Abstract
Objective
To systematically evaluate therapeutic success of the ketogenic diet (KD) as a treatment option for epilepsy.
Methods
Using MEDLINE and Google Scholar search, we searched for studies investigating the therapeutic success of ketogenic diet for epilepsy. We estimated therapeutic success rate for ketogenic diet as a treatment option for epilepsy and its 95% CIs using generic inverse variance method.
Findings
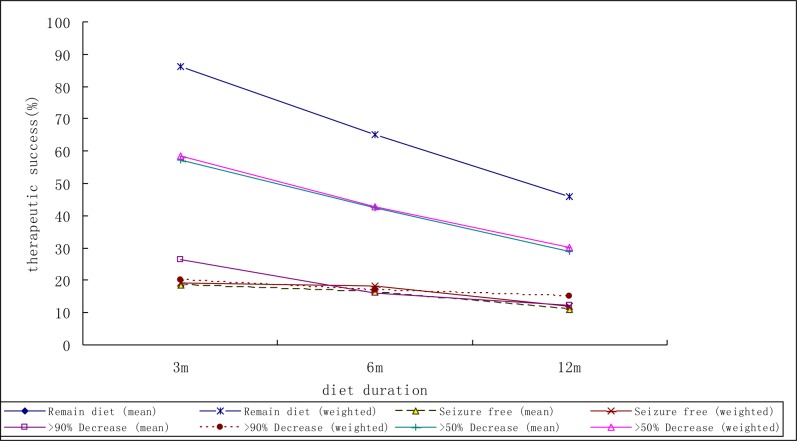
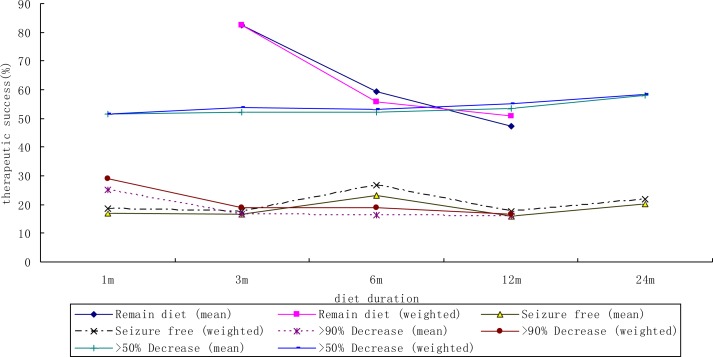
A total of 38 studies met the inclusion criteria. In retrospective studies, the weighted success rate of the patients who take the KD as a treatment option for epilepsy was 58.4% (95% confidence interval (95%CI)=48.7% – 69.9%) at 3 months (n=336); 42.8% (95%CI =36.3% – 50.3%) at 6 months (n=492), and 30.1% (95%CI =24.3% – 37.2%) at 12 months (n=387); in prospective studies, weighted success rate was 53.9% (95%CI 45.5% – 63.8%) at 3 months (n=474); 53.2% (95%CI =44.0% – 64.2%) at 6 months (n=321), and 55.0% (95%CI =45.9% – 65.9%) at 12 months (n=347).
Conclusion
This meta-analysis provides formal statistical support for the efficacy of the ketogenic diet in the treatment of epileptic patients.
Keywords: Ketogenic Diet, Epilepsy, Meta-Analysis, Children
Introduction
Epilepsy is the most common serious neurological condition in the world, with an estimated prevalence of 1% of the population[1]. Traditional epilepsy management includes pharmacological treatment, epilepsy surgery, and vagal nerve stimulation. Despite these therapies, 25% of children continue to have uncontrolled seizures. The ketogenic diet (KD), which has been in use since 1921, is a treatment option for many of these epilepsy patients. Ketogenic diet is high in fat, moderate in protein and low in carbohydrates. This combination of energy results in a sustained ketosis that somehow serves to abate seizures through an unknown mechanism. In the following years, with the use of anti-epileptic drugs such as phenytoinum natricum, ketogenic diet is not widely used in epilepsy treatment. While in the recent years, with the increase of drug resistance and adverse effect of anti-epileptic drugs, ketogenic diet was newly considered and promoted in epilepsy treatment. Recently, A review suggests that in children, the ketogenic diet results in short to medium term benefits in seizure control, the effects of which are comparable to modern antiepileptic drugs[2].
