Abstract
Aims/Introduction
The aim of the present study was to investigate whether non‐surgical periodontal treatment reduces glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) levels in diabetic patients.
Materials and Methods
An electronic search was carried out on MEDLINE (through PubMed interface), EMBASE and the Cochrane Central Register of Controlled Trials. Randomized controlled trials with a minimum of 3 months follow up were included. The risk of bias was assessed for each study. A meta‐analysis was carried out to evaluate the effect of non‐surgical periodontal treatment on HbA1c and FPG levels. The effect of the adjunctive use of antimicrobials was also assessed.
Results
A total of 15 studies were included. A reduction of −0.38% (95% confidence interval [CI] −0.23 to −0.53) after 3–4 months (P < 0.001) and of −0.31% (95% CI 0.11 to −0.74) after 6 months (P = 0.15) of follow‐up was found for HbA1c, favoring the treatment group. Similarly, in treated patients, a significantly greater decrease in FPG was observed in respect to control participants. Such difference amounted to −9.01 mg/dL (95% CI −2.24 to −15.78) after 3–4 months (P = 0.009) and −13.62 mg/dL (95% CI 0.45 to −27.69) after 6 months (P = 0.06) from treatment, respectively. In participants treated with adjunctive antimicrobials, a non‐significant increase of HbA1c was observed 3 months after treatment, whereas FPG decreased by 0.27 mg/dL (95% CI 39.56 to −40.11; P = 0.99).
Conclusions
The meta‐analysis showed that non‐surgical periodontal treatment improves metabolic control in patients with both periodontitis and diabetes.
Keywords: Diabetes, Metabolic control, Periodontal diseases
Introduction
Type 2 diabetes mellitus is a highly prevalent metabolic disease that causes an impairment in glycemic control1. Such impairment can cause a decrease of polymorphonucleate leukocytes activity and damage to microvascular endothelium, either of which can increase the susceptibility to periodontal disease2.
The presence of a chronic infection, such as periodontitis, might induce an increase of circulating cytokines and soluble factors (such as C‐reactive protein [CRP], interleukin‐1β [IL‐1β], interleukin‐6 [IL‐6], tumor necrosis factor‐α [TNF‐α] and prostaglandin‐E2 [PGE2]), which in turn increases the general inflammatory burden in the organism5. These events, as a result of low‐grade chronic infection, might alter the insulin activity, impairing glycemic control9. Furthermore, recent epidemiological studies have correlated the presence of periodontal diseases to poor glycemic control in patients with diabetes11.
Since 1960, it has been hypothesized that periodontal treatment can have beneficial effects on glycemic control in diabetic patients with severe periodontal conditions13. Both surgical and non‐surgical periodontal treatment can decrease the systemic inflammatory burden, allowing better glycemic control8, even if some studies did not report a significant improvement15.
Some systematic reviews described a significant positive effect of periodontal non‐surgical treatment on glycemic control despite a lack of robustness and homogeneity among studies17.
The present study aimed at evaluating the effect of non‐surgical periodontal treatment (NSPT) on glycemic control in patients affected by diabetes and periodontal diseases.
Materials and Methods
Search Strategy
The electronic search was carried out on MEDLINE (through PubMed interface), EMBASE and the Cochrane Central Register of Controlled Trials. A search string was created ad hoc combining keywords with the use of boolean operators ‘AND’ and ‘OR’. The search string was (periodont*) AND (diabet* OR ‘non insulin dependent diabetes’ OR ‘niddm’ OR ‘insulin dependent diabetes’ OR ‘iddm’ OR ‘type 1 diabetes’ OR ‘t1dm’ OR ‘type 2 diabetes’ OR ‘t2dm’) AND (‘therapy’ OR ‘treatment’ OR ‘intervention’). Results were limited by the year of publication (from 1970), and the last search was carried out in October 2012. In addition, a manual search was carried out considering the reference lists of the selected articles and articles published in the Journal of Clinical Periodontology, Journal of Periodontology, Journal of Dental Research, Journal of Dentistry, Journal of Periodontal Research, International Journal of Periodontics and Restorative Dentistry, Periodontology 2000, Odontology, Clinical Oral Investigations, Annals of Periodontology, Journal of American Dental Association, British Dental Journal, Community Dentistry and Oral Epidemiology, Diabetes, Diabetes Care, Diabetes & Metabolic Syndrome, Diabetes & Metabolism and Annals of Internal Medicine. No language restriction was placed.
