with NASH had lower adiponectin levels compared with healthy controls, and a formula incorporating adiponectin, leptin and ghrelin yielded an area under receiver operating characteristic of 0.789 (P = 0.002), sensitivity of 82% and specificity of 76% for NASH54. As for type 2 diabetes, a large prospective, case–control study has shown that mean adiponectin concentrations were significantly lower in individuals with incidental type 2 diabetes than in controls55. Low adiponectin levels at baseline was associated with an increased risk of diabetes in Caucasians55. Low adiponectin, together with high TNF‐α at baseline, was also independently predictive of diabetes, and the combined use of serum adiponectin and TNFR2 levels were comparable to 2‐h post‐load glucose for diabetes prediction in Chinese subjects57 (Table 2). In a recent study to characterize prediagnosis trajectories of adiponectin in individuals who developed type 2 diabetes, female subjects and those with early‐onset diabetes (age at diagnosis <52 years) had a steeper decline in adiponectin levels than non‐diabetic controls58.
Table 2. Serum/hepatic adipokine levels in human subjects with non‐alcoholic fatty lover disease and/or diabetes.
| Clinical conditions | Adipokine | Associated changes | Reference |
|---|---|---|---|
| Adiponectin | |||
| Obesity | Reduced | Negative correlation with ALT | 29 |
| Obesity and NAFLD | Reduced (HMW and total) | Negative correlation with insulin resistance | 43 |
| NASH |
Reduced Reduced |
Negative correlation with necro‐inflammation |
38
54 |
| Early‐stage NASH | Reduced | 53 | |
| Advanced hepatic fibrosis | Reduced | 46 | |
| Type 2 diabetes | Reduced (ratio of HMW to total adiponectin) | 32 | |
| Type 2 diabetes | Reduced | Low baseline adiponectin and high TNF‐α are predictive of diabetes |
55
57 |
| Type 2 diabetes and NASH | Reduced | Low adiponectin and transforming growth factor‐β1 associated with advanced fibrosis in subjects with type 2 diabetes | 59 |
| A‐FABP | |||
| NAFLD | Elevated hepatic expression | In liver biopsies of NAFLD subjects | 69 |
| NAFLD | Elevated | Positive correlation with TNF‐α, HOMA‐IR and metabolic syndrome | 70 |
| NAFLD | Elevated | Positive correlation with advanced grades of necro‐inflammation and fibrosis | 72 |
| NAFLD and type 2 diabetes | Elevated | 71 | |
| Type 2 diabetes | Elevated | Positive correlation with fasting glucose and 2‐h glucose and predictor of T2DM | 78 |
| FGF21 | |||
| NAFLD | Elevated FGF21 mRNA expression |
In human liver tissues of NAFLD subjects Positive correlation with the degree of steatosis |
85 |
| IGT/type 2 diabetes | Elevated |
Negative correlation with whole body insulin sensitivity Positive correlation with hepatic insulin resistance |
88 |
| Type 2 diabetes | Elevated | Independent predictor of type 2 diabetes | 89 |
| Insulin resistance | Elevated | Associated with diabetes and insulin resistance | 90 |
A‐FABP, adipocyte‐fatty acid binding protein; ALT, alanine transaminase; FGF21, fibroblast growth factor‐21; HMW, high molecular weight; HOMA‐IR, homeostasis model assessment of insulin resistance; IGT, impaired glucose tolerance; IR, insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; TNF‐α, tumor necrosis factor‐alpha; TG, triglycerides.
In patients with both type 2 diabetes and NAFLD, low adiponectin levels were independently associated with NASH in a cross‐sectional study on type 2 diabetes patients with histologically‐diagnosed NAFLD. Low adiponectin, together with transforming growth factor (TGF)‐β1, were associated with advanced fibrosis, the more severe stage of NAFLD in subjects with type 2 diabetes. It has been postulated that type 2 diabetes patients with NAFLD might develop steatohepatitis and progressive fibrosis because of the lack of upregulation of adiponectin, which inhibits connective tissue growth factor (CTGF), a cell‐adhesion factor for hepatic stellate cells and a deciding factor for the development of fibrosis59. CTGF has been described as a profibrotic factor that mediates some TGF‐β1 responses, including apoptosis and fibrosis59. As type 2 diabetes patients have a more progressive course of NAFLD60, these results suggest that hypoadiponectinemia, present in type 2 diabetes, might play a key role in the progression of NAFLD in type 2 diabetes patients.
Adipocyte Fatty Acid Binding Protein
Adipocyte fatty acid binding protein (A‐FABP) is a cytosolic lipid‐binding chaperone mainly expressed in mature adipocytes and activated macrophages. It was initially thought to be a solely intracellular protein, but our group has recently identified the circulating form of A‐FABP in the human bloodstream61. It reversibly binds with a high affinity to hydrophobic ligands, such as saturated and unsaturated long‐chain fatty acids, and functions as a fatty acid chaperone, which facilitates fatty acid signaling by targeting and transporting fatty acid metabolites to the lipid signal transduction pathway62. Its expression is highly regulated during differentiation of adipocytes, and transcription of its mRNA is controlled by fatty acids, insulin and PPAR‐γ agonists62. Cross‐sectional and longitudinal studies have reported positive associations between A‐FABP levels and parameters of adiposity, insulin resistance and the metabolic syndrome61.