In this article, existing studies (retrospective studies and prospective studies) were collected and used to systematically evaluate therapeutic success of ketogenic diet at different duration. Individual studies were summarized using meta- analysis of generic inverse variance method.
Subjects and Methods
Search Strategy and Selection Criteria
As the basis for our analysis, we performed a MEDLINE search of the literature from 2000 to December 2011. The search themes were combined as “(ketogenic diet or ketosis or ketone) and (epilepsy seizures or focal seizures or seizures or epilepsy or refractory seizures or generalized seizures)”. In addition, we performed a Google Scholar search.
Two investigators independently reviewed titles and abstracts, and selected articles addressing ketogenic diet and epilepsy. Disagreements were resolved by discussion and consensus. On a second sift, we selected original studies on ketogenic diet and epilepsy with the following inclusion criteria:
Addressing ketogenic diet and epilepsy.
The study should provide sample size.
The study should provide the percentage of seizure reduction with the corresponding ketogenic diet duration.
KD type including johns Hopkins protocol, modified Atkins diet, classical ketogenic diet.
Definitions
For the purpose of meta-analysis, therapeutic success was defined as ≥50% seizure reduction at follow-up.
Data Extraction and Analysis
For each study included, the full text was retrieved and the following data were extracted: authors, year of publication, size, gender and mean (or range) of age of the sample, and ketogenic diet duration. For each included study, we extracted the percentage of seizure reduction with the corresponding ketogenic diet duration.
Two investigators performed the extraction of the data in duplicate to avoid errors. The percentage of seizure free, >90% decrease, >50% decrease were always selected when available; otherwise the number of seizure free, >90% decrease, >50% decrease were recorded. We pooled studies that present percentages or the subjects that could be defined as therapeutic success.
Given the high number of potential ketogenic diet duration, we restricted our analysis to those for which the therapeutic success was assessed by at least 3 studies. Therapeutic success was analyzed separately according to the duration of ketogenic diet.
We used SAS (r) Software 9.2 to analyze data. The statistical heterogeneity of the included studies was assessed by using the χ2 test and the I2 index. The latter examines the percentage of total variation across studies that are due to heterogeneity between studies rather than by randomness. A value of I2 greater than about 70% indicates a high level of heterogeneity. Since the results showed no statistical heterogeneity, we estimated therapeutic success rate for ketogenic diet as a treatment option for epilepsy and its 95% confidence interval (95%) CIs using generic inverse variance method[3]. Using generic inverse variance method, each study estimate of the success rate is given a weight that is equal to the inverse of the variance of the effect estimate.
Findings
The MEDLINE search produced 933 citations. Records after duplicates removed resulted in 933 citations. Review of titles and abstracts resulted in the selection of 55 articles, among which 38 articles met the inclusion criteria. Selected characteristics of the 38 included articles are reported individually in Table 1, [4–41].
Table 1.
Summary of 38 studies investigating ketogenic diet and epilepsy
| Study | Author, Reference | Prospective/ Retrospective | Year | Sample Size Number | Male | Median /Mean/Range Age of Study Population (years) |
|---|---|---|---|---|---|---|
| 1 | Roberto, et al | Prospective | 2011 | 24 | 16 | 6 [mean] |
