SUMMARY
This study was conducted on patients with squamous cell carcinoma of the external auditory canal and temporal bone treated with surgery alone or surgery plus postoperative radiotherapy. It was designed as a retrospective investigation with complete long-term follow-up covering the years from 1983 to 2008. The setting was a tertiary referral centre. Forty-one consecutive cases underwent surgery involving en bloc lateral or subtotal temporal bone resection, parotidectomy and neck dissection plus radiotherapy in advanced cases. The Pittsburgh staging system 7 was adopted. No cases were lost to follow-up, which ranged from 2 to 220 months, while for survivors ranged from 60 to 220 months and included clinical examinations and imaging. Outcome was expressed as NED (no evidence of disease), DOC (dead of other causes), DOD (dead of disease), AWD (alive with disease), disease-free survival (DFS) and disease-specific survival (DSS). Results were expressed with raw data and Kaplan Meyer curves. Patients with T1 and T2 disease had a DFS of 67% and a DSS of 92%. For T3 and T4 cases, the DFS was 41% and DSS was 48%. All treatment failures were due to local recurrences. The cases classified as T4 because the lesion extended from the cartilage canal to the periauricular soft tissues, or from the anterior wall to the parotid space, had a better outcome than the other T4 cases: this different prognosis suggests the need to stage tumours differently. Nodal disease coincided with a worse outcome due to local recurrence.
KEY WORDS: Temporal bone tumour, Carcinoma of the ear, Temporal bone resections, Lateral skull base surgery
RIASSUNTO
L'obiettivo di questo lavoro è valutare i risultati a lungo termine del trattamento chirurgico e chirurgico/radioterapico nel carcinoma spinocellulare primitivo del condotto uditivo esterno-osso temporale. Lo studio consiste in una analisi retrospettiva con follow-up a lungo termine. I casi chirurgici sono stati trattati tutti in un centro terziario di riferimento negli anni dal 1983 al 2008. Sono stati analizzati 41 casi di tumore spinocellulare primitivo del condotto uditivo esterno, trattati chirurgicamente con blocco resezione laterale o subtotale del temporale, parotidectomia, svuotamento laterocervicale elettivo o terapeutico. Abbiamo utilizzato la classificazione di Pittsburgh. Il follow-up si estende da 2 mesi a 220 mesi e, per quelli sopravvissuti, da un minimo di 60 mesi a 220 mesi. I risultati sono stati espressi come NED, DOD, DOC e AWD (non-evidenza di malattia, morto per malattia, morto per altre cause e vivo con malattia), con dati crudi e con curve di Kaplan Meyer. I T1-T2 hanno una sopravvivenza libera da malattia del 67%, ed una sopravvivenza specifica per malattia del 92%. I T3-T4 hanno una sopravvivenza libera da malattia del 41% e una sopravvivenza specifica del 48%. Tutti gli insuccessi sono avvenuti per recidiva locale. I casi stadiati come T4 per estensione da cartilagine ai tessuti molli o dalla parete anteriore alla parotide hanno avuto una miglior prognosi rispetto ai T4 con differente estensione mediale, inferiore o posteriore. Questa differenza di prognosi suggerisce la necessità di un cambio della stadiazione. Il coinvolgimento linfonodale implica una prognosi peggiore ma per recidiva locale e non regionale.
Introduction
Squamous cell carcinoma (SCC) of the external auditory canal is an uncommon malignancy 1 2 that arises from the external ear and spreads to the temporal bone and surrounding sites. Periauricular soft tissues, the parotid gland, temporomandibular joint and mastoid are common sites of tumour progression. The carotid canal, jugular foramen, dura, middle and posterior cranial fossae are invaded in advanced stages 2 3.
Ear pain and discharge may initially be mistaken for external otitis 5. Late diagnosis is common and worsens prognosis. Aggressive surgery with postoperative radiation is the usual treatment 6.
A retrospective study was conducted on the outcome of our consecutive series of patients assessed preoperatively using modern imaging methods who underwent surgery between 1983 and 2008 based on a consistent skull base surgery protocol. The rationale for the surgical procedure is discussed in relation to the site and extent of the tumour and its classification. The outcome is expressed using raw data as well as Kaplan Meyer curves.
Materials and methods
Forty-four patients with primary SCC of the external auditory canal extending to the temporal bone treated between 1983 and 2008 were retrospectively reviewed. Tumours with a different histology or extending secondarily into the temporal bone were not considered. Three of the 44 patients with unresectable tumour only received palliative therapy, so 41 were the subject of the present study. This series included 35 first diagnoses and 6 recurrences (Table I). Among the former tumours, 4 arose in the radical tympanomastoidectomy cavity, and 3 cases were associated with another SCC arising in the auricle or periauricular skin.
Table I.
Site of origin of tumours.
| Site of origin of tumour | Primary tumour |
|---|---|
| Bone canal | 23 |
| Cartilage canal | 4 |
| Cartilage + bone canal | 4 |
| In radical cavity | 4 |
| Recurrent tumour | 6 |
The follow-up ranged from 2 to 220 months (mean 60 months) for the complete series, while for survivors it ranged from 60 to 220 months (mean 120 months). All surgical procedures were performed by the same surgeon using similar technique.
Diagnostic work-up and classification
The diagnostic work-up included clinical examination, imaging and biopsy. High-resolution CT and contrast-enhanced MRI were performed in 41 and 30 cases, respectively. All patients had a preoperative biopsy. The sites of origin of the tumour are listed in Table I. The modified Pittsburgh classification 7 was used for staging purposes (Table II); the early cases in the series were classified retrospectively.
