Abstract
Objective
To determine the risk factors associated with lower respiratory tract infections (LRTI) related hospitalizations in preterm infants receiving palivizumab throughout the high season for respiratory syncytial virus (RSV) infection.
Methods
Premature infants who were commenced on palivizumab prophylaxis during the RSV season were included in the study following parental consent. Information on demographic, social, prenatal and postnatal clinical characteristics was recorded and risk factors associated with hospitalization were evaluated for each patient.
Findings
While 234 participants (Group 1, 92.8%) did not require hospitalization during the study period, 18 patients (Group 2, 7.2%) were hospitalized at least once for LRTI during the RSV season. The rate of moderate-severe bronchopulmonary dysplasia (BPD) was significantly higher in group 2 compared to group 1 (38.9% vs 16.2%; P=0.016). Of the 18 infants who were hospitalized, 6 (33.3%) tested positive for RSV while the remaining 12 patients (66.7%) were negative for RSV. Odds ratio (OR) analysis of several risk factors revealed the presence of BPD (OR: 3.28; 95%CI: 1.19-9), being from a family with low socioeconomic status (OR: 3.64; 95%CI 1.08-12.3) to be associated with a higher likelihood of LRTI-related hospitalization.
Conclusion
Our data demonstrated that RSV is an important LRTI agent and cause of hospitalization especially in preterm infants with additional risks such as BPD, gestational age of <28 weeks and low socioeconomic status. We suggest that improving care conditions and decreased BPD with prematurity would help in prevention of LRTI hospitalization.
Keywords: Respiratory syncytial virus, Lower respiratory tract infection, Risk Factors, Preterm Infants
Introduction
Respiratory syncytial virus (RSV) is one of the most prevalent causes of lower respiratory tract infection (LRTI) related hospitalizations among infants younger than 2 years of age[1–4]. Almost all children are infected by RSV at least once before the age of 2 years, with a recurrent infection occurring in more than 50% of cases[5]. Premature infants are at particular risk for RSV infection, requiring readmission following discharge after neonatal intensive care[6]. Other risk factors include bronchopulmonary dysplasia (BPD) and severe congenital heart disease (CHD), which contribute to the severity of a RSV infection while also prolonging hospital stay[7, 8].
Palivizumab is a monoclonal anti-RSV globulin that has been developed and marketed as a preventive measure in infants at risk for RSV infection[1]. Epidemiological studies from Turkey have shown that annually, RSV infections occur most frequently during the period between October-March[9], which translates to a 6-month window for palivizumab prophylaxis. Current international guidelines reserve the use of RSV prophylaxis in infants considered to be at high-risk for developing severe RSV infections, namely those with prematurity, BPD or CHD. Previously reported RSV-related hospitalization rates range from 3% to 37%[10, 11]. Palivizumab has been shown to reduce the overall rate of hospitalization in premature infants with BPD by 55%[12]. In a previous study, we managed to demonstrate a 31% reduction in the duration of hospital stay in BDP patients treated with palivizumab[13].
RSV prophylaxis is mainly used in high-risk patients with prematurity, BPD or CHD, however despite the use of palivizumab, subsequent hospitalization rates are mainly affected by socioeconomic status of families[1, 4]. The aim of this study was to determine the risk factors associated with LRTI-related hospitalizations in preterm infants receiving palivizumab throughout the high season for RSV infection.