| 2 | Mara, et al | Prospective | 2011 | 18 | 9 | 29 [median] |
| 3 | Maria, et al | Prospective | 2010 | 50 | 23 | 4.6 [mean] |
| 3 | Maria, et al | Prospective | 2010 | 33 | 15 | 8.25 [mean] |
| 4 | Yoon, et al | Retrospectively | 2011 | 20 | 12 | 6.9 [mean] |
| 5 | Rima, et al | Prospective | 2011 | 15 | NR | 5 [mean] |
| 6 | Amnon, et al | Prospective | 2009 | 9 | 2 | 28 [mean] |
| 7 | Pavel, et al | Prospective | 2010 | 12 | 4 | 24–65 [range] |
| 8 | Eric, et al | Prospective | 2010 | 5 | 3 | 4–18 [range] |
| 9 | Eric | Prospective | 2011 | 30 | 11 | 7 [mean] |
| 10 | Dekker | Retrospective | 2010 | 43 | NR | 2–19 [range] |
| 11 | Amanda | Prospective | 2010 | 104 | 59 | 1.2 [mean] |
| 12 | Beniczky | Retrospective | 2010 | 50 | 27 | 1–14.5 [range] |
| 13 | Anastasia, et al | Retrospective | 2010 | 50 | 22 | 4.5 [mean] |
| 14 | Giangennaro, et al | Prospective | 2009 | 38 | 22 | 3.1 [mean] |
| 15 | Suvasini, et al | Prospective | 2009 | 27 | NR | 2.5 [mean] |
| 16 | Natacha, et al | Retrospective | 2009 | 17 | NR | 2.7 [mean] |
| 16 | Natacha, et al | Retrospective | 2009 | 10 | NR | 6.4 [mean] |
| 17 | John, et al | Prospective | 2009 | 20 | NR | 1–10 [range] |
| 18 | Elizabeth, et al | Prospective | 2008 | 73 | 40 | 2–16 [range] |
| 19 | Eric | Retrospective | 2008 | 13 | 6 | NR |
| 19 | Eric, et al | Retrospective | 2008 | 20 | 13 | NR |
| 20 | Eric, et al | Prospective | 2007 | 30 | 11 | 31 [mean] |
| 21 | Eric, et al | Prospective | 2007 | 10 | 6 | 4–25 [range] |
| 21 | Eric, et al | Prospective | 2007 | 10 | 4 | 3–16 [range] |
| 22 | Joo, et al | Prospective | 2007 | 40 | 24 | 4.3 [mean] |
| 22 | Joo, et al | Prospective | 2007 | 36 | 19 | 3.7 [mean] |
| 23 | Hoon-Chul, et al | Prospective | 2007 | 14 | NR | 7.4 [mean] |
| 24 | So-Hee, et al | Retrospective | 2006 | 43 | 24 | 1.6 [mean] |
| 25 | Eric, et al | Prospective | 2006 | 20 | 9 | 8.1 [mean] |
| 26 | Elisabeth, et al | Prospective | 2006 | 150 | 85 | 5.3 [mean] |
| 27 | Christina,et al | Prospective | 2005 | 24 | 17 | 5.8 [mean] |
| 27 | Christina,et al | Prospective | 2006 | 24 | 17 | 4.8 [mean] |
| 28 | Mark, et al | Retrospective | 2005 | 26 | 6 | 6.1 [mean] |
| 29 | Hoon-Chul, et al | Retrospective | 2005 | 199 | 110 | 4.8 [mean] |
| 30 | Seyed, et al | Prospective | 2010 | 24 | NR | 1–16 [range] |
| 31 | Giangennaro, et al | Prospective | 2002 | 56 | 36 | 10.4 [mean] |
| 32 | Bernhard, et al | Retrospective | 2001 | 134 | NR | 7.5 [mean] |
| 33 | Cheryl, et al | Prospective | 2001 | 150 | NR | NR |
| 34 | Mackenzie, et al | Retrospective | 2003 | 45 | 25 | 14.4 [mean] |
| 35 | James, et al | Retrospective | 2005 | 10 | 10 | 0.5 [mean] |
| 36 | Vykunta, et al | Prospective | 2011 | 19 | 15 | 2.5 [median] |
| 36 | Vykunta, et al | Prospective | 2011 | 19 | 16 | 3 [median] |
| 37 | Giangennaro, et al | Retrospective | 2011 | 15 | 13 | 12.4 [mean] |
| 38 | Roberto, et al | Prospective | 2006 | 11 | 7 | 5 [mean] |
*NR indicates not reported
Table 2 provides the mean and range of success, and weighted success rate and 95% CI of ketogenic diet. In retrospective studies, the weighted success rate of the patients who take the KD as a treatment option for epilepsy was 58.4% (95% confidence interval (95%CI)=48.7% – 69.9%) at 3 months (n=336); 42.8% (95%CI =36.3% – 50.3%) at 6 months (n=492), and 30.1% (95%CI = 24.3%– 37.2%) at 12 months (n=387); in prospective studies, weighted success rate was 53.9% (95%CI 45.5% – 63.8%) at 3 months (n=474); 53.2% (95%CI =44.0% – 64.2%) at 6 months (n=321), and 55.0% (95%CI =45.9% – 65.9%) at 12 months (n=347). In addition, weighted success rate for 1 month and 12 months in prospective study was 51.6% (95% CI =41.0% – 65.1%) at 1 month (n=212), 58.5% (95% CI =48.1% – 71.2%) at 12 months (n=278).