Study Selection Criteria
The following inclusion criteria had to be met:
randomized controlled studies on human subjects;
intervention studies on diabetic patients with periodontal diseases;
a minimum of 3 months of follow up after intervention;
reporting data about glycated hemoglobin (HbA1c) and/or fasting plasma glucose (FPG) modification after treatment; and
clear presentation of population demographic characteristics.
A first screening was based on title and abstract.
Study Quality Assessment
Study quality was assessed independently by two authors (SC and MDF) according to the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Library, http://www.cochrane-handbook.org, Chapter 8). The following parameters were considered: (i) random sequence generation; (ii) allocation concealment; (iii) blinding of participants and personnel; (iv) incomplete data outcome; and (v) selective reporting. If no random sequence generation was present (high risk of bias), the study was excluded. In cases of randomization method not reported, allocation concealment control or blinding, such parameters were considered at unclear risk of bias. If two or more parameters were evaluated at unclear or high risk of bias, the study was considered at moderate risk and included in the review. Otherwise, the studies were considered at low risk of bias.
Data Extraction and Statistical Analysis
The following parameters were extracted from each selected study and recorded by two authors (SC and MDF) independently: (i) demographics (age, country, sex); (ii) definition of periodontal disease and of diabetes; (iii) sample group size; (iv) characteristics of periodontal treatment; (v) follow‐up duration; and (vi) evaluated parameters (HbA1c and FPG) chosen accordingly with previous systematic reviews18.
Data regarding modifications of HbA1c and FPG between follow up and baseline examination were included in the meta‐analysis. The meta‐analysis was made using Review Manager 5.1 (Cochrane Library, http://ims.cochrane.org/revman). Non‐surgical periodontal treatment (NSPT) was compared with no treatment. A further comparison was made between NSPT alone vs NSPT with any adjunctive antimicrobial substance. Two time frames were considered: (i) 3–4 months of follow up; and (ii) 6 months.
According to a previous systematic review18; if not reported, the absolute difference of the selected parameters (∆P) between baseline (t0) and the end (t1) of the study was estimated as follows:
where P represented the chosen parameter.
The variance (and consequently the standard deviation) of ∆P, if not reported, was estimated as follows18:
where S ∆P2 is the variance of ∆P, St02 is the variance of P at baseline, St12 is the variance of P at follow‐up visit and r is the correlation between St0 and St1 (r = 0.5 as described in previous studies18).
When two or more treatment or control groups had to be pooled to be included in the comparison, the weighted mean was computed and the variance of the pooled group was computed as follows21:
where n is the number of observations, SC2 is the combined variance, S12 and S22 are the variance of the two groups, X1 and X2 are the mean values of the two groups and XC is the mean value of the combined group.
The meta‐analysis was carried out using an inverse variance statistical method and random effects model comparing weighted mean difference.
Results
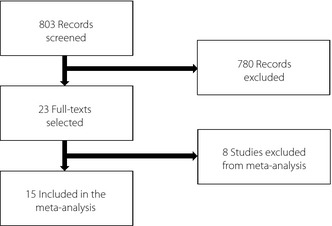
The flowchart of the article selection process is shown in Figure 1. The initial research retrieved 803 articles. After title and abstract screening, 23 articles were identified as potentially relevant. The full text of these articles was obtained and evaluated for inclusion. Six articles were excluded, because they were not coherent with the methods of the present study. The risk of bias evaluation of the included articles is shown in Figure 2. One article was then excluded after the risk of bias evaluation, because no random allocation was described22. Another study was excluded because of the impossibility of the calculation of standard deviations in the meta‐analysis23. A total of 15 articles were finally considered for the meta‐analysis24. The main characteristics of the selected studies are summarized in Table 1.
Figure 1.

Flow chart of article selection process.
Figure 2.
Risk of bias evaluation graph.