Subjects and Methods
This prospective study was undertaken in Zekai Tahir Burak Maternity Teaching Hospital in Ankara, which is located in central Anatolia with the approval of the local ethics committee. This hospital has 150 incubators and serves as a referral Level III NICU with about 4000 newborn admissions per year. Premature infants who were commenced on palivizumab prophylaxis during the RSV season between October 2011 and March 2012 were included in the study following parental consent. Prior to initiation of treatment, information on social and demographic characteristics of the patients’ families, as well as relevant patient data such as birth weight, gestational age (GA), presence of a medical history of respiratory distress syndrome (RDS), patent ductus arteriosus (PDA), intracranial hemorrhage (Grade III-IV), necrotizing enterocolitis (NEC), BPD and retinopathy of prematurity (ROP) were recorded on pre-prepared forms. A diagnosis of BPD was made based on criteria put forth by the US National Institutes of Health [15]. Each form also included information on indication for palivizumab prophylaxis as well as planned and administered number of doses. Monthly income was provided in United States Dollars (USD) using the official exchange rate of the Central Bank of the Republic of Turkey. As per manufacturer's instructions, palivizumab was administered intramuscularly at a standard dose of 15 mg/kg, repeated every 30 days. In Turkey, according to the records of the Turkish Pharmacist's Association, one 50 mg-vial of palivizumab costs 516 USD. Palivizumab is applied free to patients who have insurance.
Additionally, socioeconomic status of each family based on monthly income (<300 USD=low, 300-900 USD=middle, 900-1500 USD=high, >1500 USD=very high), along with details regarding family lodgings were also recorded, taking into account the type of community inhabited (urban, suburban, rural), type of heating (stove, radiator, etc.), exposure to smoke (cigarette smoking by at least one of the parents), number of rooms, and the number of individuals in the household including older siblings (cared for at home or in school). Monthly follow-up visits were scheduled in the prematurity outpatient clinic for each patient during which patients were subject to a detailed physical examination followed by intramuscular administration of palivizumab. RSV prophylaxis was administered taking into consideration the current recommendations of the Turkish Neonatology Society, which have been adapted from recommendations of the American Academy of Pediatrics (AAP) and the European Palivizumab Study Group.