Table 2.
The therapeutic success of ketogenic diet with the corresponding ketogenic diet duration
| 3M | 6 M | 12 M | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Summary of Therapeutic Success of Ketogenic Diet | Meta-analysis Studies Included | Total Number of Cases | Mean and Range of Success Rates (%) | Weighted Success Rates and 95%CI (%) | Meta-analysis Studies Included | Total Number of Cases | Mean and Range of Success Rates (%) | Weighted Success Rates and 95%CI (%) | Meta-analysis Studies Included | Total Number of Cases | Mean and Range of Success Rate (%) | Weighted Success Rate and 95%CI (%) | |
| Remain diet | 24, 34, 35 | 101 | 86.1 (81.4–100.0) |
86.1 (64.7–100.0) (Q=0.0001, P=0.9990) |
13, 24, 29, 34, 35 | 350 | 65.1 (58.1–76.9) |
65.1 (55.2–77.0) (Q=0.0004, P=1.0000) |
28, 29, 34, 35 | 283 | 45.9 (44.4–46.2) |
45.9 (37.3–56.5) (Q=0.0000, P=1.0000) |
|
| Retrospective study | Seizure free | 10, 12, 24, 32 | 370 | 18.6 (15.7–34.9) |
19.1 (14.8–24.8) (Q=0.0003, P=1.000) |
13, 24, 29, 32 | 426 | 16.2 (8.0–39.5) |
18.1 (14.0–23.5) (Q=0.0171, P=0.9998) |
28, 29, 32 | 359 | 11.1 (6.5–17.1) |
12.1 (8.7–16.9) (Q=0.0046, P=0.9977) |
| >90% Decrease | 10, 12, 32, 35 | 240 | 26.3 (16.4–53.8) |
20.1 (15.2–26.7) (Q=0.0048, P=0.9999) |
32, 34, 35 | 192 | 16.1 (12.7–46.2) |
17.0 (11.6–25.0) (Q=0.0189, P=0.9905) |
32, 34, 35 | 192 | 12.3 (11.2–46.2) |
15.0 (10.0–22.6) (Q=0.0390, P=0.9807) |
|
| >50% Decrease | 10, 12, 16, 24, 28, 32, 35 | 336 | 57.1 (20.0–100.0) |
58.4 (48.7–69.9) (Q=0.0089, P=1.0000) |
13, 16, 24, 28, 29, 32 35 | 492 | 42.3 (19.2–61.5) |
42.8 (36.3–50.3) (Q=0.0676, P=1.0000) |
28, 29, 32, 35, 37 | 387 | 28.9 (19.1–53.3) |
30.1 (24.3–37.2) (Q=0.0056, P=1.0000) |
|
| Remain diet | 14, 15, 23, 25, 31 | 155 | 82.6 (64.3–92.1) |
82.6 (65.4–100.0) (Q=0.0014, P=1.0000) |
14, 15, 17, 21, 23, 25, 31 | 195 | 59.5 (37.5–85.0) |
55.8 (46.8–66.5) (Q=0.0092, P=1.0000) |
2, 3, 5, 14, 15, 17, 31, 33 | 357 | 47.3 (8.9–77.8) |
50.8 (42.1–61.3) (Q=0.1011, P=1.0000) |
|
| Prospective study | Seizure free | 3, 11, 20, 23, 25, 27, 30, 31, 36 | 343 | 16.6 (3.3–28.6) |
17.7 (13.4–23.5) (Q=0.0089, P=1.0000) |
11, 15, 17, 20, 22, 23, 25, 31 | 347 | 23.3 (3.3–30.6) |
26.9 (20.9–34.5) (Q=1.0223, P=0.9981) |
5, 11, 15, 17, 26, 33 | 466 | 15.9 (6.6–29.8) |
17.7 (13.8–22.8) (Q=0.1562, P=0.9995) |
| >90% Decrease | 3, 11, 15, 18, 21, 25, 27, 30 | 339 | 16.5 (4.1–45.8) |
19.0 (14.2–25.4) (Q=0.9457, P=0.9986) |
3, 8, 11, 15, 17, 21, 22, 25 | 355 | 16.3 (5.0–34.0) |
19.0 (14.3–25.2) (Q=1.4530, P=0.9991) |
3, 11, 15, 17, 26, 33 | 484 | 16.1 (7.4–35.0) |
16.6 (13.1–21.2) (Q=0.0879, P=0.9999) |
|
| >50% Decrease | 2, 3, 11, 18, 20, 21, 23, 27, 30, 36 | 474 | 52.2 (10.0–66.7) |
53.9 (45.5–63.8) (Q=0.5554, P=1.0000) |
2, 3, 8, 11, 15, 20, 21, 23, 25 | 321 | 52.3 (22.2–65.0) |
53.2 (44.0–64.2) (Q=0.0798, P=1.000) |
2, 3, 5, 11, 15, 33 | 347 | 53.6 (16.7–76.9) |
55.0 (45.9–65.9) (Q=0.0528, P=0.9999) |
|
Figs. 1 and 2 show the mean weighted therapeutic success rate of ketogenic diet in included retrospective and prospective study respectively.
