SUMMARY
In patients with a cochlear implant (CI), the first critical point in processing auditory information from sound stimuli that leads to comprehension is the interface between the electrode and the cochlear nerve, which is dependent on providing appropriate current input. The purpose of this work was to evaluate the longitudinal differences in psychoacoustic fitting parameters in CI users. We studied 26 profoundly deaf adults, aged between 18 and 58 years, who had been implanted in our department between 2009 and 2011. The lowest current levels that evoked an auditory sensation (T-level) and the highest current levels that did not elicit an uncomfortable loud sensation (C-level) were recorded at the time of activation, approximately 30 days after implantation (mean 28.5 days) (T0), after one month (T1), 3 months (T3), 6 months (T6) and one year (T12). Impedance values were calculated for electrode groups: basal, middle and apical. In all cases, the same model of perimodiolar implant (Cochlear™ Nucleus® CI24RE) and the same surgical technique (cochleostomy) were used. The values of T-level and C-level showed significant incremental changes between T0 and T1 and between T1 and T3. T-levels in the basal regions of the cochlea were higher than in other sites. T-levels in the basal turn exhibited higher values consistent with a greater amount of fibrosis, as reported in other studies. Our findings suggest that fitting sessions should be scheduled more frequently during the first three months as indicated by the greater slope of T- and C- level variations during that time frame.
KEY WORDS: Cochlear implant, Adults, Fitting values
RIASSUNTO
Nei pazienti con impianto cocleare il primo punto critico del processo uditivo dallo stimolo sonoro alla comprensione può essere identificato nell'interfaccia elettrodo-coclea. Scopo di questo lavoro è quello di valutare, nei pazienti con sordità profonda e sottoposti ad impianto cocleare, le differenze longitudinali dei principali parametri psicoacustici del mappaggio. Abbiamo studiato 26 pazienti adulti di età compresa tra i 18 e 58, impiantati presso il nostro Dipartimento, nel periodo compreso tra il 2009 ed il 2011. La minima intensità di corrente necessaria ad evocare una sensazione uditiva (T-level) e la più alta intensità di corrente che non evoca sensazione acustica di fastidio (C-level) sono state registrate al momento dell'attivazione, che si è verificata circa 30 giorni dopo l'impianto (in media 28,5 giorni) (T0), dopo un mese (T1), 3 mesi (T3), 6 mesi (T6) e un anno (T12). In tutti i casi è stato utilizzato lo stesso tipo di impianto perimodiolare (Cochlear™ Nucleus® CI24RE) con la stessa tecnica chirurgica (cocleostomia). I valori di T-level e quelli di C-level mostrano variazioni significative incrementali tra T0 e T1 e tra T1 e T3. I T-level nelle regioni basali della coclea sono più alti rispetto agli elettrodi inseriti nelle partizioni cocleari mediana ed apicale. Tali valori più alti concordano con i reperti di altri autori di una prevalente neoformazione fibrosa basale. Pertanto è necessario programmare più frequentemente le sedute di mappaggio durante i primi 3 mesi, in base ad una maggiore velocità di variazione dei valori di T e C.
Introduction
Cochlear implants can restore hearing function to people with a severe or profound hearing loss using an electrode system stimulating endocochlear surviving neuronal cells. Two main variables affect the performance of cochlear implants: the processor's capacity to deliver effective bursts of electrical impulses and the ability of the patient to receive such stimuli and process them appropriately.
The fitting procedure for a cochlear implant aims to establish suitable electrical stimulation levels for each channel. It includes measuring each electrode for the lowest current level that evokes an auditory sensation (T-level) and for the highest current level that does not elicit an uncomfortable loud sensation (C-level). The objective of this investigation was to evaluate time variations of these main fitting parameters in CI users. Many authors adopt study intervals at one month after activation, followed by three months, six months and 12 months 1-3; in some cases, intervals at 24 and 36 months are also used 4 5. Thus, we followed the mostly widely-adopted timing criteria to better compare our data with other reports.
Materials and methods
Twenty-six profoundly hearing-impaired adults implanted by our department between 2009 and 2011 were studied. There were 16 males and 10 females ranging in age between 18 and 58 years who had either pre-lingual (n = 21) or post-lingual (n = 5) deafness: all were selected according to the latest criteria 6.