In relation to NAFLD, mice lacking A‐FABP were found to be strongly protected against hepatic steatosis64 and had reduced liver SCD‐1 activity, a rate‐limiting enzyme important for the conversion of saturated to monounsaturated fatty acid that contributes to hepatic fat accumulation65. Hepatic expression of A‐FABP in Kupffer cells has been shown to be elevated in chemically‐ and diet‐induced obese mice with NASH, likely forming a feed‐forward loop with JNK and c‐Jun66 (Table 1) to instigate an inflammatory response in Kupffer cells67, the hepatic macrophages that are responsible for recruiting a cluster of pro‐inflammatory cytokines to mediate transition from steatosis to steatohepatitis68. In keeping with this, elevated A‐FABP expression has been observed in subjects with NAFLD69 (Table 2). Cross‐sectional studies have shown an association of elevated A‐FABP levels with ultrasound‐diagnosed NAFLD in both healthy70 and type 2 diabetes subjects71. Furthermore, serum A‐FABP levels can distinguish NASH from steatosis, and elevated A‐FABP levels are independently associated with advanced grades of necro‐inflammation and fibrosis in liver biopsies72. These results strongly support the role of A‐FABP in the pathogenesis of obesity‐related fatty liver disease.
As for its role in diabetes, mice lacking A‐FABP are protected from development of insulin resistance, hyperinsulinemia, and hyperglycemia in the context of both dietary and genetic obesity64. Apolipoprotein E−/− mice lacking both adipocyte and macrophage fatty acid binding protein (FABP) have better insulin and glucose tolerance, and survival74. An orally active A‐FABP inhibitor has been shown to be effective in alleviating diabetes in animal models, and obesity‐induced adipose tissue JNK1 activity is attenuated in mice treated with A‐FABP inhibitor75.
In humans, significant reductions in A‐FABP concentration, together with a decrease in TNFR2 and high sensitivity C‐reactive protein, and an increase in adiponectin levels, were observed in obese individuals after bariatric surgery and intensive weight loss76. A‐FABP contributes to an improvement in HOMA‐IR index after weight loss, independent of pro‐inflammatory/anti‐inflammatory cytokine profile, thereby supporting its role in insulin‐sensitivity pathways in the morbidly obese76.
In a large population study, individuals with a genetic variant at the FABP gene locus, coinciding with the binding site for CCAAT/enhancer binding protein (C/EBP), had lower triglyceride levels and showed a reduced risk of obesity‐induced type 2 diabetes. This particular mutation was found to alter C/EBP binding and reduce the transcriptional activity of the human FABP gene promoter, as well as the adipose tissue A‐FABP expression of individuals carrying the variant77. The role of A‐FABP in predicting diabetes has also been shown in a 10‐year prospective study, whereby plasma A‐FABP level correlated positively with fasting glucose and 2‐h glucose and predicted the development of type 2 diabetes independent of the traditional risk factors that included obesity, insulin resistance, or glycemic indices78.
Like adiponectin, A‐FABP also has a dual role in the pathogenesis of NAFLD and type 2 diabetes, and would represent a useful biomarker for the prediction of NAFLD and type 2 diabetes. As animal studies have yielded promising results of A‐FABP blockade in alleviating steatosis and impaired glucose tolerance67, therapeutic inhibition of A‐FABP can potentially target the triad of obesity, diabetes and fatty liver disease.
Fibroblast Growth Factor‐21
Fibroblast growth factor 21 (FGF21), a polypeptide with 210 amino acid residues originally cloned from the mouse liver, is a metabolic hormone that regulates glucose and lipid metabolism. Obesity is associated with increased FGF21 expression in adipose tissues79. In obese rodents, adipocytes have been shown to be another important site of FGF21 production80. Thus, FGF21 can also be considered as an adipokine.
FGF21 activates cell signaling by binding to a heteromeric cell‐surface receptor tyrosine kinase complex composed of β‐Klotho and a fibroblast growth factor receptor, namely FGFR1c. Both β‐Klotho and FGFR1c are abundantly expressed in white adipose tissue (WAT), where FGF21‐regulated genes are involved in metabolic processes that include lipogenesis, lipolysis and fatty acid oxidation. Systemic administration and transgenic overexpression of FGF21 induce weight loss in obese mouse models through increases in energy expenditure without changing food intake81.
Adipose FGF21 acts as an autocrine factor in the fed state by regulating the activity of PPAR‐γ in adipose tissues. We have shown that both FGF21 mRNA expression and its protein release in vitro are markedly increased during conversion of human pre‐adipocytes into mature adipocytes, showing a differentiation dependent expression of FGF2180. Chronic treatment of the PPAR‐γ agonist, rosiglitazone, markedly enhances FGF21 production in both 3T3‐L1 murine adipocytes and human adipocytes80. In obese mice, the degree of FGF21 expression in several types of adipose tissue has been shown to be markedly raised, to levels comparable to that of its expression in the liver80.
In humans, serum FGF21 levels are also significantly elevated in obese subjects, thus providing evidence that adipose tissue is another important source of circulating FGF2180. In vivo, however, treatment with rosiglitazone leads to a reduction in circulating FGF21 levels in type 2 diabetes patients, likely as a result of the amelioration of diabetes‐related metabolic dysfunction, such as insulin resistance and raised FFA levels.