Table II.
The Pittsburgh staging system modified by Hirsch 7.
| T status | Description |
|---|---|
| T1 | Tumour limited to the external auditory canal without bony erosion or evidence of soft tissue extension |
| T2 | Tumour with limited external auditory canal bony erosion (not full thickness) or radiographic finding consistent with limited (< 0.5 cm) soft tissue involvement |
| T3 | Tumour eroding the osseous external auditory canal (full thickness) with limited (< 0.5 cm) soft tissue involvement, or tumour involving middle ear and/or mastoid |
| T 4 | Tumour eroding the cochlear, petrous apex, medial wall of the middle ear, carotid canal, jugular foramen or dura, or with extensive (> 0.5 cm) soft tissue involvement; patients presenting with facial paralysis |
| N status | Lymph node involvement is a poor prognostic sign and places the patient in advanced stage (i.e. T1 N1, stage III), and T2, T3, T4 N1 (stage IV) |
| M status | M1 disease is stage IV and is considered a very poor prognostic sign |
Surgical indications
The framework for planning surgery involved identifying the sites and subsites of tumour growth and safe resection margins. All sites and subsites were assessed for their potential for hidden tumour diffusion and the related width of resection. Spread beyond the external auditory canal to adjacent areas, full-thickness bone erosion in the canal walls and lymph nodes that were clinically positive or suspect were all considered during the planning of the procedure. Other factors such as age, general conditions and comorbidities were also taken into account.
En bloc lateral temporal bone resection (LTBR) and en bloc subtotal temporal bone resection (STBR) were judged to be the procedures combining the widest safe resection margins with a clear and reproducible approach. No piecemeal removal was performed. The principles of skull base surgery were applied to the procedure 8. For the LTBR, the block of the outer canal was contoured with an extended mastoidectomy and freed through a temporal craniotomy. The same craniotomy enabled the internal carotid artery to be exposed by drilling and displacing it from its canal down to the neck. The osteotomy from the carotid canal to the glenoid made it possible to free the block and preserve the continuity of the latter with the soft tissues of the parotid and neck dissection.
The STBR involved temporal and occipital craniotomies, freeing the carotid as mentioned above, and four osteotomies to deliver the block. One subtemporal osteotomy from the carotid canal to the posterior side of the petrous bone, with transection of the proximal third of the internal auditory canal and a second osteotomy through the occipital craniotomy, from the petrous bone to the jugular foramen (preserving its medial wall), a third osteotomy from the jugular fossa to the carotid canal, and a fourth from the carotid canal to the glenoid fossa through the temporal craniotomy. The block remained continuous with the neck dissection and the parotid without violating the safe margins at this level.
Retrograde parotidectomy was performed in both LTBR and STBR. Total parotidectomy was indicated in cases of anterior growth beyond the anterior wall of the external auditory canal, while superficial parotidectomy was performed as a prophylactic measure on the intraparotid nodes in T1 and T2 cases. Parotid involvement was suspected when imaging revealed erosion of the anterior wall of the auditory canal or an enhancing mass in the parotid area.
The neck was treated in both LTBR and STBR with prophylactic selective neck dissection for clinically-negative necks and a modified type III radical neck dissection in patients with clinically-positive lymph nodes. Neck dissection was not performed in a few elderly patients who were N0, or who had an advanced tumour with a poor prognosis. If imaging showed growth of the tumour anteriorly towards the condyle of the mandible or the temporomandibular joint, then the condyle, mandible ramus or temporomandibular joint was added to the en bloc resection. Tumour growth against or infiltrating the dura demanded wide dura resection and repair with fascia. When the tumour was confined to the lateral end of the canal with no bone erosion, the LTBR lay laterally to the eardrum.
The facial nerve was handled on the basis of general principles, i.e. it was to be included in the resection when clinical examination or imaging indicated that it was involved by the tumour, or when the safe resection margin included the Fallopius. The internal carotid artery was never resected.
T1 and T2 cases underwent LTBR, superficial parotidectomy and neck dissection. T3 and T4 patients, classified as such due to spread from the cartilage canal to the periauricular soft tissues, or anteriorly through the anterior wall of the canal into the parotid space, were treated with LTBR, total parotidectomy and neck dissection. T3 and T4 cases with growth into the mastoid, or tympanum, or medial sections of the temporal bone underwent STBR, total parotidectomy and neck dissection.
Therapy
All 41 patients underwent surgery with a curative intent, but one had an incomplete STBR as the tumour was discovered to be unexpectedly advanced. LTBR was performed in 30 cases, and STBR in 11 (including the above mentioned incomplete resection). Neck dissection was part of the treatment in 33 of the 41 patients. Retrograde parotidectomy was performed in 37 cases. Postoperative radiotherapy with 50 to 70 Gy (median 60 Gy) was administered to T3-T4 cases, and to T1-T2 patients with intraoperative findings suggestive of more extensive disease, or with multiple nodal metastases or a single nodal metastasis with extracapsular spread, and to the case with undissected neck.
Follow-up
The follow-up involved clinical examination every 3 months for the first year and then every six months for four years, then yearly. Imaging with bone window CT and contrast-enhanced MRI was repeated every six months for the first year, then yearly until the 10th year.
Outcome measures
Outcome was expressed as NED (no evidence of disease), DOD (dead of disease), DOC (dead of other causes) and AWD (alive with disease), DFS (disease-free survival) and DSS (disease-specific survival) rates.