In our patients, the aetiology of deafness remained unknown in 46% of cases, while 34% was due to environmental factors, 15% to genetic causes and 4% to other clinical features. Details of the different aetiologic causes are provided in Table I.
Table I.
Aetiologies of profound sensorineural hearing loss in our cases (percentages are rounded).
| Aetiology | Number of patients | % |
|---|---|---|
| Unknown | 12 | 46 |
| Genetic | 4 | 15 |
| Prenatal infections (Rubella) | 1 | 4 |
| Prematurity/neonatal intensive care unit stay (sepsis, hypoxia, jaundice) | 5 | 19 |
| Postnatal infections (meningitis, measles, chronic suppurative otitis media) | 3 | 11 |
| Multiple sclerosis | 1 | 4 |
| Total | 26 |
According to the fact that the aetiology of deafness contributes to outcomes only to a very small extent 7, we did not consider separate groups in collecting the results.
The degree of deafness was bilateral and profound. Cases of bilateral implantation were excluded.
Our study protocol included assessment during device activation (T0, approximately 30 days after surgery - mean: 28.5 days), after one month (T1), 3 months (T3), 6 months (T6) and a year (T12). We chose these time points to better compare our data with existing literature reports, which adopted similar timing.
All patients received the same model of perimodiolar array (CI24RE by Cochlear LTD). Furthermore, the same surgical technique (cochleostomy) was performed on all patients; i.e. a manual and progressive introduction of the array, removal of the stylet at the end of insertion in scala tympani and always applying the recommended precautions of soft surgery for this device 8-11.
For this study, we excluded patients with incomplete insertion and/or with cochlear malformations and/or impedance values more than 20 kOhm even if occurring in only one electrode of the patient's array, and also if noted at one visit during follow-up. All patients received the same fitting using the ACE strategy at the same default fitting parameters (rate = 900 pps, pulse width = 27 μsec). The T-SPL (minimum intensity input level that results in electrical stimulation) employed was set at 25 dB. Maxima were selected according to patient preference considering subjective quality of perceived sounds: variations were noted from 8 to 12.
At the beginning of each session, electrical impedance was measured and the subjective values of T-level and Clevel for each electrode were obtained. T-level was found on a channel-by-channel basis starting with an audible stimulation and reducing the energy level until the patient reported that there was no longer any sound perception. As for C-level, single channels were selected and the energy level was increased until the patient reported hearing a loud, not uncomfortable sound.
Overall mean values were evaluated for the full array and for grouped data by considering basal electrodes (E1 to E7), middle (E8 to E14) and apical (E15 to E22) named according to anatomical cochlear segments.
The progression of the values of T-level and C-level at observational periods was assessed using a repeated-measures analysis of variance (ANOVA). Post-hoc comparisons utilised the Bonferroni method of confidence interval adjustment. The probability error accepted for significant values was p < 0.05 after Bonferroni correction, which adds a very restrictive criteria for significance.
Speech audiometry was not considered as to its variability was mostly noted in preverbal patients, which are numerous in our cohort.
We prefer a subjective method for fitting T and C values, as we had no non-collaborating patients and to optimize compliance throughout the total time of study. An alternative method such as neural response telemetry is objective, but correlates only to a certain extent with subjective measures. In fact, in another study 12, prediction of the contour of T- and C-levels from the contour of NRT thresholds across electrodes would not be appropriate for half of subjects.
Results
Raw data showed increasing mean values for T-levels and C-levels, with a reduction of standard deviations in the final stages, T6 and T12 (Table II) comparing the results for the full complement of all electrodes for all subjects. Figures 1a and 1b illustrate these changes as mean values for T- and C-levels. The repeated measures analysis of variance showed significant differences for TLevel [F = 44.11; p < 0.001] and for C-Level [F = 93.69; p < 0.001]. The Bonferroni post-hoc comparison yielded significant incremental changes for T0 to T1 in T-level [p < 0.0001] and C-level [p < 0.0001], and from T1 to T3 in T-level [p = 0.0013] and C-level [p = 0.0023]. No significant differences were obtained for incremental changes for the T3 to T6 measurements or from T6 to T12.
Table II.