Table 1. Main characteristics of the study.
| Study | Year | Population | Definition of diabetes | Definition of periodontal disease |
|---|---|---|---|---|
| Rocha et al.23 | 2001 | Type 2 diabetes mellitus | Type 2 diabetes mellitus diagnosis ‘confirmed’ | More than one tooth with PD ≥3 mm |
| Al‐Mubarak et al.24 | 2002 | Type 1 and type 2 diabetes mellitus | Type 1 or type 2 diabetes mellitus for more than 1 year | At least 14 teeth; with calculus in at least four teeth in two different quadrants. PD ≥5 mm, but <8 mm in at least one site in four teeth in at least two different quadrants |
| Rodrigues et al.25 | 2003 | Type 2 diabetes mellitus | Type 2 diabetes mellitus diagnosis ‘confirmed’ | More than one site with PD ≥5 mm and more than two teeth with CAL ≥6 mm |
| Kiran et al.26 | 2005 | Type 2 diabetes mellitus | 6% < HbA1c < 8% | NR |
| Jones et al.27 | 2007 | Poorly controlled diabetes | HbA1c > 8.5% within the last 6 months | CPITN scores of ≥3 in at least two sextants |
| Singh et al.30 | 2008 | Type 2 diabetes mellitus | Type 2 diabetes mellitus diagnosis ‘confirmed’ | More than 30% teeth with PD ≥4 mm |
| Llambes et al.28 | 2008 | Type 1 diabetes | Definition of American Diabetes Association (1994) | More five sites with PD ≥5 mm and CAL ≥3 mm |
| O'Connell et al.29 | 2008 | Type 2 diabetes mellitus | HbA1c > 8% | More than one tooth with PD ≥5 mm and more than 2 teeth with CAL ≥6 mm |
| Katagiri et al.32 | 2009 | Type 2 diabetes mellitus | 6.5% < HbA1c < 10% | More than 11 remaining teeth; more than 2 sites with PD ≥4 mm |
| Al‐Zahrani et al.31 | 2011 | Type 2 diabetes mellitus | Type 2 diabetes mellitus diagnosis ‘confirmed’ | CAL ≥3 mm at 30% sites: ≥20 remaining teeth |
| Koromantzos et al.35 | 2011 | Type 2 diabetes mellitus | Type 2 diabetes mellitus diagnosis ‘confirmed’ | More than eight sites with PD ≥6 mm and more than four sites with CAL ≥5 mm |
| Chen et al.33 | 2011 | Type 2 diabetes mellitus | NR | Mean CAL ≥1 mm |
| Sun et al.36 | 2011 | Type 2 diabetes mellitus | 7.5% ≤ HbA1c ≤ 9.5% | More than 30% teeth with AL >4 mm or more than 60% teeth with PD >4 mm and AL >3 mm |
| Engebretson et al.34 | 2011 | Type 2 diabetes mellitus | Type 2 diabetes mellitus diagnosis ‘confirmed’ | Loss of clinical attachment greater than 5 mm in at least one site in each jaw quadrant |
| Moeintaghavi et al.37 | 2012 | Type 2 diabetes mellitus | HbA1c > 7% | Armitage49; American Academy of Periodontology |
AL, attachment loss; CAL, clinical attachment loss; CPITN, Community Peridontal Index of Treatment Needs; Hba1c, glycated hemoglobin; NR, not reported; PD: probing depth.
Periodontal Treatment and HbA1c
Periodontal non‐surgical treatment was compared with no treatment in eight studies. Seven of them (accounting for a total of 678 participants)25 presented data at 3–4 months follow up after treatment, and three studies (235 participants)33 presented data at 6 months follow up. At the 3–4 months follow up, the computed weighted mean difference was −0.38% (95% confidence interval [CI] −0.23 to −0.53; Figure 3), and at 6 months it was −0.31% (95% CI 0.11 to −0.74; Figure 4), favoring the treatment groups. Considering the meta‐analysis, heterogeneity of the study outcomes was statistically significant for both comparisons.
Figure 3.

Non‐surgical periodontal treatment vs no treatment: 3‐month glycated hemoglobin (%) difference between baseline and end of treatment. CI, confidence interval; df, degrees of freedom; SD, standard deviation.