In mice, FGF21 plays a physiological role in suppressing the rate of lipolysis, functioning as a metabolic regulator of lipid metabolism in concert with growth hormone82. Its role in alleviating hepatic steatosis has been shown by the effect of systemic administration of FGF21 in diet‐induced obese mice (Table 1). Furthermore, adenovirus‐mediated knockdown of hepatic FGF21 leads to the development of fatty liver and dyslipidemia as a result of the altered expression of several key genes involved in hepatic lipid metabolism83. Chronic treatment with recombinant FGF21 also reduces serum and hepatic triglyceride levels, and reverses fatty liver disease in diet‐induced obese mice through the inhibition of SREBP‐1, the key transcription factor for lipogenesis84. In human liver tissues, FGF21 mRNA expression increases with the degree of steatosis85. These findings might suggest a compensatory increase in hepatic FGF21 expression in response to FGF21 resistance, and that FGF21 resistance might have contributed to the pathogenesis of NAFLD. Alternatively, as in the case of type 2 diabetes, the increase in FGF21 levels might be secondary to the metabolic perturbations associated with insulin resistance. A recent study suggested that adipose tissue inflammation in obesity, involving the JNK1 pathway, can lead to the suppression of β‐Klotho expression by TNF‐α and hence impaired FGF21 action in adipocytes86. This might also explain the mechanism that leads to FGF21 resistance in NAFLD.
The role of FGF21 in glucose metabolism was first suggested by the finding of a high throughput screening that FGF21 was one of the agents capable of increasing glucose uptake in 3T3‐L1 adipocytes81. The addition of recombinant FGF21 to adipocytes was found to induce insulin‐independent glucose uptake by enhancing the expression of glucose transporter 1 (GLUT1). Subsequently, treatment with recombinant FGF21 was found to reduce blood glucose and triglycerides to near normal levels in both ob/ob mice and db/db diabetic mice81 and chronic treatment with FGF21 in diabetic rhesus monkeys also ameliorated triglyceride and glucose controls87.
Despite beneficial effects of FGF21 on glucose and lipid homeostasis in animal models, elevated circulating FGF21 levels are present in obese diabetic db/db mice and obese/overweight humans80. This elevation in FGF21 levels were also found in humans with IGT and type 2 diabetes, and correlated directly with hepatic insulin resistance and inversely with whole‐body insulin sensitivity88 (Table 2). A high FGF21 level in non‐diabetic subjects has been shown to predict diabetes development during long‐term follow up in the Hong Kong Cardiovascular Risk Factor Prevalence Study89, suggesting that FGF21 resistance also occurs early in the course of dysglycemia and predisposes to diabetes development. The elevated serum FGF21 levels might be consequential to other metabolic disturbances, such as hyperinsulinemia or increased circulating FFA levels, in subjects with insulin resistance90.
In summary, these findings show that FGF21, together with adiponectin and A‐FABP, might serve as biomarkers for both NAFLD and dysglycemia.
Therapeutic Implications
Metformin
Various antidiabetic agents have been shown to confer beneficial effects on NAFLD. Metformin has been shown to reduce insulin resistance and aminotransferase levels associated with NAFLD91. Like adiponectin, metformin exerts its insulin‐sensitizing and antisteatotic effects, at least in part, through the AMPK‐mediated pathway (Table 3). In adipose tissues, it has recently been shown that metformin improves insulin resistance by enhancing glucose transporter 4 (GLUT4) translocation through AMPK‐mediated Cbl/c‐Cbl‐associated protein (CAP) signaling, thereby inhibiting differentiation of pre‐adipocytes to adipocytes. Knockdown of AMPK and JNK blocks metformin‐induced expression of CAP, implying that metformin stimulates the AMPK‐JNK‐CAP axis pathway92. Metformin also activates AMPK and reduces ACC protein levels in human adipose tissue93. In the liver, metformin acts through AMPK to stimulate fatty acid oxidation and decrease hepatic glucose production. Furthermore, metformin has been shown to induce hepatic FGF21 expression through AMPK activation. A strong dose‐dependent increase in FGF21 expression was observed in both rat and human hepatocytes treated with metformin, an effect that was blocked by the addition of an AMPK‐inhibitor94. Further studies are required to investigate if induction of hepatic FGF21 by metformin plays a significant role in mediating the metabolic benefits of metformin.
Table 3. Mechanisms of action of current antidiabetic agents on non‐alcoholic fatty liver disease.
| Class of antidiabetic agent | Example | Primary mechanism | Effects on liver or adipose tissue hormone expression | Actions in NAFLD | Reference |
|---|---|---|---|---|---|
| Biguanides | Metformin | Activates AMPK | Induces FGF21 expression in hepatocytes |
Improves insulin resistance Reduces aminotransferase levels Reduces hepatic glucose production Stimulates fatty acid oxidation in liver |
91 |
| Thiazolidinediones | Pioglitazone | Activates nuclear transcription factor PPAR‐γ |
Increases circulating adiponectin level Induces FGF21 expression in adipocytes |
Reduces aminotransferase levels Reduces hepatic steatosis, inflammation and fibrosis Improves hepatic insulin sensitivity |
96 |
| DPP‐4 Inhibitors | Sitagliptin, vildaglitin, linagliptin, saxagliptin | Inhibits DPP‐4 activity, increasing postprandial GLP‐1 concentrations |
Improves liver enzyme levels and hepatocyte ballooning Reduces plasma glucose and liver enzyme levels |
102 | |
| GLP‐1 Receptor Agonists | Exenatide, liraglutide | Activates AMPK in hepatocytes | Increases hepatic FGF21 expression and plasma FGF21 level |
Reduces hepatic lipogenesis Reduces diet‐induced hepatic pro‐inflammatory response Improves insulin sensitivity |
100 |
AMPK, adenosine monophosphate‐activated protein kinase; CAP, Cbl/c‐Cbl‐associated protein; DPP‐4, dipeptidyl peptidase‐4; FGF‐21, fibroblast growth factor‐21; GLP‐1, Glucagon‐like peptide‐1; GLUT‐4, glucose transporter 4; NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis; PPAR‐γ, peroxisome proliferator‐activated receptor‐gamma.