Raw data are reported because the follow-up was long and none of the patients were lost. Survivors were followedup for 5 to 18 years (median 10 years).
DFS refers to all patients still alive and without disease (NED) after a median follow-up of 10 years (range 5-18 years) and to those who died of other causes (DOC) after a follow-up of at least 5 years. DSS refers to all patients who did not die of the disease (the whole series minus the DOD cases). Overall survival (OS) is expressed in terms of NED as none of the patients in our series were alive with disease at the end of follow-up (February 2013, after a follow-up of 5-18 years).
Results
Outcomes
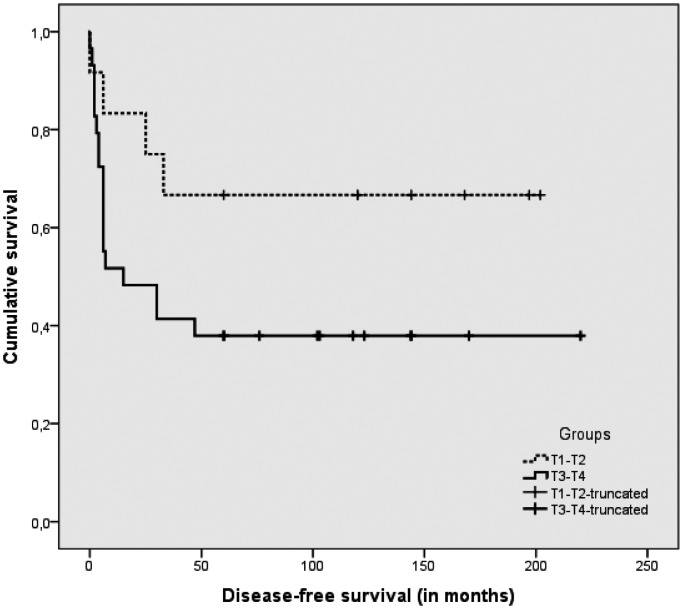
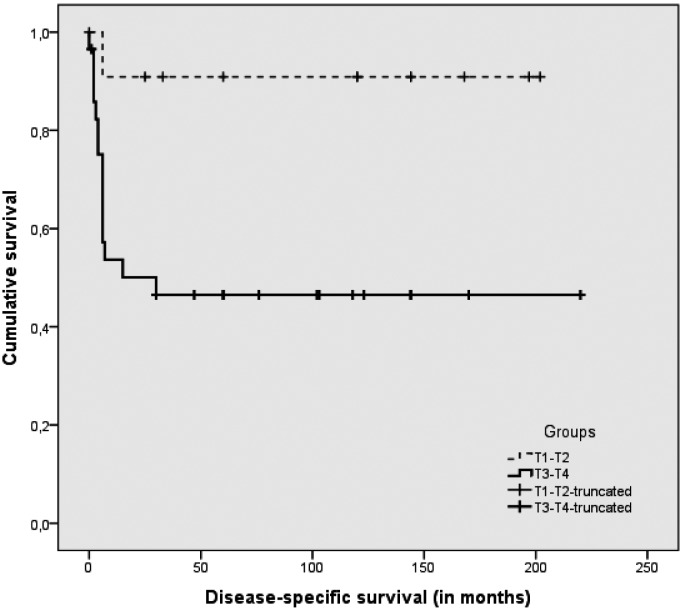
The TNM staging of the surgical series is shown in Table III. Among the 36 of 41 patients who were graded, 20 were G1, 15 were G2 and one was G3. Tables III and IV show the complete case material with the T stage-related outcome and survival. The overall treatment failure rate was 39% (16/41), after a median follow-up of 10 years (range 5-18 years). The DFS (NED + DOC > 5 years, after a median 10-year follow-up, range 5-18 years) for the series as a whole was 49% (20/41), and the DSS was 61% (25/41). The failure rate for T1-T2 tumours was 8% (1/12), while for T3-T4 cases it was 52% (15/29). The T-stage related DFS and DSS are also shown in Figures 1 and 2.
Table III.
Stage-related outcome results.
| T | No. of cases | NED | DOD | DOC < 5 years | DOC > 5 years | DSS% | DFS% |
|---|---|---|---|---|---|---|---|
| T1 | 6 | 2 | / | 3 | 1 | 100% | 50% |
| T2 | 6 | 4 | 1 | / | 1 | 83.3% | 83.3% |
| T3 | 8 | 4 | 2 | / | 2 | 75% | 75% |
| T4 | 21 | 1 | 13 | 2 | 5 | 38% | 29.3% |
| Total | 41 | 11 | 16 | 5 | 9 | 61% | 49% |
| T1+T2 | 12 | 6 | 1 | 3 | 2 | 92% | 67% |
| T3+T4 | 29 | 5 | 15 | 2 | 7 | 48% | 41% |
Table IV.
T stage and subsites of the external auditory canal walls originating the tumour.
| T stage | No. of cases | Anterior wall | Other single wall | ≥ 2 walls |
|---|---|---|---|---|
| T1 | 6 | 1 | 1 | 4 |
| T2 | 6 | 3 | 2 | 1 |
| T3 | 8 | 2 | 4 | 2 |
| T4 | 21 | 8 | 2 | 11 |
Fig. 1.
Disease-free survival (DFS) in T1-T2 vs. T3-T4 tumours.
Fig. 2.
Disease-specific survival (DSS) in T1-T1 vs. T3-T4 tumours.