Parents were informed in detail regarding the study protocol, and in the event any of the participants developed symptoms suggestive of a lower respiratory infection, parents were instructed to bring their children to designated health facilities depending on their community of residence (urban, suburban or rural). For infants requiring hospitalization, RSV status was evaluated using a RSV strip test (RSV Respi-Strip kit, Coris Bioconcept, Belgium). This test is based on the interaction between conjugated RSV complex and anti-RSV antibody, which appears as a second line on the test strip. Tests were performed as per manufacturer's instructions. In the literature, RSV Respi-strip test kits have been reported to have a sensitivity of 92%, specificity of 98% and a diagnostic accuracy of 95%[16].
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) for Microsoft Windows, version 20.0 (SPSS Inc., Chicago, IL, USA). Comparisons of categorical variables between groups were made using the Chi-square test. Differences between two groups were examined by the independent samples t-test for normally distributed variables and Mann Whitney U test for abnormally distributed variables. Following univariate analysis, factors that were found to be associated with an increased risk for LRTI-related hospitalization and for the hospitalization to be due to an RSV infection were subjected to odds ratio analysis. A P-value of less than 0.05 was considered indicative of statistical significance.
Findings
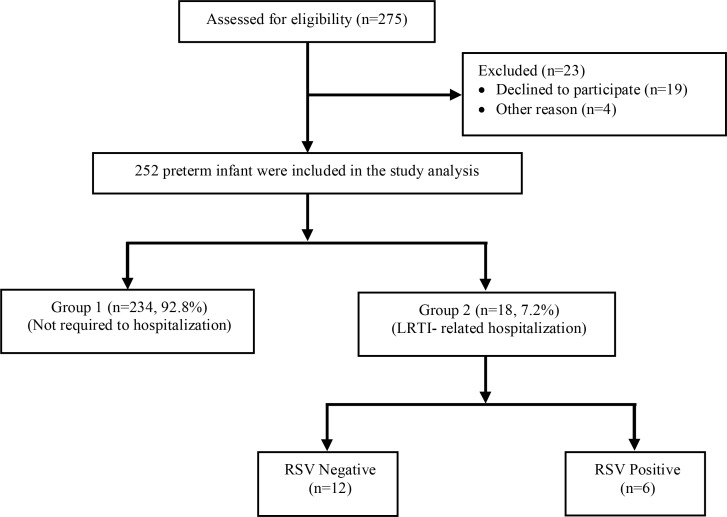
A total of 275 premature infants who were given palivizumab prophylaxis were initially included in the study, although 23 were excluded from the final analysis due to non-compliance with follow-up visits. Overall, 234 (92.8%) participants did not require hospitalization (Group 1) whereas 18 (7.2%) patients were hospitalized at least once due to LRTI (Group 2) during the study period (Fig. 1). Demographic and clinical characteristics of the study population are presented in Table 1. There was no significant difference between groups in terms of gestational age, birth weight, gender, mode of delivery, comorbid conditions (RDS, PDA, NEC and ROP), number of cases with intracranial hemorrhage (Grade III-IV), planned and administered number of palivizumab doses, socioeconomic status, type of community inhabited, type of heating (stove, radiator, etc.), exposure to smoke (cigarette smoking by at least one of the parents), number of rooms, and the number of individuals in the household including older siblings (Table 1). The rate of moderate-severe BPD was significantly higher in group 2 compared to group 1 (38.9% vs 16.2%; P=0.02).