Thalizolidinediones
Pioglitazone, a PPAR‐γ agonist, has been recommended to treat steatohepatitis in patients with biopsy‐proven NASH95. Pioglitazone treatment in patients with NASH and dysglycemia (IGT or type 2 diabetes) was associated with improved aminotransferase levels, steatosis, inflammation and hepatocyte ballooning96. It exerts its therapeutic actions partly through adiponectin, with a 2.3‐fold increase in plasma levels significantly associated with improved hepatic insulin sensitivity and histological improvement in hepatic steatosis, necro‐inflammation and fibrosis in vitro97. Pioglitazone has also been shown to induce FGF21 expression in mouse and human adipocytes98, and animal studies have shown its role as an autocrine factor regulating the activity of PPAR‐γ in adipose tissues99. Whether FGF21 is involved in the increase in adiponectin expression by PPAR‐γ and hence the protection against NASH remains speculative.
Glucagon‐Like Peptide‐1 Agonists and Enhancers
Glucagon‐like peptide‐1 (GLP‐1) suppresses hepatic lipogenesis through activation of the AMPK pathway in hepatocytes. The inhibitory effects of GLP‐1 on hepatic fat accumulation and diet‐induced hepatic pro‐inflammatory response suggest a therapeutic role of GLP‐1 agonists in NAFLD100.
Liraglutide, a long‐acting GLP‐1 agonist, increases FGF‐21, FGFR mRNA and protein expression, and improved insulin sensitivity, in a mouse model of insulin resistance induced by a combination of adiponectin and apolipoprotein E deficiency, and high fat101. In addition, preliminary evidence suggests that dipeptidyl peptidase IV (DPP‐4) inhibitors, which enhances endogenous GLP‐1 levels by inhibiting its rapid degradation by DPP‐4, ameliorate liver enzymes and hepatocyte ballooning in NASH patients with type 2 diabetes102. In a pilot study, significant reduction in plasma glucose, hemoglobin A1c (HbA1c) and liver enzyme levels were observed after 4 months of treatment with sitagliptin in NAFLD patients with type 2 diabetes103. More studies are required to determine the role of DDP‐4 inhibitors on adipokines, which may or may not be similar to that of GLP‐1 agonists.
Future Therapeutic Targets
Agents that enhance adiponectin production might represent potential targets for the treatment or prevention of NAFLD and diabetes. Such agents might be derived from natural products, as exampled by the identification of two naturally‐occurring compounds (astragaloside II and isoastragaloside I) from the widely used medicinal herb, Radix Astragali, which can selectively increase adiponectin secretion in primary adipocytes. The two compounds further enhance adiponectin production in addition to the effect of rosiglitazone. These changes are associated with an alleviation of hyperglycemia, glucose intolerance and insulin resistance, and might also provide beneficial effects for NAFLD104. Recently, A‐FABP blockade has also shown promising results in animal models in alleviating obesity‐related NAFLD62. Therefore, therapeutic targets based on selective A‐FABP inhibition are also a promising area for further investigation. Whether FGF21, which is being actively researched in preclinical studies for the treatment of diabetes, can ameliorate NAFLD in humans remains to be investigated.
Role of Adipokines in the Screening for Dysglycemia in NAFLD
Importance of Screening
NAFLD renders a person 1.6‐times more likely to develop diabetes105. Obesity also increases the risk of diabetes in people with NAFLD, as the incidence in an urban Chinese population was shown to be highest in obese subjects with NAFLD (23.2%), when compared with the non‐obese group with NAFLD (11.1%) and those without NAFLD (4.3%)16. In addition, diabetes mellitus, obesity and old age were significant predictors of severe liver fibrosis60. Mortality amongst community‐diagnosed NAFLD patients was higher than the general population, and was associated with impaired fasting glucose, old age and cirrhosis10. Screening for dysglycemia in NAFLD should include an oral glucose tolerance test (OGTT) to diagnose prediabetes (impaired fasting glucose [IFG] and IGT), as individuals with prediabetes are already at risk of developing diabetes‐related complications106. Furthermore, NAFLD patients with prediabetes had worse hepatic insulin resistance than NAFLD patients with normal glucose tolerance and those without NAFLD107. Indeed, NAFLD patients with prediabetes had a similar degree of muscle and liver insulin resistance as NAFLD patients with type 2 diabetes107.
Oral Glucose Tolerance Test for Diagnosis of Dysglycemia in NAFLD
To detect prediabetes and type 2 diabetes, 75‐g OGTT, rather than fasting glucose alone, has been recommended in NAFLD patients, as fasting glucose (≥7.0 mmol/L) has been found to considerably underestimate the diabetes prevalence in Hong Kong Chinese108, and IGT with normal fasting plasma glucose is common (47%) among Hong Kong Chinese with biopsy‐proven NAFLD109. However, OGTT is notorious for being cumbersome to carry out, and has poor reproducibility with large intra‐individual variation in glucose responses110. The alternative use of HbA1c is also limited by its lower sensitivity in identifying prediabetes and type 2 diabetes than OGTT111. The measurement of adipokines could potentially serve to provide biomarkers that can enhance the detection of dysglycemia in NAFLD without the use of OGTT.