Fig. 1.
The mean weighted therapeutic success rate according to the duration of ketogenic diet in included retrospective study
Fig. 2.
The mean weighted therapeutic success rate according to the duration of ketogenic diet in included prospective study
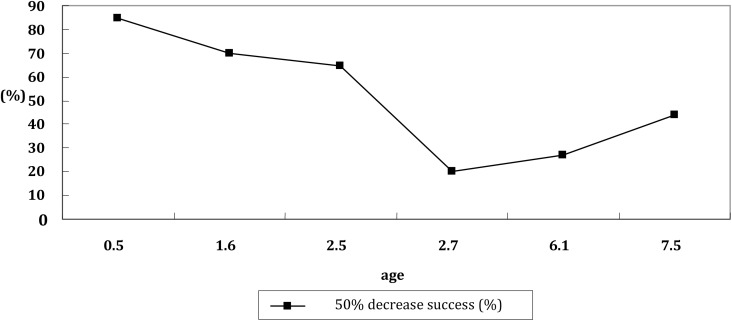
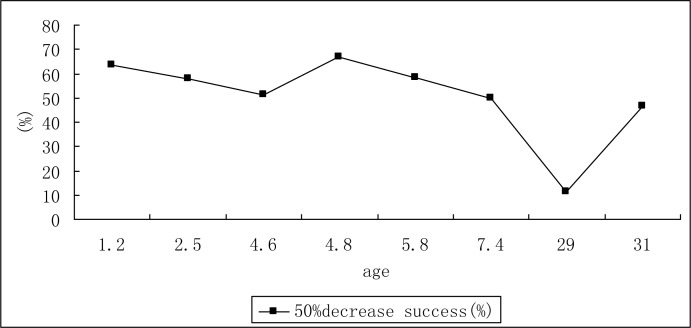
The Pearson's correlation coefficient (rho) of therapeutic success rate and age in ketogenic diet treatment is shown in Figs. 3 and 4. There was a negative correlation between therapeutic success rate and age at 3 months in retrospective study.
Fig. 3.
The relationship of success rate of ketogenic diet and age at 3 month in included retrospective study
Fig. 4.
The relationship of success rate of ketogenic diet and age at 3 month in included prospective study
Discussion
Over the past 90 years since its introduction, the ketogenic diet has been shown to be extremely successful in existing studies. Although many studies have investigated the therapeutic success of ketogenic diet, a comprehensive and quantitative summary at different diet duration has been lacking. In most instances, however, results were fairly consistent in the direction of therapeutic success of ketogenic diet, even though studies differed in the estimation of effect extent. In this meta-analysis, compliance of patients was found to be decreased with the prolonged ketogenic diet duration. It is reported that the initial 3 months on the diet are typically considered a trial period. So the surveillance for the compliance should be carried out especially in the initial 3 months. Vining et al reported that 71% of patients with 50% seizure at 3 months could stay on the diet until the KD duration reached one year[42].