Fig. 1.
Flow chart of preterm infants who were received palivizumab prophylaxis for RSV
RSV: Respiratory syncytial virus
Table 1.
Demographic and clinical characteristics of the study population
| Parameters | Group 1 (n=234) | Group 2 (n=18) | P. value | |
|---|---|---|---|---|
| Gestational age, weeks* | 28.3 (1.8) | 28 (1.5) | 0.5 | |
| Birth weight, g* | 1195 (339) | 1097 (308) | 0.2 | |
| Male gender ‡ | 131 (56) | 11 (61.1) | 0.7 | |
| Mode of delivery (Caesarean) ‡ | 206 (88) | 15 (83.3) | 0.5 | |
| Respiratory distress syndrome ‡ | 177 (75.6) | 13 (72.2) | 0.7 | |
| Patent Ductus Arteriosus ‡ | 95 (40.6) | 9 (50) | 0.4 | |
| Intracranial hemorrhage (Grade III-IV) ‡ | 10 (4.3) | 1 (5.6) | 0.8 | |
| Necrotizing Enterocolitis ‡ | 3 (1.3) | 1 (5.6) | 0.2 | |
| Retinopathy of Prematurity ‡ | 45 (19.2) | 5 (27.8) | 0.4 | |
| Moderate-severe BPD ‡ | 38 (16.2) | 7 (38.9) | 0.02 | |
| Indication for palivizumab prophylaxis ‡ | <28 weeks | 90 (38.6) | 9 (50) | 0.007 |
| 29–32 weeks | 106 (45.3) | 2 (11.1) | ||
| BPD | 38 (16.2) | 7 (38.9) | ||
| Number of palivizumab doses | Planned † | 5 (2–5) | 5 (4–5) | 0.7 |
| Administered † | 5 (2–5) | 5 (1–5) | 0.9 | |
| Socioeconomic status ‡ | Low | 17 (7.3) | 4 (22.2) | 0.09 |
| Middle | 179 (76.5) | 12 (66.7) | ||
| High | 31 (13.2) | 2 (11.1) | ||
| Very high | 7 (3) | 0 (0) | ||
| Type of community inhabited ‡ | Rural | 16 (6.8) | 2 (11.1) | 0.3 |
| Suburban | 49 (20.9) | 5 (27.8) | ||
| Urban | 169 (72.2) | 11 (61.1) | ||
| Type of heating ‡ | Stove | 86 (36.8) | 7 (38.9) | 0.7 |
| Radiator | 143 (61.1) | 11 (61.1) | ||
| Other | 5 (2.1) | 0 (0) | ||
| Exposure to smoke ‡ | 148 (63.2) | 13 (72.2) | 0.4 | |
| Home characteristics | Number of rooms † | 3 (1–5) | 3 (2–4) | 0.8 |
| Number of inhabitants † | 4 (3–9) | 4 (3–7) | 0.1 | |
| Number of older siblings† | 1 (0–5) | 1 (0–3) | 0.3 | |
Values provided as mean ± standard deviation
Values provided as number and percentage of patients
Values provided as median (min-max); BPD: Bronchopulmonary dysplasia
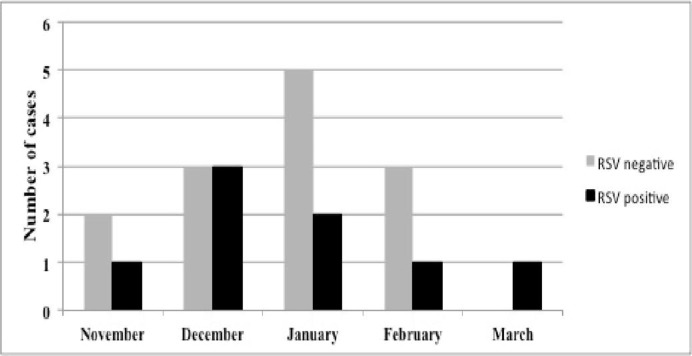
Of the 18 infants who were hospitalized, 6 (33.3%) tested positive for RSV while the remaining 12 patients (66.7%) were negative for RSV. Demographic and clinical characteristics of RSV-positive and RSV-negative patients are summarized in Table 2. Five of the 6 (83.3%) patients who were RSV-positive presented with pneumonia, whereas 1 (16.7%) patient was hospitalized due to bronchiolitis. Half of the patients who were found to be RSV positive required hospitalization for a second time during the RSV season compared to only 1 (8.3%) patient in the RSV-negative group. The difference in re-hospitalization rates between groups was statistically significant (P=0.04). Median duration of hospital stay was significantly longer in the RSV-positive group compared to the RSV-negative group [12 (5-23) days vs.4 (2-20) days; P=0.02)]. The distribution of hospitalized patients with RSV-negative and RSV-positive LRTI according to month is depicted in Fig. 2. Hospitalization rates for both RSV-negative and RSV-positive patients were highest in December and January. Odds ratio (OR) analysis of several risk factors revealed presence of BPD (OR: 3.28; 95%CI: 1.19 to 9), a GA <28 weeks (OR: 1.6; 95%CI: 0.61 to 4.18), history of exposure to smoke (OR: 1.51; 95%CI: 0.52 to 4.38), being from a family with low socioeconomic status (OR: 3.64; 95% CI: 1.08 to 12.3), having older siblings (OR: 1.35; 95%CI: 0.46 to 3.92) and living in a suburban or rural area (OR: 1.65; 95%CI: 0.61 to 4.45) to be associated with a higher likelihood of LRTI-related hospitalization. Results of OR analysis have been summarized in Table 3.
Table 2.