Role of Adipokines in Detecting Dysglycemia in NAFLD
We have discussed the potential diagnostic and prognostic roles of adipokines in detecting diabetes, as well as their effects in currently available antidiabetic agents in the treatment of NAFLD. In essence, low adiponectin, together with high TNF‐α at baseline, is independently predictive of diabetes57, with a performance comparable to that of 2‐h plasma glucose post OGTT. High A‐FABP and FGF21 levels are also a strong predictor of diabetes78. In the context of NAFLD, low adiponectin levels were independently associated with NASH in type 2 diabetes patients with NAFLD59. Further studies will be required to evaluate the diagnostic roles of adipokines specifically in patients with NAFLD.
Conclusion
Adipose tissue dysfunction is characterized by inflammation and adipokine dysregulation, and subsequent ectopic fat deposition in the abdominal viscera and liver, and insulin resistance. It significantly contributes to the development of obesity‐related conditions, including NAFLD and diabetes mellitus. Adipokines are important mediators of both lipid and glucose homeostasis. Adiponectin has antisteatotic, anti‐inflammatory and insulin‐sensitizing properties by promoting free fatty acid oxidation, reducing fatty acid influx to liver and de novo lipogenesis, as well as suppressing the action of pro‐inflammatory cytokines and gluconeogenesis. A‐FABP facilitates fatty acid signaling, which promotes hepatic fat accumulation and inhibition of A‐FABP in mice, has been shown to alleviate NAFLD and diabetes. Recombinant FGF21 administration has been shown to reverse fatty liver disease and improve glucose control in animal models. These adipokines have been implicated in currently‐available antidiabetic agents with beneficial effects on NAFLD. Adipokine‐based therapeutic agents for NAFLD and diabetes would represent a promising area for further investigation.
Acknowledgement
The authors declare no conflict of interest.
(J Diabetes Invest, doi: 10.1111/jdi.12093, 2013)
References
- 1.Ludwig J, Viggiano TR, McGill DB, et al Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980; 55: 434–438 [PubMed] [Google Scholar]
- 2.Ekstedt M, Franzen LE, Mathiesen UL, et al Long‐term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44: 865–873 [DOI] [PubMed] [Google Scholar]
- 3.Farrell GC, van ??????? RD, Gan L, et al NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 2012; 6: 149–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan JG, Zhu J, Li XJ, et al Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol 2005; 43: 508–514 [DOI] [PubMed] [Google Scholar]
- 5.Yun JW, Cho YK, Park JH, et al Abnormal glucose tolerance in young male patients with nonalcoholic fatty liver disease. Liver Int 2009; 29: 525–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, et al Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40: 1387–1395 [DOI] [PubMed] [Google Scholar]
- 7.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 2006; 40(Suppl 1): S5–S10 [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Xia B, Ma C, et al Prevalence and risk factors of fatty liver disease in the Shuiguohu district of Wuhan city, central China. Postgrad Med J 2007; 83: 192–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan JG, Li F, Cai XB, et al The importance of metabolic factors for the increasing prevalence of fatty liver in Shanghai factory workers. J Gastroenterol Hepatol 2007; 22: 663–668 [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Sanderson S, Lindor KD, et al The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005; 42: 132–138 [DOI] [PubMed] [Google Scholar]
- 11.Zhou YJ, Li YY, Nie YQ, et al Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. J Dig Dis 2012; 13: 153–160 [DOI] [PubMed] [Google Scholar]
- 12.Dam‐Larsen S, Becker U, Franzmann MB, et al Final results of a long‐term, clinical follow‐up in fatty liver patients. Scand J Gastroenterol 2009; 44: 1236–1243 [DOI] [PubMed] [Google Scholar]
- 13.Shibata M, Kihara Y, Taguchi M, et al Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle‐aged Japanese men. Diabetes Care 2007; 30: 2940–2944 [DOI] [PubMed] [Google Scholar]
- 14.He S, Bao W, Shao M, et al Risk factors for non‐alcoholic fatty liver disease in a Chinese population. Acta Gastroenterol Belg 2011; 74: 503–508 [PubMed] [Google Scholar]
- 15.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol 2007; 17: 863–869 [DOI] [PubMed] [Google Scholar]
- 16.Fan JG, Li F, Cai XB, et al Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol 2007; 22: 1086–1091 [DOI] [PubMed] [Google Scholar]
- 17.Chon CW, Kim BS, Cho YK, et al Effect of nonalcoholic Fatty liver disease on the development of type 2 diabetes in nonobese, nondiabetic korean men. Gut Liver 2012; 6: 368–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younossi ZM, Gramlich T, Matteoni CA, et al Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004; 2: 262–265 [DOI] [PubMed] [Google Scholar]
- 19.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010; 52: 1836–1846 [DOI] [PubMed] [Google Scholar]
- 20.Marchesini G, Brizi M, Bianchi G, et al Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001; 50: 1844–1850 [DOI] [PubMed] [Google Scholar]
- 21.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998; 114: 842–845 [DOI] [PubMed] [Google Scholar]
- 22.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363: 1341–1350 [DOI] [PubMed] [Google Scholar]
- 23.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol 2009; 3: 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bugianesi E, Gastaldelli A, Vanni E, et al Insulin resistance in non‐diabetic patients with non‐alcoholic fatty liver disease: sites and mechanisms. Diabetologia 2005; 48: 634–642 [DOI] [PubMed] [Google Scholar]