In this study, the therapeutic success of ketogenic diet was underscored using generic inverse variance method. This method is applied in meta-analysis when the results show no statistical heterogeneity. Each study estimate of the success rate is given a weight that is equal to the inverse of the variance of the effect estimate. Using this method, we found that in retrospective study, the success rate for patients who achieved therapeutic success (>50% reduction in seizure frequency) were 58.4% at 3 month, 42.8% at 6 month, and 30.1% at 12 month respectively; and in prospective study, the success rates were 53.9% at 3 month, 53.2% at 6 month, and 55.0% at 12 month respectively. It is consistent with reported efficacy of ketogenic diet in controlling seizures that 56% had a >50% reduction[43]. When the ketogenic diet duration prolonged, the efficacy was found decreased. The decreased success rates may be related to the decrease of compliance. In addition, the influence of lost to follow up may be considered when in the designing period, especially for long observation period, and measures to control the loss of follow up may be carried out in retrospective studies. Therefore, the reported rates were lower in retrospective studies than in prospective studies at 12 months. Furthermore, we cannot calculate the efficacy for ketogenic diet considering the anti-epilepsy drugs treatment when taking the ketogenic diet, so there is a need for further studies to explore the interaction effect.
Guidance on the duration of dietary treatment is limited, and one study looked into the effect of the KD after its discontinuation, observing reduced benefit following cessation of treatment[10]. Levy RG et al also point out the problem of poor persistence[1]. The education level and care of the patient's family, the knowledge of epilepsy treatment, and the complicated recipe for daily diet treatment as well as the side effect of ketogenic diet result in poor compliance. Common side effects of the ketogenic diet are gastrointestinal complaints and unfavorable lipid profiles. But Kessler et al reported that side effects are usually transient and the most common reason for discontinuation of treatment is lack of effectiveness[44]. Anyway, for the ketogenic diet to be clinically meaningful, persistence or compliance would be quiet important. And to improve therapeutic success of the ketogenic diet, some measures such as effectiveness evaluation, health education, supervision of compliance, awareness of epilepsy treatment, side effect minimizing, user-friendly recipe developing should be taken into consideration.
The ketogenic diet has been used with infants as young as 3 months and adults as old as 58 years. Although our analysis indicates the negative correlation between therapeutic success rate and age, this relationship was only found statistically significant at 3 month in retrospective study. There is a tendency for younger children to have higher success rate although this trend was not statistically significant. Ketogenic diet is currently used mainly for children who continue to have seizures despite treatment with antiepileptic drugs. Children's stomach capacity is small and the liver glycogen storage quantity is limited, so the energy storage level of children is lower than that of adults. Due to the higher energy storage level of adults and comparable stable blood glucose levels, the adult ketogenic diet practice is not as successful as in children. The results on less than 20% seizure free after treating with ketogenic diet for epileptic patients may be related to the age factor.
Conclusion
This meta-analysis provides formal statistical support for the efficacy of the ketogenic diet in the treatment of epileptic patients. There is a tendency for younger age patients to have higher success rate although this trend was not statistically significant. It shows the need for further research on interaction effect considering both ketogenic diet and anti-epilepsy drugs.
Acknowledgment
None of the authors of the present paper have information to declare regarding conflicts of interest
Conflict of Interest
None
References
- 1.Zupec-Kania BA, Spellman E. An overview of the ketogenic diet for pediatric epilepsy. Nutr Clin Pract. 2008;23(6):589–96. doi: 10.1177/0884533608326138. [DOI] [PubMed] [Google Scholar]
- 2.Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2012;3:CD001903. doi: 10.1002/14651858.CD001903.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Sutton AJ, Abrams KR, Jones DR, et al. Methods for meta-analysis in medical research. Chichester: England John Wiley & Sons Ltd; 2000. [Google Scholar]