Demographic and clinical characteristics of RSV-positive and RSV-negative patients who were hospitalized
| Parameters | RSV (−) (n=12) | RSV (+) (n=6) | P. value | |
|---|---|---|---|---|
| Gestational age, weeks * | 27.8 (1.8) | 28.5 (0.8) | 0.4 | |
| Birth weight, g * | 1025 (260) | 1240 (371) | 0.2 | |
| Male gender ‡ | 7 (58.3) | 4 (66.7) | 0.6 | |
| Indication for palivizumab prophylaxis ‡ | <28 weeks | 6 (50) | 3 (50) | 0.5 |
| 29–32 weeks | 2 (16.7) | 0 (0) | ||
| BPD | 4 (33.3) | 3 (50) | ||
| Number of palivizumab doses | Planned † | 5 (4–5) | 5 (4–5) | 0.6 |
| Administered † | 5 (4–5) | 4.5 (1–5) | 0.1 | |
| Indication for hospitalization ‡ | Bronchiolitis | 7 (58.3) | 1 (16.7) | 0.09 |
| Pneumonia | 5 (41.7) | 5 (83.3) | ||
| Number of same-season re-hospitalizations ‡ | 1 (8.3) | 3 (50) | 0.04 | |
| Duration of hospital stay, day† | 4 (2–20) | 12 (5–23) | 0.02 | |
| Socioeconomic status ‡ | Low | 2 (16.7) | 2 (33.3) | 0.5 |
| Middle | 8 (66.7) | 4 (66.7) | ||
| High | 2 (16.7) | 0 (0) | ||
| Very high | 0 (0) | 0 (0) | ||
| Type of community inhabited ‡ | Rural | 1 (8.3) | 1 (16.7) | 0.8 |
| Suburban | 3 (25) | 2 (33.3) | ||
| Urban | 8 (66.7) | 3 (50) | ||
| Type of heating ‡ | Stove | 5 (41.7) | 2 (33.3) | 0.6 |
| Radiator | 7 (58.3) | 4 (66.7) | ||
| Other | 0 (0) | 0 (0) | ||
| Exposure to smoke ‡ | 8 (66.7) | 5 (83.3) | 0.4 | |
| Home characteristics | Number of rooms † | 3 (2–4) | 2 (2–4) | 0.2 |
| Number of inhabitants † | 4 (3–7) | 4 (4–6) | 0.7 | |
| Number of older siblings † | 1 (0–3) | 1 (1–2) | 0.6 | |
Values provided as mean ± standard deviation
Values provided as number and percentage of patients
Values provided as median (min-max); RSV: respiratory syncytial virus; BPD: Bronchopulmonary dysplasia
Fig. 2.
The distribution of hospitalized patients with RSV-negative and RSV-positive LRTI according to month for the season between November 2011-March 2012
Table 3.
Summary of odds ratio analysis for several risk factors associated with an increased likelihood for LRTI-related hospitalizations
| Parameters | All hospitalized patients (n=18) | |
|---|---|---|
| Odds ratio | 95% Confidence interval | |
| Male gender | 1.23 | 0.46–3.29 |
| Gestational age <28 weeks | 1.6 | 0.61–4.18 |
| Bronchopulmonary dysplasia | 3.28 | 1.19–9 |
| Older siblings | 1.35 | 0.46–3.92 |
| Low socioeconomic status | 3.64 | 1.08–12.3 |
| Non-urban community | 1.65 | 0.61–4.45 |
| Stove heating | 1.09 | 0.4–2.93 |
| Exposure to smoke | 1.51 | 0.52–4.38 |
Discussion
The main outcome of this study was that it was the most widely conducted study for determining with or without RSV related hospitalization risk factors that increase hospitalization of the preterm newborns under the influence of palivizumab prophylaxis that were closely observed during the season in our country. With this study, it wasdetermined that the preterm newborns who were under the influence of palivizumab prophylaxis during the season would have higher hospitalization rate if such cases had BPD at the outset and low socioeconomic status. LRTIs are one of the leading causes of death in children worldwide, with RSV being the most common viral pathogen in young children[17, 18]. The likelihood of RSV being the cause of LRTI increases with decreasing age[19]. The aim of this study was to identify risk factors associated with an increased risk of LRTI-related hospitalizations, regardless of causative pathogen, in preterm infants under close follow-up while on palivizumab prophylaxis. Preterm infants are at high risk for developing BPD, which makes them susceptible to ventilation-induced volutrauma. Oxygen toxicity as well as pre- and post-natal infections triggers a pulmonary inflammatory response, which can eventually lead to impaired lung development[18]. In a retrospective study by Greenough, et al[20] on a cohort of 235 premature infants (<32 weeks of gestation) it was observed that 19% of neonates who require oxygen treatment beyond 28 days were subsequently re-hospitalized with a proven RSV infection. In our study, 38.9% of patient who were hospitalized for an LRTI had BPD, compared to 16.2% in patients who were not hospitalized, a statistically significant difference (P=0.02). Patients who were hospitalized were 3.28 times more likely to have BPD compared to infants who were not hospitalized.