All 16 failures were due to local recurrences, and one patient had a lung metastasis. In 12 patients, the tumour recurred within a mean 13.2 months (median 6 months, range 1-17 months) and all died of their disease (DOD) within 2 to 34 months. The other 4 patients survived longer: 2 had a longer time to recurrence and survived for 38 and 41 months; 2 were cases of tumour in a radical cavity and survived for 50 and 62 months.
Among the 14 DOC patients, 5 died before the fifth year of follow-up, and the other 9 died without disease after a follow-up of five years or more, and were considered cured.
Six of the 41 patients had recurrent tumour after previous treatment: their outcome was a treatment failure in 5 cases, while one died of other causes after 144 months.
The DFS rate was 17% (1/6) in the group of recurrences and 51% (18/35) in the group with a first diagnosis.
Tumours arising in a radical cavity, tumours of the canal with synchronous or metachronous SCC of the periauricular skin (regional cases) and tumours arising from the cartilage canal were considered as special subgroups. They were included among the whole series and are considered separately here in terms of their outcome.
There were 4 cases of SCC originating in an old regular cavity of a radical tympanomastoidectomy for cholesteatoma, where an area of SCC developed in the skin: 2 cases with tumour limited to the skin (considered as T1) were NED after 13 and 17 years; 2 with bone involvement (considered as T4) were DOD after 50 and 62 months (with the longest survival in the DOD group). There were 3 cases of synchronous or metachronous SCC of the auditory canal and periauricular skin: one T3 was NED at 127 months, one T3 was DOD after 16 months and one T4 was DOD after 34 months.
The eight tumours arising in the cartilage canal had the following outcome: one T2 and two T3 were NED at 113, 120 and 127 months; one T3 (also belonging to the regional SCC group) was DOD at 16 months; one T1 of the cartilage and bone canals was DOC at 33 months; three T4 (one of them was also a regional tumour) were DOD at 15, 34 and 50 months. The patients with intrinsic tumours survived, while all those with extrinsic tumours died except for one T3.
Postoperative radiotherapy
Twenty-three of the 41 patients received postoperative radiotherapy, including 19 of 29 advanced (T3-T4) cases and 4/12 T1-T2 cases. The median dosage was 60 Gy, range 50-70 Gy.
The indications for radiotherapy in T1-T2 patients varied: in one T2 case, intraoperative evidence of tumour aggressiveness prevailed over a negative finding at pathology; one patient had a positive node with extracapsular spread; one was a case of recurrence after primary surgery; and one case was a T1Nx with an undissected neck.
The outcome differed between the two groups treated with and without RT. Among the 4 T1-T2 cases given radiotherapy, there was one DOC after less than 5 years, and the others were all NED. Among the 19 T3-T4 cases given RT, there were 9 DOD and one DOC with a followup of less than 5 years, 4 DOC with a more than 5-years of follow-up and 5 NED. The DFS and DSS were 75% and 100% for the T1-T2 cases, and 47% (9/19) and 53% (10/19) for the T3-T4 cases. One of the 10 patients given no RT postoperatively had already received this treatment preoperatively, and died after 12 years of other causes (DOC > 5 years). Among the other 9 cases, one was NED, 7 were DOD and 1 was DOC < 5 years. The DFS for these patients was 20% (2/10) and the DSS was 30% (3/10).
Surgical procedures
The surgical procedures were LTBR in 30 cases and STBR in 11 (one of the latter was deliberately not completed due to uncontrollable tumour). Both types of resection were combined with retrograde parotidectomy in 37 of 41 cases. Parotidectomy was not performed in 4 cases due to patients' age or advanced tumour: one 82-year-old patient had postoperative RT and was DOC 33 months later (DOC < 5 years); two were cases of T4 recurring after surgery alone or surgery and radiotherapy (one underwent STBR and was DOD after 41 months, the other was the case of incomplete STBR, who was DOD after 14 months); and the fourth was the previously-mentioned T4 in a radical cavity who was DOD at 50 months.
LTBR was performed in T1-T2 cases and in T3-T4 disease involving the cartilage canal with periauricular extension, or the anterior wall extending into the parotid space. STBR was performed in T3-T4 cases of other origins and extensions. The DFS and DSS were 57% (17/30) and 73% (22/30) in the LTBR group, and 18% (2/11) and 27% (3/11) in the STBR group.
The treatment failed in 30% (9/30) of the LTBR group and 73% (8/11) of the STBR group.
It is worth noting the outcome in the T4 cases (Table V). The 21 patients in the T4 group included 8 cases of tumour of the anterior bone wall extending anteriorly > 0.5 cm, and 13 cases spreading medially and posteriorly to the temporal bone. The Pittsburgh classification draws no distinction between these two groups, but we found a difference in terms of survival. In the group of T4 cases with anteriorly extending disease the DFS was 62.5% (5/8 cases) and the DSS 75% (6/8), while the 13 T4 patients with a medial and posterior spread into the temporal bone had a DFS of 0% (0/13) and a DSS of 15% (2/13). If we exclude the cases with tumour in a radical cavity and recurrent tumour, a 6 case group homogeneous to the anterior extension group had similar results.
Table V.