- 4.Caraballo RH. Nonpharmacologic treatments of Dravet syndrome: focus on the ketogenic diet. Epilepsia. 2011;52(suupl 2):79–82. doi: 10.1111/j.1528-1167.2011.03009.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith M, Politzer N, Macgarvie D, et al. Efficacy and tolerability of the modified Atkins diet in adults with pharmacoresistant epilepsy: a prospective observational study. Epilepsia. 2011;52(4):775–80. doi: 10.1111/j.1528-1167.2010.02941.x. [DOI] [PubMed] [Google Scholar]
- 6.Miranda MJ, Mortensen M, Povlsen JH, et al. Danish study of a modified Atkins diet for medically intractable epilepsy in children: can we achieve the same results as with the classical ketogenic diet? Seizure. 2011;20(2):151–5. doi: 10.1016/j.seizure.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Kim YM, Vaidya VV, Khusainov T, et al. Various indications for a modified Atkins diet in intractable childhood epilepsy. Brain Dev. 2011;34(7):570–5. doi: 10.1016/j.braindev.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Nabbout R, Copioli C, Chipaux M, et al. Ketogenic diet also benefits Dravet syndrome patients receiving stiripentol: a prospective pilot study. Epilepsia. 2011;52(7):e54–7. doi: 10.1111/j.1528-1167.2011.03107.x. [DOI] [PubMed] [Google Scholar]
- 9.Mosek A, Natour H, Neufeld MY, et al. Ketogenic diet treatment in adults with refractory epilepsy: a prospective pilot study. Seizure. 2009;18(1):30–3. doi: 10.1016/j.seizure.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Klein P, Janousek J, Barber A, et al. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav. 2010;19(4):575–9. doi: 10.1016/j.yebeh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Kossoff EH, Borsage JL, Comi AM. A pilot study of the modified Atkins diet for Sturge-Weber syndrome. Epilepsy Res. 2010;92(2–3):240–3. doi: 10.1016/j.eplepsyres.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Kossoff EH, Dorward JL, Turner Z, et al. Prospective study of the modified atkins diet in combination with a ketogenic liquid supplement during the initial month. J Child Neurol. 2011;26(2):147–51. doi: 10.1177/0883073810375718. [DOI] [PubMed] [Google Scholar]
- 13.Dekker CF, van den Hurk TA, van Nieuwenhuizen O. Does a preference for fatty foods prior to commencing treatment with the ketogenic diet predict the efficacy of this diet? Seizure. 2010;19(7):421–5. doi: 10.1016/j.seizure.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Hong AM, Turner Z, Hamdy RF, et al. Infantile spasms treated with the ketogenic diet: prospective single-center experience in 104 consecutive infants. Epilepsia. 2010;51(8):1403–7. doi: 10.1111/j.1528-1167.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- 15.Beniczky S, Jose Miranda M, Alving J, et al. Effectiveness of the ketogenic diet in a broad range of seizure types and EEG features for severe childhood epilepsies. Acta Neurol Scand. 2010;121(1):58–62. doi: 10.1111/j.1600-0404.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 16.Dressler A, Stöcklin B, Reithofer E, et al. Long-term outcome and tolerability of the ketogenic diet in drug-resistant childhood epilepsy--the Austrian experience. Seizure. 2010;19(7):404–8. doi: 10.1016/j.seizure.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Coppola G, Verrotti A, Ammendola E, et al. Ketogenic diet for the treatment of catastrophic epileptic encephalopathies in childhood. Eur J Paediatr Neurol. 2010;14(3):229–34. doi: 10.1016/j.ejpn.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Gulati S, Kalra V, et al. Seizure control and biochemical profile on the ketogenic diet in young children with refractory epilepsy - Indian experience. Seizure. 2009;18(6):446–9. doi: 10.1016/j.seizure.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Porta N, Vallée L, Boutry E, et al. Comparison of seizure reduction and serum fatty acid levels after receiving the ketogenic and modified Atkins diet. Seizure. 2009;18(5):359–64. doi: 10.1016/j.seizure.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Freeman JM. The ketogenic diet: additional information from a crossover study. J Child Neurol. 2009;24(4):509–12. doi: 10.1177/0883073808324776. [DOI] [PubMed] [Google Scholar]
- 21.Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–6. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 22.Kossoff EH, Hedderick EF, Turner Z, et al. A case-control evaluation of the ketogenic diet versus ACTH for new-onset infantile spasms. Epilepsia. 2008;49(9):1504–9. doi: 10.1111/j.1528-1167.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 23.Kossoff EH, Rowley H, Sinha SR, et al. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008;49(9):316–9. doi: 10.1111/j.1528-1167.2007.01256.x. [DOI] [PubMed] [Google Scholar]