Full array, n = 26, raw mean values and standard deviation for T-levels, C-levels at various times of observation are shown for activation at approximately one month after surgery (T0), after one month (T1), 3 months (T3), 6 months (T6) and a year (T12).
| T-level average | Std dev | C-level average | Std dev | |
|---|---|---|---|---|
| T0 | 106.20 | 4.63 | 143.97 | 3.19 |
| T1 | 130.74 | 4.14 | 181.26 | 4.04 |
| T3 | 142.92 | 3.21 | 192.95 | 3.14 |
| T6 | 143.99 | 2.89 | 195.65 | 2.48 |
| T12 | 147.51 | 2.60 | 198.70 | 2.23 |
Figs. 1a-b.
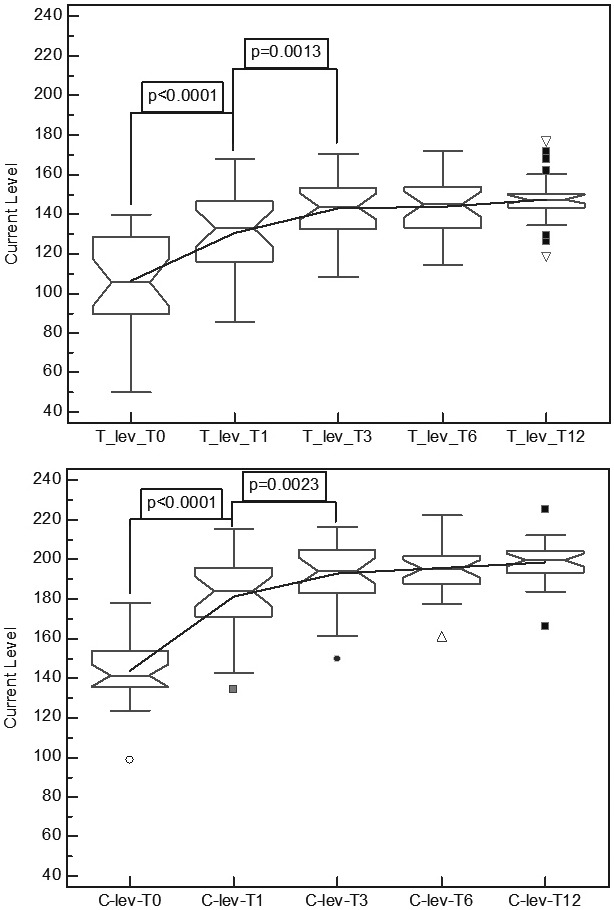
(1a) Overall variation in mean T-levels for the full array, (1b) showing the overall variation in mean C-levels for the full array, n = 26. Significant differences for the test periods are shown (p-values are Bonferroni corrected).
Figs. 2a-f.
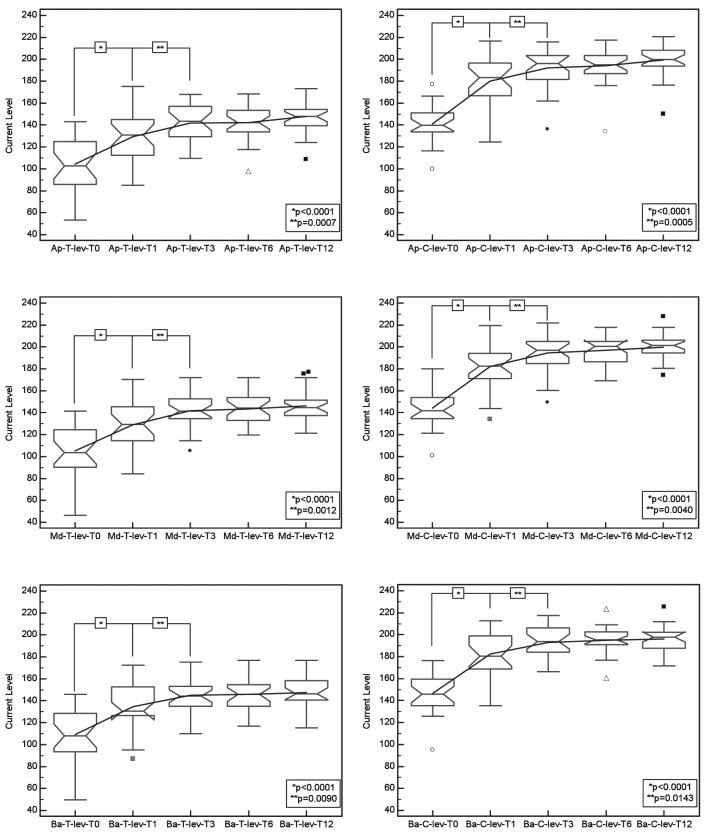
Time sequence of the values of T (left figures, a, c, e) and C-level (right figures, b, d, f) differentiated by apical (top figures), middle and basal (down figures) electrode groups.