- 25.Byrne CD. Dorothy Hodgkin Lecture 2012* Non‐alcoholic fatty liver disease, insulin resistance and ectopic fat: a new problem in diabetes management. Diabet Med 2012; 29: 1098–1107 [DOI] [PubMed] [Google Scholar]
- 26.Donnelly KL, Smith CI, Schwarzenberg SJ, et al Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115: 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirosumi J, Tuncman G, Chang L, et al A central role for JNK in obesity and insulin resistance. Nature 2002; 420: 333–336 [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Xu A, Chung SK, et al Selective inactivation of c‐Jun NH2‐terminal kinase in adipose tissue protects against diet‐induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes 2011; 60: 486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu A, Wang Y, Keshaw H, et al The fat‐derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 2003; 112: 91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoo RL, Chow WS, Yau MH, et al Adiponectin mediates the suppressive effect of rosiglitazone on plasminogen activator inhibitor‐1 production. Arterioscler Thromb Vasc Biol 2007; 27: 2777–2782 [DOI] [PubMed] [Google Scholar]
- 31.Tsuchida A, Yamauchi T, Takekawa S, et al Peroxisome proliferator‐activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity‐related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes 2005; 54: 3358–3370 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Lam KS, Chan L, et al Post‐translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem 2006; 281: 16391–16400 [DOI] [PubMed] [Google Scholar]
- 33.Stumvoll M, Tschritter O, Fritsche A, et al Association of the T‐G polymorphism in adiponectin (exon 2) with obesity and insulin sensitivity: interaction with family history of type 2 diabetes. Diabetes 2002; 51: 37–41 [DOI] [PubMed] [Google Scholar]
- 34.Stefan N, Hennige AM, Staiger H, et al Alpha2‐Heremans‐Schmid glycoprotein/fetuin‐A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006; 29: 853–857 [DOI] [PubMed] [Google Scholar]
- 35.Brix JM, Stingl H, Hollerl F, et al Elevated Fetuin‐A concentrations in morbid obesity decrease after dramatic weight loss. J Clin Endocrinol Metab 2010; 95: 4877–4881 [DOI] [PubMed] [Google Scholar]
- 36.Sun Q, Cornelis MC, Manson JE, et al Plasma levels of fetuin‐a and hepatic enzymes and risk of type 2 diabetes in women in the u.s. Diabetes 2013; 62: 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masaki T, Chiba S, Tatsukawa H, et al Adiponectin protects LPS‐induced liver injury through modulation of TNF‐alpha in KK‐Ay obese mice. Hepatology 2004; 40: 177–184 [DOI] [PubMed] [Google Scholar]
- 38.Hui JM, Hodge A, Farrell GC, et al Beyond insulin resistance in NASH: TNF‐alpha or adiponectin? Hepatology 2004; 40: 46–54 [DOI] [PubMed] [Google Scholar]
- 39.Polyzos SA, Toulis KA, Goulis DG, et al Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Metabolism 2011; 60: 313–326 [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Minokoshi Y, et al Adiponectin stimulates glucose utilization and fatty‐acid oxidation by activating AMP‐activated protein kinase. Nat Med 2002; 8: 1288–1295 [DOI] [PubMed] [Google Scholar]
- 41.Awazawa M, Ueki K, Inabe K, et al Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 2009; 382: 51–56 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Zhou M, Lam KS, et al Protective roles of adiponectin in obesity‐related fatty liver diseases: mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol 2009; 53: 201–212 [DOI] [PubMed] [Google Scholar]
- 43.Pettinelli P, Videla LA. Up‐regulation of PPAR‐gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP‐1c induction. J Clin Endocrinol Metab 2011; 96: 1424–1430 [DOI] [PubMed] [Google Scholar]
- 44.Zhou M, Xu A, Tam PK, et al Mitochondrial dysfunction contributes to the increased vulnerabilities of adiponectin knockout mice to liver injury. Hepatology 2008; 48: 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tietge UJ, Boker KH, Manns MP, et al Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab 2004; 287: E82–E89 [DOI] [PubMed] [Google Scholar]
- 46.Savvidou S, Hytiroglou P, Orfanou‐Koumerkeridou H, et al Low serum adiponectin levels are predictive of advanced hepatic fibrosis in patients with NAFLD. J Clin Gastroenterol 2009; 43: 765–772 [DOI] [PubMed] [Google Scholar]
- 47.Ikejima K, Okumura K, Kon K, et al Role of adipocytokines in hepatic fibrogenesis. J Gastroenterol Hepatol 2007; 22(Suppl 1): S87–S92 [DOI] [PubMed] [Google Scholar]
- 48.Zhou M, Xu A, Lam KS, et al Rosiglitazone promotes fatty acyl CoA accumulation and excessive glycogen storage in livers of mice without adiponectin. J Hepatol 2010; 53: 1108–1116 [DOI] [PubMed] [Google Scholar]
- 49.Berg AH, Combs TP, Du X, et al The adipocyte‐secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001; 7: 947–953 [DOI] [PubMed] [Google Scholar]
- 50.Pajvani UB, Hawkins M, Combs TP, et al Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione‐mediated improvement in insulin sensitivity. J Biol Chem 2004; 279: 12152–12162 [DOI] [PubMed] [Google Scholar]
- 51.Iwabu M, Yamauchi T, Okada‐Iwabu M, et al Adiponectin and AdipoR1 regulate PGC‐1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010; 464: 1313–1319 [DOI] [PubMed] [Google Scholar]
- 52.Wang CH, Wang CC, Huang HC, et al Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J 2012; 280: 1039–1050 [DOI] [PubMed] [Google Scholar]
- 53.Shimada M, Kawahara H, Ozaki K, et al Usefulness of a combined evaluation of the serum adiponectin level, HOMA‐IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol 2007; 102: 1931–1938 [DOI] [PubMed] [Google Scholar]