Sites of origin and outcome of T4 tumours*.
| No. of T4 cases | Site | NED | DOD | DOC < 5 years | DOC > 5 years | DFS% | DSS% |
|---|---|---|---|---|---|---|---|
| 8 | Anterior wall | 1 | 2 | 1 | 4 | 62.5 | 75. |
| 13 | Other walls | / | 11 | 2 | / | 0 | 15.3 |
| 6/13 | Selected other walls* | / | 5 | 1 | / | 0 | 16.6 |
Selected other walls = tumour arising from walls other than the anterior wall, excluding recurrent or radical cavity cases.
All 7 patients with pT4 disease and tumour infiltrating the dura, who underwent STBR, dura resection and fascia grafting, died of local recurrence at sites other than the dura or brain.
Nodal disease
Thirty-three of our 41 cases underwent neck dissection. The neck was clinically positive in 4 and negative in the remaining 37 patients. Pathology identified nodal disease in 9 cases, i.e. in the four clinically-positive necks and another five of the 29 cN0 cases that underwent neck dissection. The overall rate of lymph node metastases was 27% (9/33), and for occult metastases in the cN0 dissected necks was 17% (5/29). The remaining 24 cases were all pN0. The DFS in the positive neck cases was 33.% (3/9), and the DSS was 33% (3/9). In the negative neck cases, the DFS was 62.5% (15/24), and the DSS was 71% (17/24). The two disease-free patients in the N+ group were staged T1N2 and T3N2b. There were no regional recurrences in the neck, and all treatment failures were due to local recurrences.
Discussion
SCC of the external auditory canal is quite rare and the variability of the clinical pictures encountered makes it difficult to obtain homogeneous groups with sufficient statistical power. The complex anatomy and changing relationships between the tumour and contiguous tissues within a limited space make it complicated for surgery to achieve tumour-free margins. Individual surgeons take years to accumulate a number of cases, and surgical expertise changes in the meantime. All these factors limit the advances made in the treatment of this condition. Progress has nonetheless been made since the historical works by Lewis 9 and Conley 10, thanks to small steps forward achieved by several contributions. Staging with the Arriaga classification modified by Moody 7 is still the ground for progress.
The aim of this retrospective study was to provide outcome results based on complete long-term follow-up. The discussion focuses on the value of en bloc resection based on principles of oncological radicality and expertise in skull base surgery 8, as well as the outcome of treatment in relation to the tumour's sites and subsites of origin and direction of growth.
Outcomes
Cure rates have improved since the 1970s thanks to progress in diagnostic imaging and skull base microsurgery. In Conley's series 9, the 5-year cure rate was 18%, and in Lewis' 10 it was 25%; Moody and Hirsch 7 reported a 2- to 3-year cure rate of 20-30%. In 2005, Moffat 6 reported an overall survival rate of 43%, with a median follow-up of 7 years (range 6 months to 16 years). Later publications showed a trend towards better outcome. Yin 11 reported an overall survival of 66.8%, with 100% for T1-T2 tumours, and 67% and 29.55% for T3 and T4 cases, respectively. Adjuvant chemo-radiotherapy improved outcomes to 72%. Gidley 12 reported a 5-year survival of 48% for T1- T2 disease, and 28% for T3-T4. Dean 13 reported a 5-year DFS of 50% in a series of mainly recurrent tumours, and no worsening outcome in cases with intracranial extension. According to the author, sequential aggressive piecemeal resection in a sequential manner appeared to be more effective than the traditional en bloc approach. Morris 14 studied predictors of survival/recurrence in temporal bone resections: for 31 SCC cases of the external auditory canal, the 5-year OS was 62.2%, DSS was 67.7% and recurrence-free survival was 53.5%. Predictors were the status of the surgical margins, metastatic nodes in the neck or parotid and parotid invasion.
In our series, DFS and DSS rates were 49% and 61%, respectively. We believe that patients surviving more than 5 years without disease can reasonably be considered as cured. A follow-up of less than 2 years, as is often reported in the literature 6 15-21, seems to be too short for the purpose of assessing DFS.
When patients died of their disease, the time elapsing varied considerably, from 2 to 62 months, with the first signs of recurrence being detected after 1 to 36 months. A long-term survival with a late recurrence was seen in both the two cases of T4 in radical cavity and 2 of the 5 recurrences after surgery or RT.
T stage and outcomes
Lower-stage tumours, T1 and T2 according to the Pittsburgh staging system, had a higher rate of success after LTBR, while T3 and T4 tumours had less favourable outcome
(Table III). Failures were all due to local recurrences and correlated with sites of secondary tumour growth and lymph node involvement. Tumours spreading to periauricular soft tissues and parotid space had a different effect on outcome than when they extended to the mastoid and deeper parts of the temporal bone. Tumours staged as T4 due to periauricular or anterior growth and treated with LTBR had a better outcome than T4 cases extending posteriorly, medially and inferiorly treated with STBR. For the 8 T4 cases extending anteriorly, the DFS rate was 62.5% (5/8), and DSS was 75% (6/8), while the DFS was 0% (0/13) and DSS was 15% (2/13) for the 13 T4 cases extending medially or posteriorly. Among 12 cases with extensive soft tissue vs. bone involvement, Ito 23 reported that only extensive bone involvement correlated with a worse prognosis. This author also mentioned 23 that Moore 24 reported a similar outcome in groups of T1, T2 and T4 cases with extensive soft tissue involvement. On the other hand, Moody 7 reported good outcome in a few cases with spread to the mastoid.