The same analysis was conducted for each cochlear partition differentiating the electrodes by groups for apical, middle and basal regions compared to the entire cochlear array. The results for the apical cochlear segment for the repeated measures analysis of variance reached significant differences for T-Level [F = 44.92; p < 0.001] and C-Level [F = 81.70; p < 0.001]; Bonferroni post-hoc comparison yielded significant incremental changes from T0 to T1 in T-level [p < 0.0001] and C-level [p < 0.0001], and from T1 to T3 in T-level [p = 0.0007] and C-level [p = 0.0005]. We obtained non-significant incremental changes from T3 to T6 and from T6 to T12. In the middle cochlear array segment, the repeated measures analysis of variance showed significant differences for T-Level [F = 42.73; p < 0.001] and for C-Level [F = 96.54; p < 0.001]; Bonferroni post-hoc comparison showed significant incremental changes from T0 to T1 in T-level [p < 0.0001] and C-level [p < 0.0001], and from T1 to T3 in T-level [p = 0.0012] and C-level [p = 0.0040]. Incremental changes from T3 to T6 and from T6 to T12 were not significant. In the basal cochlear array segment, repeated measures analysis of variance revealed significant differences for T-Level [F = 33.56; p < 0.001] and C-Level [F = 77.09; p < 0.001]; Bonferroni post-hoc comparison yielded significant incremental changes from T0 to T1 in T-level [p < 0.0001] and C-level [p < 0.0001], and from T1 to T3 in T-level [p = 0.0090] and C-level [p = 0.0143]. No significant differences in incremental changes from T3 to T6 and from T6 to T12 time intervals were observed.
Table III summarises the results of comparison between the electrode groups at different follow-up points: in T0 for T-Level significant differences [F = 7.82; p = 0.001] with Bonferroni post-hoc comparison significant difference in Apical vs Basal [p = 0.022] and Middle vs Basal [p = 0.0006], no statistical difference in Apical vs Middle; for C-Level significant differences [F = 4.57; p = 0.015], but with Bonferroni post-hoc comparison no significant difference in Apical vs Basal [p = 0.0847], Middle vs Basal [p = 0.371] and Apical vs Middle [p = 0.1371]; in T1 for T-Level significant differences [F = 5.39; p = 0.008] with Bonferroni post-hoc comparison no significant difference in Apical vs Basal [p = 0.1285] and Apical vs Middle [p = 1], statistical difference in Middle vs Basal [p = 0.0056]; for C-Level, no significant differences [F = 0.77; p = 0.468]; in T3, no significant differences for T-Level [F = 2.27; p = 0.114] and C-Level [F = 0.70; p = 0.503]; in T6, no significant differences for T-Level [F = 1.91; p = 0.159] and C-Level [F = 0.85; p = 0.434]; in T12, no significant differences for T-Level [F = 0.18; p = 0.833] and C-Level [F = 1.79; p = 0.177]. Figure 3 shows that the basal T-level values were slightly higher compared to middle and apical, unlike the C- level values that were always overlapping. C-level values showed no significant differences.
Table III.
Comparison between electrode groups at different follow-up times of T-and C-levels (significant difference are highlighted).
| T-Level | T0 | T1 | T3 | T6 | T12 |
| Apical vs Middle vs Basal | F = 7.82 p = 0.001 |
F = 5.39 p = 0.008 |
F = 2.27 p = 0.114 |
F = 1.94 p = 0.159 |
F = 0.18 p = 0.833 |
| Apical vs Middle | pa=1 | pa=1 | |||
| Middle vs Basal | pa = 0.0006 | pa = 0.0056 | |||
| Apical vs Basal | pa = 0.0220 | pa = 0.1290 | |||
| C-Level | T0 | T1 | T3 | T6 | T12 |
| Apical vs Middle vs Basal | F = 4.57 p = 0.015 |
F = 0.77 p = 0.468 |
F = 0.70 p = 0.503 |
F = 0.85 p = 0.434 |
F = 1.79 p = 0.177 |
| Apical vs Middle | pa = 0.137 | ||||
| Middle vs Basal | pa = 0.371 | ||||
| Apical vs Basal | pa = 0.085 |
Bonferroni corrected
Figs. 3a-b.