Figure 4.

Non‐surgical periodontal treatment vs no treatment: 6‐month glycated hemoglobin (%) difference between baseline and end of treatment. CI, confidence interval; df, degrees of freedom; SD, standard deviation.
The comparison between periodontal non‐surgical treatment alone vs treatment with adjunctive antimicrobial devices was made in five studies (208 participants)26, which presented data at 3–4 months follow up. One study presented data for this comparison at 6 months follow up, showing 2.3 ± 2.69% increase of HbA1c in the control group and 2.5 ± 2.77% increase in the test group without significant difference24. After 3–4 months follow up, the computed weighted mean difference was 0.13% (95% CI 0.35 to −0.10; Figure 5), favoring the control group. The heterogeneity was not significant (P = 0.47).
Figure 5.

Non‐surgical periodontal treatment and adjunctive antimicrobials vs non‐surgical periodontal treatment: 3‐month glycated hemoglobin (%) difference between baseline and end of treatment. Study heterogeneity cannot be observed (P = 0.47). CI, confidence interval; df, degrees of freedom; SD, standard deviation.
Periodontal Treatment and FPG
Periodontal non‐surgical treatment was compared with no treatment in six studies. Five of these studies (412 participants)27 presented data at 3–4 months follow up, and two studies (175 participants)33 presented data at 6 months follow up. At 3–4 months, the computed weighted mean difference was −9.01 mg/dL (95% CI −2.24 to −15.78; Figure 6), and at 6 months it was −13.62 mg/dL (95% CI 0.45 to −27.69; Figure 7), favoring the treatment groups. The heterogeneity calculated in the meta‐analysis was not significant in both comparisons (P = 0.09 comparing no treatment vs treatment at 3–4 months and P = 0.50 at 6 months).
Figure 6.

Non‐surgical periodontal treatment vs no treatment: 3‐month fasting plasma glucose (mg/dL) difference between baseline and end of treatment. CI, confidence interval; df, degrees of freedom; SD, standard deviation.
Figure 7.

Non‐surgical periodontal treatment vs no treatment: 6‐month glycated hemoglobin (%) difference between baseline and end of treatment. Study heterogeneity cannot be observed (P = 0.50). CI, confidence interval; df, degrees of freedom; SD, standard deviation.
Two studies (60 participants)26 with a 3 to 4‐month follow up evaluated the effect of adjunctive antimicrobial devices on periodontal treatment. No studies presented data for this comparison at 6 months follow up. After 3–4 months, the computed weighted mean difference was −0.27 mg/dL (95% CI 39.56 to −40.11; Figure 8), favoring the control group (heterogeneity P = 0.46).
Figure 8.

Non‐surgical periodontal treatment and adjunctive vs Non‐surgical periodontal treatment: 3‐month fasting plasma glucose (mg/dL) difference between baseline and end of treatment. Study heterogeneity cannot be observed (P = 0.46). CI, confidence interval; df, degrees of freedom; SD, standard deviation.
Discussion
The present study aimed at evaluating if non‐surgical periodontal treatment alone or with the adjunctive use of antimicrobials had an influence on clinical parameters related with glycemic control in patients affected by both diabetes and periodontitis.
As compared with previous reviews, a higher number of articles could be included and different comparisons were carried out (NSPT vs no treatment and NSPT + antimicrobials vs NSPT) for the two clinical parameters considered.
In the included studies, the majority of patients were affected by uncontrolled type 2 diabetes mellitus, whereas only one of the studies involved patients with type 1 diabetes29.
The meta‐analysis showed that periodontal treatment significantly reduces the levels of HbA1c and FPG in patients with diabetes. Though, it is difficult to quantify the clinical relevance of such findings in terms of improved glycemic control. The mean decrease of HbA1c after periodontal treatment (−0.38% after 3 months and −0.31% after 6 months) can be hypothesized to have a clinical relevance. Considering that the decrease of such a parameter after the therapeutic administration of some antidiabetes agents can range from 0.4% to more than 3.0%39, the effect estimated in the present study can be considered relevant, as its order of magnitude is similar.