- 24.Kossoff EH, Turner Z, Bluml RM, et al. A randomized, crossover comparison of daily carbohydrate limits using the modified Atkins diet. Epilepsy Behav. 2007;10(3):432–6. doi: 10.1016/j.yebeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Seo JH, Lee YM, Lee JS, et al. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios -- comparison of 3:1 with 4:1 diet. Epilepsia. 2007;48(4):801–5. doi: 10.1111/j.1528-1167.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 26.Kang HC, Lee HS, You SJ, et al. Use of a modified Atkins diet in intractable childhood epilepsy. Epilepsia. 2007;48(1):182–6. doi: 10.1111/j.1528-1167.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- 27.Eun SH, Kang HC, Kim DW, et al. Ketogenic diet for treatment of infantile spasms. Brain Dev. 2006;28(9):566–71. doi: 10.1016/j.braindev.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Kossoff EH, McGrogan JR, Bluml RM, et al. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47(2):421–4. doi: 10.1111/j.1528-1167.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 29.Marsh EB, Freeman JM, Kossoff EH, et al. The outcome of children with intractable seizures: a 3- to 6-year follow-up of 67 children who remained on the ketogenic diet less than one year. Epilepsia. 2006;47(2):425–30. doi: 10.1111/j.1528-1167.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 30.Bergqvist AG, Schall JI, Gallagher PR, et al. Fasting versus gradual initiation of the ketogenic diet: a prospective, randomized clinical trial of efficacy. Epilepsia. 2005;46(11):1810–9. doi: 10.1111/j.1528-1167.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 31.Mackay MT, Bicknell-Royle J, Nation J, et al. The ketogenic diet in refractory childhood epilepsy. J Paediatr Child Health. 2005;41(7):353–7. doi: 10.1111/j.1440-1754.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 32.Kang HC, Kim YJ, Kim DW, et al. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005;46(2):272–9. doi: 10.1111/j.0013-9580.2005.48504.x. [DOI] [PubMed] [Google Scholar]
- 33.Tonekaboni SH, Mostaghimi P, Mirmiran P, et al. Efficacy of the Atkins diet as therapy for intractable epilepsy in children. Arch Iran Med. 2010;13(6):492–7. [PubMed] [Google Scholar]
- 34.Coppola G, Veggiotti P, Cusmai R, et al. The ketogenic diet in children, adolescents and young adults with refractory epilepsy: an Italian multicentric experience. Epilepsy Res. 2002;48(3):221–7. doi: 10.1016/s0920-1211(01)00315-1. [DOI] [PubMed] [Google Scholar]
- 35.Maydell BV, Wyllie E, Akhtar N, et al. Efficacy of the ketogenic diet in focal versus generalized seizures. Pediatr Neurol. 2001;25(3):208–12. doi: 10.1016/s0887-8994(01)00310-1. [DOI] [PubMed] [Google Scholar]
- 36.Hemingway C, Freeman JM, Pillas DJ, et al. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001;108(4):898–905. doi: 10.1542/peds.108.4.898. [DOI] [PubMed] [Google Scholar]
- 37.Mady MA, Kossoff EH, McGregor AL, et al. The ketogenic diet: adolescents can do it, too. Epilepsia. 2003;44(6):847–51. doi: 10.1046/j.1528-1157.2003.57002.x. [DOI] [PubMed] [Google Scholar]
- 38.Rubenstein JE, Kossoff EH, Pyzik PL, et al. Experience in the use of the ketogenic diet as early therapy. J Child Neurol. 2005;20(1):31–4. doi: 10.1177/08830738050200010501. [DOI] [PubMed] [Google Scholar]
- 39.Raju KN, Gulati S, Kabra M, et al. Efficacy of 4:1 (classic) versus 2.5:1 ketogenic ratio diets in refractory epilepsy in young children: a randomized open labeled study. Epilepsy Res. 2011;96(1–2):96–100. doi: 10.1016/j.eplepsyres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Coppola G, D'Aniello A, Messana T, et al. Low glycemic index diet in children and young adults with refractory epilepsy: first Italian experience. Seizure. 2011;20(7):526–8. doi: 10.1016/j.seizure.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Caraballo RH, Cersósimo RO, Sakr D, et al. Ketogenic diet in patients with myoclonic-astatic epilepsy. Epileptic Disord. 2006;8(2):151–5. [PubMed] [Google Scholar]
- 42.Vining EP, Freeman JM, Ballaban-Gil K, et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55(11):1433–7. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 43.Zupec-Kania BA, Spellman E. An overview of the ketogenic diet for pediatric epilepsy. Nutr Clin Pract. 2008;23(6):589–96. doi: 10.1177/0884533608326138. [DOI] [PubMed] [Google Scholar]
- 44.Kessler SK, Neal EG, Camfield CS, et al. Dietary therapies for epilepsy: future research. Epilepsy Behav. 2011;22(1):17–22. doi: 10.1016/j.yebeh.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]