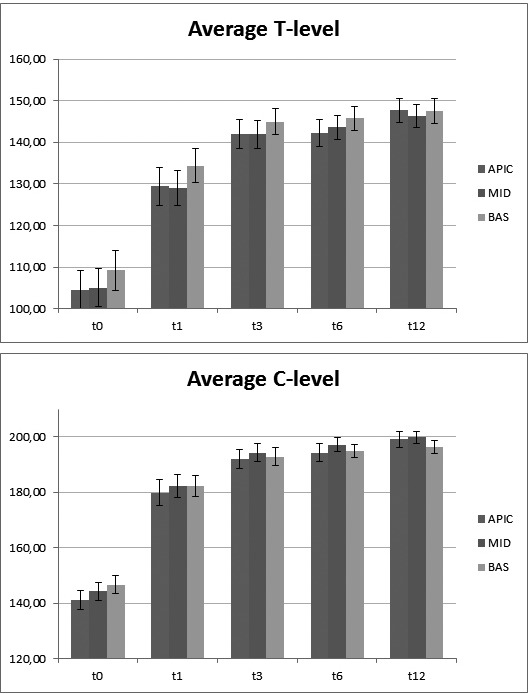
Time sequence of the values of T (3a) and C-level (3b) differentiated by apical, middle and basal electrodes.
Discussion
Previous changes over time in electrical stimulation levels have been reported for adults 13-15. In particular, Hughes 13 stated that C-levels and T-levels stabilised within 12 months of use. The results of the present study indicate that T-levels and C-levels tend to increase up to T12, with significant changes seen up to T3. Later changes observed up to T12 were not considered significant. These results appear as a slowing, although not a cessation, of change after T3. The significant difference between T1 and T12 for T- and C-levels in all electrodes may be due to a more conservative approach in setting the T1 values in patients seen at first mapping. In particular, the important interval differences observed between T1 and T12 may be due to excessive reduction of T1 values rather than a real increase in T12 values.
The non-significant values noted after T3 agree with an earlier intervention of cochlear factors of variations.
The differences noted in the basal T-level responses compared with middle and apical suggest involvement of anatomical factors in that location. Fayad 16 reported a basal prevalence of fibrosis and newly-formed bone after implantation in humans. Our results agree with such a basal anatomical modification, which can be related to the different mean basal T-levels compared to middle and apical findings.
In our patients, the same surgical technique was always implemented; therefore, the result of higher T-values in basal electrodes could be due the insertion technique compared to the round window route. Against this hypothesis, there are the histological findings of Fayad 16, who reported that five round-window surgeries compared to five cochleostomies did not yield significant differences in the amount of fibrosis, bone or, in general, of the newly-formed tissue or in residual sensorineural cells. According to Kawano 17, T-values correlate with the level of fibrous tissue and new bone, particularly with the former. The trend for average T-levels to be lower in the middle and apical segments is consistent with a more robust middle and apical turn survival of sensorineural cells and/ or a closer distance between array and modiolus. Average C-levels did not exhibit any substantial differences between various electrodes at different evaluation periods. This finding agrees with a prevalence of extracochlear factors in defining C-levels; e.g. a) the prolonged activating effect of electrical stimulation on the fibres of the auditory nerve, b) the intervention of central auditory pathways, c) purely cortical factors such as learning or patient preference of a more or less robust stimulation.
Only one study 7 deals specifically with the statistical optimization of speech processor readjustment by considering fitting time intervals to maintain the maximal variation in 90% of recipients between two consecutive fittings within 6 current units.
Clinically, the data presented may be useful when scheduling patients for fitting sessions where appointments are more frequent in the first months after activation when there are more intensive fitting parameters variations.
Conclusions
T-levels in the basal turn exhibit higher values consistent with a greater amount of fibrosis, as reported in other studies. Fitting sessions should be scheduled more frequently during the first three months as indicated by the relatively greater changes seen in the slopes of early T- and C-levels.