The current Pittsburgh classification 7 draws no distinction between the sites and subsites of a T4 tumour, whereas the Manolidis 22 staging system differentiates outgrowth to the parotid and infratemporal "fossa" from spread to the mastoid. Different outcomes are not further divided in the literature because the T4 stage includes all advanced tumours. It is reasonable to speculate whether further removal from the remains of an en bloc resection might be useful. STBR in our series was performed with neat, narrow cuts, leaving the carotid artery, petrous apex and neural section of the jugular foramen in place. The questions remains if a supplemental, aggressive, sequential removal 13 is indicated in such cases.
Involvement of the dura is considered an indicator of a poor prognosis 6 17-19, and this was also confirmed in our experience. It is noteworthy that our 6 cases who underwent STBR with an ample resection of the dura (and brain in one case) all developed a local recurrence away from the dura or brain. The temporal bone resection seemed to be less efficacious than on the dura.
Prognosis in T4 tumours
The different outcomes in T4 tumours extending from the cartilage canal to the periauricular soft tissues, or from the anterior bone wall to the parotid space compared to the other T4 tumours raises some questions about the anatomical and surgical factors behind these different results, and the possible need to introduce new steps in the oncological severity of the clinical picture.
Resecting tumours extending into the periauricular tissues enables safer and wider tumour-free margins to be achieved with no vital structures to sacrifice and with a choice of reconstruction techniques.
The resections under discussion are the LTBR used for T4 tumours growing into the parotid area and periauricular tissues, and the STBR for the T4 tumours in other areas. The question is why STBR fails to prevent local recurrences of tumours having bone growth as their main feature. The natural foramina, fissures and channels for nerves and vessels have been thought to provide the routes for dissemination of the tumour: these include the branches for the auditory canal and the posterior auricular nerve for the facial nerve, the posterior auricular nerve from the vagus nerve in the jugular fossa to the Fallopius, a branch from the mandibular nerve to the auditory canal and tympanic membrane, the auriculo-temporal nerve of the mandibular nerve and the chorda tympani. The vascular channels are the numerous veins running from the mucosa and bone to the petrosal sinuses and the jugular bulb, the tympanic arteries from the carotid and the subarcuate artery.
The above-mentioned bone channels may be a crucial anatomical factor related to the severity of T4 tumours. Our experience suggests that the amount of bone invasion may be an essential factor in the prognosis of every tumour. The resection could be enlarged, but this carries a burden of morbidity for the lower cranial nerves and carotid artery, which is unacceptable to the patient and may not be supported by preoperative imaging.
The different prognostic values of the various sites of T3 and T4 tumours warrants a critical reconsideration of the current staging system that could benefit from integration of new data. In our experience, there are increasingly severe steps, depending on the sit of origin of the tumour and its secondary growth pattern, as outlined below:
The skin of the auditory canal.
Skin and bone and/or cartilage involvement, but not full thickness (the term 'full thickness' is appropriate for the anterior bone wall, while it needs to be defined for the other bone walls).
Anterior extension from anterior wall to parotid space, or from cartilage canal to periauricular soft tissues.
Extension from canal to mastoid and other sites of the temporal bone.
A tumour outgrowing the canal cannot be classified solely on quantitative measures of extension. Anatomic variations of amount and structure of bone as well as diffusion routes and critical tissues are factors affecting severity.
Our view on staging and treatment reflects the general consensus on the Arriaga-Moody 7 21 system, while introducing a few changes concerning the different severity of advanced tumours growing into soft tissue or bone as follows:
T1 Tumour in skin without bone involvement;
T2 Tumour in skin with bone/cartilage involvement, but not full thickness;
T3a Tumour extending < 5 mm from cartilage to periauricular soft tissues, or Tumour strictly limited to the anterior bone wall and growing < 5 mm into the parotid space;
T3b Same as for T3a, but extending > 5 mm;
T4a Tumour growing into mastoid, without 7th nerve paresis;
T4b Tumour growing into mastoid with facial paresis, or infratemporal space, or medial wall of tympanum, labyrinth, petrous bone (jugular foramen, internal carotid canal, petrous apex).
The multifaceted reality of temporal bone SCC includes several conditions that should be considered in the staging systems:
tumour persistence or recurrence after surgery or radiotherapy;
tumour in a radical cavity (only skin or skin plus extension to bone);
tumour of the auditory canal as a site of synchronous or metachronous regional cancer of the auricular-periauricular skin;
the area considered as "soft tissues" should be mentioned explicitly.
The staging system is based on the site and extent of the lesion, and the outcome of treatment, but biological markers may come into play as well. There is preliminary evidence from recent research 27 to suggest that cytoplasmic MASPIN expression is a promising prognostic indicator and that recombinant MASPIN may be a viable therapeutic agent. Such developments deserve attention as they may have a role in both staging and treatment.
Surgical procedure vis-à-vis T stage
Numerous authors agree 1 2 6 9 15 16 19 that LTBR is the appropriate procedure for T1-T2 tumours, while LTBR and STBR may be used for T3 and T4 cases. The high rate of failures is a cause of concern, however. A local recurrence may be due both to an underestimation of the tumour's extent and erroneous or ineffectual resection. Preoperative imaging may be imprecise in identifying the extent of a tumour, and microscopic tumour dissemination also needs to be better understood. The less severe outcome of T4 cases extending anteriorly may be related to a more rational surgical procedure, since retrograde parotidectomy and carotid artery dissection from the middle fossa down to the neck stay away from the area of tumour growth. The lateral approach to the carotid canal with drilling of the tympanic bone plate may endanger the safe margins around the tumour.