Other systematic reviews have investigated the effects of periodontal treatment on glycemic control in patients with diabetes and periodontitis17. Although most of these reviews concluded that periodontal therapy causes a statistically significant improvement of glycemic control18, some authors reported that the significance is low and does not allow for generalization of the results to the entire population17. One review reported a positive effect of the use of adjunctive antimicrobials, which was not observed in the present meta‐analysis19. The authors, however, stated that such an effect was not statistically significant19.
Some novel aspects emerged from the present article. First, in relation to previous reviews, a higher number of articles could be considered. Then, different comparisons were carried out (NSPT vs no treatment and NSPT + antimicrobials vs NSPT) for two clinical parameters, which were not carried out in the previous published systematic reviews.
In the present study, some studies that did not fulfill the inclusion criteria reported positive effects of periodontal treatment on glycemic control41.
Conversely, other studies failed to show that periodontal treatment might improve glycemic control, despite an improvement in clinical periodontal parameters in patients with type 1 diabetes mellitus44.
Other studies reported that extraction of ‘hopeless’ teeth might be beneficial to glycemic control, because it might guarantee a complete elimination of periodontal infection, which is difficult to obtain with either non‐surgical or surgical periodontal treatment alone47.
Some limitations should be recognized in the present study. First, the estimation of standard deviations using the cited methods could be valid from a mathematical point of view, but it might not correspond to actual values. Furthermore, no distinction was made in outcomes analysis between controlled and uncontrolled diabetes, and between studies carried out in developing countries where other major risk factors could have acted as confounding factors. Finally, it has to be underlined that the robustness of results of some comparisons could be weakened by the heterogeneity among the studies, as described in the literature18.
In order to clarify the effect of non‐surgical periodontal treatment on glycemic control in patients affected by diabetes, it is important that further randomized controlled trials with a large sample size report detailed information about initial and final parameters. Finally, as shown in a previous review19, the positive effect of periodontal treatment must be clearly demonstrated.
Despite the limitations of the present study, it can be concluded that periodontal treatment might be effective in improving metabolic control in terms of reduction of HbA1c and FPG concentrations in patients with diabetes. However, the significance of this improvement is questionable and should be further investigated.
Periodontal non‐surgical treatment is important in periodontal patients affected by diabetes because, in addition to the negligible side‐effects, it leads to the reduction of one potential factor impairing glycemic control, while preserving dental and periodontal health.
Acknowledgements
None of the authors received any funding for writing this article. The authors declare that they are free from any conflict of interest.
(J Diabetes Invest, doi: 10.1111/jdi.12088, 2013)
References
- 1.Danaei G, Finucane MM, Lu Y, et al National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet 2011; 378: 31–40 [DOI] [PubMed] [Google Scholar]
- 2.Lakschevitz F, Aboodi G, Tenenbaum H, et al Diabetes and periodontal diseases: interplay and links. Curr Diabetes Rev 2011; 7: 433–439 [DOI] [PubMed] [Google Scholar]
- 3.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol 2006; 77: 1289–1303 [DOI] [PubMed] [Google Scholar]
- 4.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol 2001; 6: 99–112 [DOI] [PubMed] [Google Scholar]
- 5.Nesse W, Abbas F, van der Ploeg I, et al Periodontal inflamed surface area: quantifying inflammatory burden. J Clin Periodontol 2008; 35: 668–673 [DOI] [PubMed] [Google Scholar]
- 6.Nibali L, D'Aiuto F, Griffiths G, et al Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case‐control study. J Clin Periodontol 2007; 34: 931–937 [DOI] [PubMed] [Google Scholar]