Male gender is a well-established risk factor for severe RSV-related LRTI, and a review of relevant studies of the past 30 years revealed a boy-to-girl ratio of 1.425:1[14]. It has been postulated that the increased risk could be attributed to boys having shorter and narrower airways making them more likely to develop bronchial obstruction during an RSV infection[21]. In our study, however, the difference between group 1 and group 2 in terms of male gender distribution was insignificant (56% vs 61.1%; 0.67). Nevertheless, male gender was found to be associated with a 1.23-fold increased risk of LRTI-related hospitalization.
Low socioeconomic status is another frequently reported risk factor for RSV infections [22]. In a study from Sweden in 2002 [23], univariate analysis revealed the presence of a significant positive correlation between percentage of immigrants, per capita income and an increased risk of developing RSV. In our study, a low socioeconomic status was associated with a 3.64-fold increased risk for LRTI-related hospitalizations.
Crowded living conditions and the presence of other siblings in the home have also been reported as important risk factors for severe RSV infection and LRTI-related hospitalization. The obvious reason behind this is the increased likelihood of exposure to the virus in a crowded home setting[21]. In the Canadian PICNIC study[24] the presence of preschool-aged siblings was found to be associated with a significantly increased risk for RSV-related hospitalization, while a weaker association was observed in the presence of school-aged siblings. In our study, hospitalized infants were 1.35 times more likely to have an older sibling.
The influence of smoking in the household on the risk of bronchiolitis and hospitalization due to RSV-related LRTI has been a focus of research, albeit with conflicting results[14]. Bivariate analysis in the FLIP[25] and FLIP-2[26] studies from Spain established that the presence of more than two smokers at home was associated with a higher risk of RSV-related hospitalization. A similar finding was reported in the Canadian PICNIC- Study [24] in that household exposure to cigarette smoke from 2 or more smokers was found to be a predictive factor for subsequent RSV-related hospitalization. In our study, we considered cigarette smoking by at least 1 parent as indicative of smoke exposure, and the difference between group 1 and group 2 in terms of exposure to smoke was statistically insignificant (P=0.4). Overall, exposure to smoke was found to be associated with a 1.51-fold increased risk of LRTI-related hospitalization.
Our study has some limitations, the most important of which is that infants hospitalized for LRTIs were not enough positive for RSV infection. So, RSV specific risk analysis for LRTIs cannot be made. Furthermore, the study group did not include any patients with CHD. Nevertheless, our study is the first of its kind as well as being the most comprehensive study from Turkey to evaluate risk factors for LRTI-related hospitalizations in preterm infants receiving palivizumab prophylaxis who were followed up for a whole RSV-season.
Conclusion
Although the rate of hospitalization decreases by palivizumab prophylaxis, our data demonstrated that RSV is an important LRTI agent and the cause of hospitalization especially in preterm infants with additional risks such as BPD, gestational age of <28 weeks and low socioeconomic status. We suggest that improving care conditions and decreased BPD with prematurity would help preventing LRTI hospitalization.
Acknowledgment
We are grateful to Beyhan Küçükbayrak, Emine Amirova Uçan, Şaban Yüksel, Ömer Çimen, Yurdanur Ülkü Özdemir, and Hakan Aylanç in the Department of Pediatrics in Bolu İzzetbaysal State Hospital, Zonguldak Maternity and Child Hospital, Kırşehir Ali Evran State Hospital, Aksaray Maternity and Child Hospital, Kastamonu Maternity and Child Hospital, and Yozgat State Hospital, respectively for follow-up and testing the Respi-strip test of the patients.