- 54.Machado MV, Coutinho J, Carepa F, et al How adiponectin, leptin, and ghrelin orchestrate together and correlate with the severity of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2012; 24: 1166–1172 [DOI] [PubMed] [Google Scholar]
- 55.Spranger J, Kroke A, Mohlig M, et al Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003; 361: 226–228 [DOI] [PubMed] [Google Scholar]
- 56.Tabak AG, Brunner EJ, Miller MA, et al Low serum adiponectin predicts 10‐year risk of type 2 diabetes and HbA1c independently of obesity, lipids, and inflammation: Whitehall II study. Horm Metab Res 2009; 41: 626–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woo YC, Tso AW, Xu A, et al Combined use of serum adiponectin and tumor necrosis factor‐alpha receptor 2 levels was comparable to 2‐hour post‐load glucose in diabetes prediction. PLoS One 2012; 7: e36868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabak AG, Carstensen M, Witte DR, et al Adiponectin trajectories before type 2 diabetes diagnosis: Whitehall II study. Diabetes Care 2012; 35: 2540–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leite NC, Salles GF, Cardoso CR, et al Serum biomarkers in type 2 diabetic patients with non‐alcoholic steatohepatitis and advanced fibrosis. Hepatol Res 2012; doi: 10.1111/j.1872‐034X.2012.01106.x [DOI] [PubMed] [Google Scholar]
- 60.Angulo P, Keach JC, Batts KP, et al Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999; 30: 1356–1362 [DOI] [PubMed] [Google Scholar]
- 61.Xu A, Wang Y, Xu JY, et al Adipocyte fatty acid‐binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 2006; 52: 405–413 [DOI] [PubMed] [Google Scholar]
- 62.Furuhashi M, Hotamisligil GS. Fatty acid‐binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 2008; 7: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu A, Tso AW, Cheung BM, et al Circulating adipocyte‐fatty acid binding protein levels predict the development of the metabolic syndrome: a 5‐year prospective study. Circulation 2007; 115: 1537–1543 [DOI] [PubMed] [Google Scholar]
- 64.Uysal KT, Scheja L, Wiesbrock SM, et al Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology 2000; 141: 3388–3396 [DOI] [PubMed] [Google Scholar]
- 65.Maeda K, Cao H, Kono K, et al Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 2005; 1: 107–119 [DOI] [PubMed] [Google Scholar]
- 66.Hui X, Li H, Zhou Z, et al Adipocyte fatty acid‐binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c‐Jun NH2‐terminal kinases and activator protein‐1. J Biol Chem 2010; 285: 10273–10280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoo RL, Lee IP, Zhou M, et al Pharmacological inhibition of adipocyte fatty acid binding protein alleviates both acute liver injury and non‐alcoholic steatohepatitis in mice. J Hepatol 2012; 58: 358–364 [DOI] [PubMed] [Google Scholar]
- 68.Kremer M, Thomas E, Milton RJ, et al Kupffer cell and interleukin‐12‐dependent loss of natural killer T cells in hepatosteatosis. Hepatology 2010; 51: 130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westerbacka J, Kolak M, Kiviluoto T, et al Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin‐resistant subjects. Diabetes 2007; 56: 2759–2765 [DOI] [PubMed] [Google Scholar]
- 70.Kim YC, Cho YK, Lee WY, et al Serum adipocyte‐specific fatty acid‐binding protein is associated with nonalcoholic fatty liver disease in apparently healthy subjects. J Nutr Biochem 2011; 22: 289–292 [DOI] [PubMed] [Google Scholar]
- 71.Koh JH, Shin YG, Nam SM, et al Serum adipocyte fatty acid‐binding protein levels are associated with nonalcoholic fatty liver disease in type 2 diabetic patients. Diabetes Care 2009; 32: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milner KL, van der Poorten D, Xu A, et al Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology 2009; 49: 1926–1934 [DOI] [PubMed] [Google Scholar]
- 73.Hotamisligil GS, Johnson RS, Distel RJ, et al Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 1996; 274: 1377–1379 [DOI] [PubMed] [Google Scholar]
- 74.Boord JB, Maeda K, Makowski L, et al Combined adipocyte‐macrophage fatty acid‐binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E‐deficient mice. Circulation 2004; 110: 1492–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furuhashi M, Tuncman G, Gorgun CZ, et al Treatment of diabetes and atherosclerosis by inhibiting fatty‐acid‐binding protein aP2. Nature 2007; 447: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simon I, Escote X, Vilarrasa N, et al Adipocyte fatty acid‐binding protein as a determinant of insulin sensitivity in morbid‐obese women. Obesity (Silver Spring) 2009; 17: 1124–1128 [DOI] [PubMed] [Google Scholar]
- 77.Tuncman G, Erbay E, Hom X, et al A genetic variant at the fatty acid‐binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci USA 2006; 103: 6970–6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tso AW, Xu A, Sham PC, et al Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10‐year prospective study in a Chinese cohort. Diabetes Care 2007; 30: 2667–2672 [DOI] [PubMed] [Google Scholar]
- 79.Mraz M, Bartlova M, Lacinova Z, et al Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor‐21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009; 71: 369–375 [DOI] [PubMed] [Google Scholar]
- 80.Zhang X, Yeung DC, Karpisek M, et al Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–1253 [DOI] [PubMed] [Google Scholar]
- 81.Kharitonenkov A, Shiyanova TL, Koester A, et al FGF‐21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen W, Hoo RL, Konishi M, et al Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem 2011; 286: 34559–34566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Badman MK, Pissios P, Kennedy AR, et al Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007; 5: 426–437 [DOI] [PubMed] [Google Scholar]