- 7.D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C‐reactive protein‐associated cardiovascular risk. J Periodontal Res 2004; 39: 236–241 [DOI] [PubMed] [Google Scholar]
- 8.Tonetti MS, D'Aiuto F, Nibali L, et al Treatment of periodontitis and endothelial function. N Engl J Med 2007; 356: 911–920 [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimi A, Nabipour I, Vahdat K, et al High sensitivity C‐reactive protein is associated with the metabolic syndrome independent to viral and bacterial pathogen burden. Diabetes Res Clin Pract 2009; 84: 296–302 [DOI] [PubMed] [Google Scholar]
- 10.Hung CH, Lee CM, Chen CH, et al Association of inflammatory and anti‐inflammatory cytokines with insulin resistance in chronic hepatitis C. Liver Int 2009; 29: 1086–1093 [DOI] [PubMed] [Google Scholar]
- 11.Morita I, Inagaki K, Nakamura F, et al Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res 2012; 91: 161–166 [DOI] [PubMed] [Google Scholar]
- 12.Bandyopadhyay D, Marlow NM, Fernandes JK, et al Periodontal disease progression and glycemic control among Gullah African Americans with type‐2 diabetes. J Clin Periodontol 2010; 37: 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams RC Jr, Mahan CJ. Periodontal disease and diabetes in young adults. J Am Med Assoc 1960; 172: 776–778 [DOI] [PubMed] [Google Scholar]
- 14.Seinost G, Wimmer G, Skerget M, et al Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J 2005; 149: 1050–1054 [DOI] [PubMed] [Google Scholar]
- 15.Elter JR, Hinderliter AL, Offenbacher S, et al The effects of periodontal therapy on vascular endothelial function: a pilot trial. Am Heart J 2006; 151: 47. [DOI] [PubMed] [Google Scholar]
- 16.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta‐analyses on C‐reactive protein in relation to periodontitis. J Clin Periodontol 2008; 35: 277–290 [DOI] [PubMed] [Google Scholar]
- 17.Simpson TC, Needleman I, Wild SH, et al Treatment of periodontal disease for glycemic control in people with diabetes. Cochrane Database Syst Rev 2010; (5): CD004714. [DOI] [PubMed] [Google Scholar]
- 18.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta‐analysis. Diabetes Care 2010; 33: 421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janket SJ, Wightman A, Baird AE, et al Does periodontal treatment improve glycemic control in diabetic patients? A meta‐analysis of intervention studies. J Dent Res 2005; 84: 1154–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioannidou E, Kao D, Chang N, et al Elevated serum interleukin‐6 (IL‐6) in solid‐organ transplant recipients is positively associated with tissue destruction and IL‐6 gene expression in the periodontium. J Periodontol 2006; 77: 1871–1878 [DOI] [PubMed] [Google Scholar]
- 21.Combined variance, authors. 2012 http://www.emathzone.com/tutorials/basic-statistics/combined-variance.html
- 22.Matsumoto S, Ogawa H, Soda S, et al Effect of antimicrobial periodontal treatment and maintenance on serum adiponectin in type 2 diabetes mellitus. J Clin Periodontol 2009; 36: 142–148 [DOI] [PubMed] [Google Scholar]
- 23.Lin SJ, Tu YK, Tsai SC, et al Non‐surgical periodontal therapy with and without subgingival minocycline administration in patients with poorly controlled type II diabetes: a randomized controlled clinical trial. Clin Oral Investig 2012; 16: 599–609 [DOI] [PubMed] [Google Scholar]
- 24.Rocha M, Nava LE, Vazquez de la Torre C, et al Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: a randomized, placebo‐controlled trial. J Periodontol 2001; 72: 204–209 [DOI] [PubMed] [Google Scholar]
- 25.Al‐Mubarak S, Ciancio S, Aljada A, et al Comparative evaluation of adjunctive oral irrigation in diabetics. J Clin Periodontol 2002; 29: 295–300 [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues DC, Taba MJ, Novaes AB, et al Effect of non‐surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol 2003; 74: 1361–1367 [DOI] [PubMed] [Google Scholar]
- 27.Kiran M, Arpak N, Unsal E, et al The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol 2005; 32: 266–272 [DOI] [PubMed] [Google Scholar]
- 28.Jones JA, Miller DR, Wehler CJ, et al Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. J Clin Periodontol 2007; 34: 46–52 [DOI] [PubMed] [Google Scholar]