Our retrospective study lacks an analysis of recurrences, although it should be noted that this is also lacking in the literature. More precise data on the sites of recurrence are needed. Correlating the extent of the original tumour with the extent of the resection and the site of recurrence may shed light on the mechanism of recurrence. In this regard, MRI studies should be planned every three months for the first three years.
Since STBR so often fails, it should be established how the STBR can be enlarged. The inferior aspect of the surgical field, at the interface between the skull base and the neck, jugular fossa and the posterior and medial aspects of the temporal bone may be where the infiltration of the tumour is undetected and uncontrolled. Once it is reached by the tumour, the periosteum may also be a source of uncontrolled spread.
Postoperative radiotherapy
Postoperative radiotherapy is commonly recommended for T3-T4 cases 1 6 11 14 17 19. Although our experience is inconclusive concerning the benefits of RT, we prefer to use it in advanced cases.
Nodal disease and neck dissection
Lymph node involvement is acknowledged as an indicator of a poor prognosis 6 11 14 19 25 and, in our experience, outcome was poor for cases with positive nodes. Their treatment failed as a result of the disease recurring locally, and not in the neck or at distant sites. Clinical or intraoperative signs of positive nodes can be considered a sign of tumour aggressiveness and may prompt a wider resection of the tumour.
The role of neck dissection is generally accepted for cN+ cases, but is still debated for cN0 cases 6 19 25. In the literature, there are reported rates of 4.5% to 31.8% of positive nodes after neck dissection, with a cumulative rate of 17.7% 6. We found 27% of pN+ cases, including 4 patients with clinically positive nodes, and 5 with clinically negative nodes.
The rate of micrometastases in clinically-negative necks was 17%. We found a DFS of 22% in the group of 9 patients with positive nodes, and 6 of these died due to local failures. In the group with pathologically-negative nodes, the DFS was 62.5% (10/24). A 17% rate of micrometastases is considered too low to convincingly support the recommendation that elective neck dissection be performed in the cN0 neck 25, but there is no evidence to support the decision not to treat the neck, either in our experience or in the literature 6 19 25 26, and so prophylactic neck dissection may be advisable as the safer option. The type of neck dissection to perform in the clinically-negative neck is a debated issue 6 19 25 26. A planned dissection of levels Ib to III enables en bloc resection of the tumour, parotid and lymph nodes. The risk of metastases at level IV should be considered in cases with positive nodes at higher levels, or in tumours recurring after surgery and/or radiotherapy. Extending the dissection to include level V is generally recommended 25 in cases of therapeutic neck dissection and intraoperative positive nodes. Intraoperative frozen section pathology is mandatory 25.
Conclusions
Skull base surgical principles and expertise were applied to en bloc resection, plus radiotherapy in advanced cases, to treat squamous cell carcinoma of the external auditory canal extending in various ways to the temporal bone and periauricular tissues. The minimum follow-up was 5 years (mean 10, range 5-18 years) for all cases, demonstrating the validity of long-term outcomes reported herein.
Most of the results reported in the literature were confirmed, e.g. the high rate of recovery for lesions lying within the bone or cartilage walls of the canal, the still limited success of treatment for tumours outgrowing the canal walls, and the dismal prognosis in advanced cases, for which postoperative radiotherapy is recommended due to poor prognosis.
In our series, all treatment failures were due to local recurrences, suggesting that the resection was not achieving free margins despite the pathology findings. Nodal disease was a marker of tumour severity and poor prognosis with local recurrences, possibly warranting more aggressive treatment. Enlarging the resection entails a disproportionate increase in the morbidity of the procedure, however, and it may be more appropriate to associate surgery with other therapies such as chemotherapy and SCC targeted therapy. Although it is uncommon, the risk of a positive node going undetected might tilt the balance in favour of prophylactic neck dissection in cN0 cases.
It is generally agreed that prognosis is worse for tumours outgrowing the canal than for those lying within the canal walls. In our experience, the difference also lies in worse outcome for tumours extending into the temporal bone, rather than for tumours growing into soft tissues and the parotid space. The amount of tumour growth in bone seems to correlate with poorer outcome, and appears to be the most important prognostic factor. This difference may raise the question of whether the current staging of T4 tumours needs to be revised.
Acknowledgements
The authors thank Frances Coburn for the review of the English version and Laura Girasoli for statistical analyses.