- 84.Xu J, Lloyd DJ, Hale C, et al Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet‐induced obese mice. Diabetes 2009; 58: 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H, Fang Q, Gao F, et al Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol 2010; 53: 934–940 [DOI] [PubMed] [Google Scholar]
- 86.Diaz‐Delfin J, Hondares E, Iglesias R, et al TNF‐alpha represses beta‐Klotho expression and impairs FGF21 action in adipose cells: involvement of JNK1 in the FGF21 pathway. Endocrinology 2012; 153: 4238–4245 [DOI] [PubMed] [Google Scholar]
- 87.Kharitonenkov A, Wroblewski VJ, Koester A, et al The metabolic state of diabetic monkeys is regulated by fibroblast growth factor‐21. Endocrinology 2007; 148: 774–781 [DOI] [PubMed] [Google Scholar]
- 88.Chavez AO, Molina‐Carrion M, Abdul‐Ghani MA, et al Circulating fibroblast growth factor‐21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009; 32: 1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C, Cheung BM, Tso AW, et al High plasma level of fibroblast growth factor 21 is an Independent predictor of type 2 diabetes: a 5.4‐year population‐based prospective study in Chinese subjects. Diabetes Care 2011; 34: 2113–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Semba RD, Sun K, Egan JM, et al Relationship of serum fibroblast growth factor 21 with abnormal glucose metabolism and insulin resistance: the Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2012; 97: 1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marchesini G, Brizi M, Bianchi G, et al Metformin in non‐alcoholic steatohepatitis. Lancet 2001; 358: 893–894 [DOI] [PubMed] [Google Scholar]
- 92.Lee JO, Lee SK, Kim JH, et al Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP‐activated protein kinase (AMPK)‐Mediated Cbl/CAP Signaling in 3T3‐L1 Preadipocyte cells. J Biol Chem 2012; 287: 44121–44129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boyle JG, Logan PJ, Jones GC, et al AMP‐activated protein kinase is activated in adipose tissue of individuals with type 2 diabetes treated with metformin: a randomised glycaemia‐controlled crossover study. Diabetologia 2011; 54: 1799–1809 [DOI] [PubMed] [Google Scholar]
- 94.Nygaard EB, Vienberg SG, Orskov C, et al Metformin Stimulates FGF21 Expression in Primary Hepatocytes. Exp Diabetes Res 2012; 2012: 465282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chalasani N, Younossi Z, Lavine JE, et al The diagnosis and management of non‐alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55: 2005–2023 [DOI] [PubMed] [Google Scholar]
- 96.Belfort R, Harrison SA, Brown K, et al A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006; 355: 2297–2307 [DOI] [PubMed] [Google Scholar]
- 97.Gastaldelli A, Harrison S, Belfort‐Aguiar R, et al Pioglitazone in the treatment of NASH: the role of adiponectin. Aliment Pharmacol Ther 2010; 32: 769–775 [DOI] [PubMed] [Google Scholar]
- 98.Oishi K, Tomita T. Thiazolidinediones are potent inducers of fibroblast growth factor 21 expression in the liver. Biol Pharm Bull 2011; 34: 1120–1121 [DOI] [PubMed] [Google Scholar]
- 99.Dutchak PA, Katafuchi T, Bookout AL, et al Fibroblast growth factor‐21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell 2012; 148: 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ben‐Shlomo S, Zvibel I, Shnell M, et al Glucagon‐like peptide‐1 reduces hepatic lipogenesis via activation of AMP‐activated protein kinase. J Hepatol 2011; 54: 1214–1223 [DOI] [PubMed] [Google Scholar]
- 101.Yang M, Zhang L, Wang C, et al Liraglutide increases FGF‐21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One 2012; 7: e48392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yilmaz Y, Yonal O, Deyneli O, et al Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg 2012; 75: 240–244 [PubMed] [Google Scholar]
- 103.Iwasaki T, Yoneda M, Inamori M, et al Sitagliptin as a novel treatment agent for non‐alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology 2011; 58: 2103–2105 [DOI] [PubMed] [Google Scholar]
- 104.Xu A, Wang H, Hoo RL, et al Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology 2009; 150: 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kasturiratne A, Weerasinghe S, Dassanayake AS, et al Influence of non‐alcoholic fatty liver disease on the development of diabetes mellitus. J Gastroenterol Hepatol 2012; ???: ???–??? [DOI] [PubMed] [Google Scholar]
- 106.DeFronzo RA, Abdul‐Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol 2011; 108: 3B–24B [DOI] [PubMed] [Google Scholar]
- 107.Ortiz‐Lopez C, Lomonaco R, Orsak B, et al Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care 2012; 35: 873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Janus ED, Watt NM, Lam KS, et al The prevalence of diabetes, association with cardiovascular risk factors and implications of diagnostic criteria (ADA 1997 and WHO 1998) in a 1996 community‐based population study in Hong Kong Chinese. Hong Kong Cardiovascular Risk Factor Steering Committee. American Diabetes Association. Diabet Med 2000; 17: 741–745 [DOI] [PubMed] [Google Scholar]
- 109.Wong VW, Hui AY, Tsang SW, et al Prevalence of undiagnosed diabetes and postchallenge hyperglycaemia in Chinese patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2006; 24: 1215–1222 [DOI] [PubMed] [Google Scholar]
- 110.Ko GT, Chan JC, Woo J, et al The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem 1998; 35(Pt 1): 62–67 [DOI] [PubMed] [Google Scholar]
- 111.Bianchi C, Miccoli R, Bonadonna RC, et al Pathogenetic mechanisms and cardiovascular risk: differences between HbA1c and oral glucose tolerance test for the diag‐nosis of glucose tolerance. Diabetes Care 2012; 35: 2607–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]