- 29.Llambes F, Silvestre FJ, Hernandez‐Mijares A, et al The effect of periodontal treatment on metabolic control of type 1 diabetes mellitus. Clin Oral Investig 2008; 12: 337–343 [DOI] [PubMed] [Google Scholar]
- 30.O'Connell PA, Taba M, Nomizo A, et al Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol 2008; 79: 774–783 [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Kumar V, Kumar S, et al The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Diabetes Dev Ctries 2008; 28: 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al‐Zahrani MS, Bamshmous SO, Alhassani AA, et al Short‐term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J Periodontol 2009; 80: 1568–1573 [DOI] [PubMed] [Google Scholar]
- 33.Katagiri S, Nitta H, Nagasawa T, et al Multi‐center intervention study on glycohemoglobin (HbA1c) and serum, high‐sensitivity CRP (hs‐CRP) after local anti‐infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res Clin Pract 2009; 83: 308–315 [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Luo G, Xuan D, et al Effects of non‐surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in type 2 diabetic patients: a randomized study. J Periodontol 2012; 83: 435–443 [DOI] [PubMed] [Google Scholar]
- 35.Engebretson SP, Hey‐Hadavi J. Sub‐antimicrobial doxycycline for periodontitis reduces hemoglobin A1c in subjects with type 2 diabetes: a pilot study. Pharmacol Res 2011; 64: 624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koromantzos PA, Makrilakis K, Dereka X, et al A randomized, controlled trial on the effect of non‐surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycemic control. J Clin Periodontol 2011; 38: 142–147 [DOI] [PubMed] [Google Scholar]
- 37.Sun WL, Chen LL, Zhang SZ, et al Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med 2011; 50: 1569–1574 [DOI] [PubMed] [Google Scholar]
- 38.Moeintaghavi A, Arab HR, Bozorgnia Y, et al Non‐surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust Dent J 2012; 57: 31–37 [DOI] [PubMed] [Google Scholar]
- 39.Kurukulasuriya LR, Sowers JR. Therapies for type 2 diabetes: lowering HbA1c and associated cardiovascular risk factors. Cardiovasc Diabetolog 2010; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darre L, Vergnes JN, Gourdy P, et al Efficacy of periodontal treatment on glycemic control in diabetic patients: a meta‐analysis of interventional studies. Diabetes Metab 2008; 34: 497–506 [DOI] [PubMed] [Google Scholar]
- 41.Calabrese N, D'Aiuto F, Calabrese A, et al Effects of periodontal therapy on glucose management in people with diabetes mellitus. Diabetes Metab 2011; 37: 456–459 [DOI] [PubMed] [Google Scholar]
- 42.Montoya‐Carralero JM, Saura‐Perez M, Canteras‐Jordana M, et al Reduction of HbA1c levels following nonsurgical treatment of periodontal disease in type 2 diabetics. Med Oral Patol Oral Cir Bucal 2010; 15: e808–e812 [DOI] [PubMed] [Google Scholar]
- 43.Correa FO, Goncalves D, Figueredo CM, et al Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol 2010; 37: 53–58 [DOI] [PubMed] [Google Scholar]
- 44.da Cruz GA, de Toledo S, Sallum EA, et al Clinical and laboratory evaluations of non‐surgical periodontal treatment in subjects with diabetes mellitus. J Periodontol 2008; 79: 1150–1157 [DOI] [PubMed] [Google Scholar]
- 45.Tervonen T, Lamminsalo S, Hiltunen L, et al Resolution of periodontal inflammation does not guarantee improved glycemic control in type 1 diabetic subjects. J Clin Periodontol 2009; 36: 51–57 [DOI] [PubMed] [Google Scholar]
- 46.Skaleric U, Schara R, Medvescek M, et al Periodontal treatment by Arestin and its effects on glycemic control in type 1 diabetes patients. J Int Acad Periodontol 2004; 6: 160–165 [PubMed] [Google Scholar]
- 47.Khader YS, Al Habashneh R, Al Malalheh M, et al The effect of full‐mouth tooth extraction on glycemic control among patients with type 2 diabetes requiring extraction of all remaining teeth: a randomized clinical trial. J Periodontal Res 2010; 45: 741–747 [DOI] [PubMed] [Google Scholar]
- 48.Stewart JE, Wager KA, Friedlander AH, et al The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol 2001; 28: 306–310 [DOI] [PubMed] [Google Scholar]
- 49.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999; 4: 1–6 [DOI] [PubMed] [Google Scholar]