References
- 1.Pensak ML, Gleich LL, Gluckman JK, et al. Temporal bone carcinoma: contemporary perspectives in the skull base surgical era. Laryngoscope. 1996;106:1234–1237. doi: 10.1097/00005537-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Arena S, Keen M. Carcinoma of the middle ear and temporal bone. Am J Otol. 1998;9:351–356. [PubMed] [Google Scholar]
- 3.Leonetti JP, Smith PG, Kletzker GR, et al. Invasion patterns of advanced temporal bone malignancies. Am J Otol. 2000;122:882–886. [PubMed] [Google Scholar]
- 4.Grandis JR, Hirsch B, Yu VL. Simultaneous presentation of malignant external otitis and temporal bone cancer. Arch Otolaryngol Head Neck Surg. 1993;119:687–689. doi: 10.1001/archotol.1993.01880180107021. [DOI] [PubMed] [Google Scholar]
- 5.Al-Shihabi A. Carcinoma of temporal bone presenting as malignant otitis externa. J Laryngol Otol. 1992:908–910. doi: 10.1017/s0022215100121255. [DOI] [PubMed] [Google Scholar]
- 6.Moffat DA, Wagstaff SA, Hardy DG. The outcome of radical surgery and postoperative radiotherapy for squamous cell carcinoma of the temporal bone. Laryngoscope. 2005;115:341–347. doi: 10.1097/01.mlg.0000154744.71184.c7. [DOI] [PubMed] [Google Scholar]
- 7.Moody SA, Hirsch BE, Myers EN. Squamous cell carcinoma of the external auditory canal: an evaluation of a staging system. Am J Otol. 2000:21582–21588. [PubMed] [Google Scholar]
- 8.Zanoletti E, Martini A, Emanuelli E, et al. Lateral approaches to the skull base. Acta Otolaryngol Ital. 2012;22:281–287. [PMC free article] [PubMed] [Google Scholar]
- 9.Conley JJ, Novak AJ. The surgical treatment of tumors of the ear and temporal bone. Arch Otolaryngol. 1960;71:623–635. doi: 10.1001/archotol.1960.03770040035006. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JS. Temporal bone resection: review of 100 cases. Arch Otolaryngol. 1975;101:23–25. doi: 10.1001/archotol.1975.00780300027006. [DOI] [PubMed] [Google Scholar]
- 11.Yin M, Ishikawa K, Honda K. Analysis of 95 cases of squamous cell carcinoma of the external and middle ear. Auris Nasus Larynx. 2006;33:251–257. doi: 10.1016/j.anl.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Gidley PW, Roberts DB, Sturgis EM. Squamous cell carcinoma of the temporal bone. Laryngoscope. 2010;120:1144–1151. doi: 10.1002/lary.20937. [DOI] [PubMed] [Google Scholar]
- 13.Dean NR, White HN, Carter DS, et al. Outcomes following temporal bone resections. Laryngoscope. 2010;12:1516–1522. doi: 10.1002/lary.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris LG, Mehra S, Shah JP, et al. Predictors of survival and recurrence after temporal bone resection for cancer. Head Neck. 2012;34:1231–1239. doi: 10.1002/hed.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arena S. Tumor surgery of the temporal bone. Laryngoscope. 1974;84:645–670. doi: 10.1288/00005537-197404000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Austin JR, Stewart KL, Fawzi N. Squamous cell carcinoma of the external auditory canal: therapeutic prognosis based on a proposed staging system. Arch Otolaryngol Head Neck Surg. 2000;120:1228–1232. doi: 10.1001/archotol.1994.01880350036007. 1994. [DOI] [PubMed] [Google Scholar]
- 17.Moffat DA, Grey P, Ballagh RH, et al. Extended temporal bone resection for squamous cell carcinoma. Otolaryngol Head Neck Surgery. 1997;116:617–623. doi: 10.1016/S0194-59989770237-7. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T, Kumamoto Y, Natori Y, et al. Squamous cell carcinoma of the external auditory canal and middle ear: an operation combined with preoperative chemoradiotherapy and a free surgical margin. Otol Neurotol. 2006;27:242–248. doi: 10.1097/01.mao.0000190463.88873.3d. discussion 249. [DOI] [PubMed] [Google Scholar]
- 19.Prasad S, Janecka IP. Efficacy of surgical treatments for squamous cell carcinoma of the temporal bone: a literature review. Otolaryngol Head Neck Surg. 1994;110:270–280. doi: 10.1177/019459989411000303. [DOI] [PubMed] [Google Scholar]
- 20.Lim LHY, Goh YHG, Chan YM, et al. Malignancy of the temporal bone and external auditory canal. Otolaryngol Head Neck Surg. 2000;122:882–886. doi: 10.1016/S0194-59980070018-0. [DOI] [PubMed] [Google Scholar]
- 21.Arriaga M, Curtin H, Takahashi H, et al. Staging proposal for external auditory meatus carcinoma based on preoperative clinical examination and computed tomography findings. Ann Otol Rhinol Laryngol. 1990;99:714–721. doi: 10.1177/000348949009900909. [DOI] [PubMed] [Google Scholar]
- 22.Manolidis S., Pappas D, Jr, Doersten P, et al. Temporal bone and lateral skull base malignancy: experience and results with 81 patients. Am J Otol. 1998;19:S1–S15. [PubMed] [Google Scholar]
- 23.Ito M, Hatano M, Yoshizaki T. Prognostic factors for squamous cell carcinoma of the temporal bone: extensive bone involvement or extensive soft tissue involvement? Acta Otolaryngol. 2009;129:1313–1319. doi: 10.3109/00016480802642096. [DOI] [PubMed] [Google Scholar]
- 24.Moore MG, Deschler DG, McKenna MJ, et al. Management outcome following lateral temporal bone resection for ear and temporal bone malignancies. Otolaryngol Head Neck Surg. 2007;137:893–898. doi: 10.1016/j.otohns.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldo A, Ferlito A, Suarez C, et al. Nodal disease in temporal bone squamous carcinoma. Acta Otolaryngol. 2005;125:5–8. doi: 10.1080/00016480410018287. [DOI] [PubMed] [Google Scholar]
- 26.Choy JY, Choi EC, Lee HK, et al. Mode of parotid involvement in external auditory carcinoma. J Laryngol Otol. 2003;117:951–954. doi: 10.1258/002221503322683821. [DOI] [PubMed] [Google Scholar]
- 27.Marioni G, Zanoletti E, Stritoni P, et al. Expression of the tumor-suppressor maspin in temporal bone carcinoma. Histopathology. 2013;63:242–249. doi: 10.1111/his.12151. [DOI] [PubMed] [Google Scholar]