References
- 1.Henkin Y, Kaplan-Neeman R, Muchnik C, et al. Changes over time in electrical stimulation levels and electrode impedance values in children using the Nucleus 24M cochlear implant. Int J Pediatr Otorhinolaryngol. 2003;67:873–880. doi: 10.1016/s0165-5876(03)00131-9. [DOI] [PubMed] [Google Scholar]
- 2.Henkin Y, Kaplan-Neeman R, Kronenberg J, et al. Electrical stimulation levels and electrode impedance values in children using the Med-El Combi 40+ cochlear implant: a one year follow-up. J Basic Clin Physiol Pharmacol. 2005;16:127–137. doi: 10.1515/jbcpp.2005.16.2-3.127. [DOI] [PubMed] [Google Scholar]
- 3.Vargas JL, Sainz M, Roldan C, et al. Long-term evolution of electrical stimulation levels for cochlear implant patients. Clin Exp Otorhinolaryngol. 2012;5:194–200. doi: 10.3342/ceo.2012.5.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henkin Y, Kaplan-Neeman R, Muchnik C, et al. Changes over time in psycho-electric parameters in children with cochlear implant. Int J Audiol. 2003;42:274–278. doi: 10.3109/14992020309078346. [DOI] [PubMed] [Google Scholar]
- 5.Jia H, Venail F, Piron JP, et al. Effect of surgical technique on electrode impedance after cochlear implantation. Ann Otol Rhinol Laryngol. 2011;120:529–534. doi: 10.1177/000348941112000807. [DOI] [PubMed] [Google Scholar]
- 6.Berrettini S, Arslan E, Baggiani A, et al. Analysis of the impact of professional involvement in evidence generation for the HTA Process, subproject "cochlear implants": methodology, results and recommendations. Acta Otorhinolaryngol Ital. 2011;31:273–280. [PMC free article] [PubMed] [Google Scholar]
- 7.Smoorenburg G. Cochlear implant ear marks. Utrecht: University Medical Centre Ed.; 2007. pp. 1–3. [Google Scholar]
- 8.Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41:356–359. [PubMed] [Google Scholar]
- 9.Cohen NL. Cochlear implant soft surgery: fact or fantasy? Otolaryngol Head Neck Surg. 1997;117:214–216. doi: 10.1016/s0194-5998(97)70176-1. [DOI] [PubMed] [Google Scholar]
- 10.Laszig R. Cochlear implants in children (soft surgery) Adv Otorhinolaryngol. 2000;57:87–89. doi: 10.1159/000059157. [DOI] [PubMed] [Google Scholar]
- 11.James C, Albegger K, Battmer R, et al. Preservation of residual hearing with cochlear implantation: how and why. Acta Otolaryngol. 2005;125:481–491. doi: 10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- 12.Potts LG, Skinner MW, Gotter BD, et al. Relation between neural response telemetry thresholds, T- and C-levels, and loudness judgments in 12 adult nucleus 24 cochlear implant recipients. Ear Hear. 2007;28:495–511. doi: 10.1097/AUD.0b013e31806dc16e. [DOI] [PubMed] [Google Scholar]
- 13.Hughes ML, Vander KR, Brown CJ, et al. A longitudinal study of electrode impedence, the electrically evoked compound action potential, and behavioral measures in Nucleus 24 cochlear implant users. Ear Hear. 2001;22:471–486. doi: 10.1097/00003446-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kubo T, Iwaki T, Ohkusa M, et al. Auditory plasticity in cochlear implant patients. Acta Otolaryngol. 1996;116:224–227. doi: 10.3109/00016489609137829. [DOI] [PubMed] [Google Scholar]
- 15.Butts SL, Hodges AV, Dolan-Ash S, et al. Gants GJ, Tyler RS, Rubistein JT, editors. Changes in stimulation levels over time in Nucleus 22 cochlear implant users. 7th Symposium on cochlear implant in children. Ann Otol Rhinol Laryngol Suppl. 2000;109:53–56. doi: 10.1177/0003489400109s1222. [DOI] [PubMed] [Google Scholar]
- 16.Fayad JN, Makarem AO, Linthicum FH, et al. Histopathological assessment of fibrosis and new bone formation in implanted human temporal bones using 3D-reconsruction. Otolaryngol Head Neck Surg. 2009;141:247–252. doi: 10.1016/j.otohns.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawano A, Seldon HL, Clark GM, et al. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]