Conflict of Interest
None
References
- 1.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127–32. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 2.Carbonell-Estrany X, Quero J, Bustos G, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatr Infect Dis J. 2000;19(7):592–7. doi: 10.1097/00006454-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Broughton S, Roberts A, Fox G, et al. Prospective study of healthcare utilisation and respiratory morbidity due to RSV infection in prematurely born infants. Thorax. 2005;60(12):1039–44. doi: 10.1136/thx.2004.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22(Suppl 2):S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 5.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi district, Kenya: infection during the first year of life. J Infect Dis. 2004;190(10):1828–32. doi: 10.1086/425040. [DOI] [PubMed] [Google Scholar]
- 6.Joffe S, Escobar GJ, Black SB, et al. Rehospitalization for respiratory syncytial virus among premature infants. Pediatrics. 1999;104(4 Pt 1):894–9. doi: 10.1542/peds.104.4.894. [DOI] [PubMed] [Google Scholar]
- 7.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113(6):1758–64. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 8.Dizdar EA, Aydemir C, Erdeve O, et al. Respiratory syncytial virus outbreak defined by rapid screening in a neonatal intensive care unit. J Hosp Infect. 2010;75(4):292–4. doi: 10.1016/j.jhin.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkish Neonatal Society. The seasonal variations of respiratory syncytial virus infections in Turkey: a 2-year epidemiological study. Turk J Pediatr. 2012;54(3):216–22. [PubMed] [Google Scholar]
- 10.Boyce TG, Mellen BG, Mitchel EF, Jr, et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–70. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 11.Holman RC, Shay DK, Curns AT, et al. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J. 2003;22(6):483–90. doi: 10.1097/01.inf.0000069765.43405.3b. [DOI] [PubMed] [Google Scholar]
- 12.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3 Pt 1):531–7. [PubMed] [Google Scholar]
- 13.Oncel MY, Mutlu B, Kavurt S, et al. Respiratory syncytial virus prophylaxis in preterm infants: A cost-effectiveness study from Turkey. Turk J Pediatr. 2012;54(4):344–51. [PubMed] [Google Scholar]
- 14.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(Suppl 5):118–26. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 15.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 16.Gregson D, Lloyd T, Buchan S, et al. Comparison of the RSV respi-strip with direct fluorescent-antigen detection for diagnosis of respiratory syncytial virus infection in pediatric patients. J Clin Microbiol. 2005;43(11):5782–3. doi: 10.1128/JCM.43.11.5782-5783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang AB, Chang CC, O'Grady K, et al. Lower respiratory tract infections. Pediatr Clin North Am. 2009;56(6):1303–21. doi: 10.1016/j.pcl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medici MC, Arcangeletti MC, Merolla R, et al. Incidence of respiratory syncytial virus infection in infants and young children referred to the emergency departments for lower respiratory tract diseases in Italy. Acta Biomed. 2004;75(1):26–33. [PubMed] [Google Scholar]
- 20.Greenough A, Cox S, Alexander J, et al. Health care utilisation of infants with chronic lung disease, related to hospitalization for RSV infection. Arch Dis Child. 2001;85(6):463–8. doi: 10.1136/adc.85.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommer C, Resch B, Simões EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J. 2011;5:144–54. doi: 10.2174/1874285801105010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glezen WP, Paredes A, Allison JE, et al. Risk factors for respiratory syncytial virus infection for infants from low-income families in relation to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98(5):708–15. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 23.Jansson L, Nilson P, Olsson M. Socioeconomic enviromental factors and hospitalization for acute bronchiolitis during infancy. Acta Paediatr. 2002;91(3):335–8. doi: 10.1080/08035250252834021. [DOI] [PubMed] [Google Scholar]
- 24.Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23(9):806–14. doi: 10.1097/01.inf.0000137568.71589.bd. [DOI] [PubMed] [Google Scholar]
- 25.Figueras-Aloy J, Carbonell-Estrany X, Quero J, et al. Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33-35 weeks in Spain. Pediatr Infect Dis J. 2004;23(9):815–20. doi: 10.1097/01.inf.0000136869.21397.6b. [DOI] [PubMed] [Google Scholar]
- 26.Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J, et al. IRIS Study Group. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J. 2008;27(9):788–93. doi: 10.1097/INF.0b013e3181710990. [DOI] [PubMed] [Google Scholar]