We review recent evidence that non-native and invasive plant species may have distinct timings of their seasonal life history characteristics (such as date of leaf out or flowering, that is, their phenology) that allow them to establish in new communities. In particular we examine how invasions may be bolstered by the longer growing seasons associated with climate change. Based on our current of plant phenology and growth strategies—especially rapid growing, early-flowering species versus later-flowering species that make slower-return investments in growth—we project optimal periods for invasions across three distinct systems under current climate change scenarios.
Keywords: Alien or exotic species, climate change, invasions, non-native, phenology, plasticity, temperate systems.
Abstract
In recent years, research in invasion biology has focused increasing attention on understanding the role of phenology in shaping plant invasions. Multiple studies have found non-native species that tend to flower distinctly early or late in the growing season, advance more with warming or have shifted earlier with climate change compared with native species. This growing body of literature has focused on patterns of phenological differences, but there is a need now for mechanistic studies of how phenology contributes to invasions. To do this, however, requires understanding how phenology fits within complex functional trait relationships. Towards this goal, we review recent literature linking phenology with other functional traits, and discuss the role of phenology in mediating how plants experience disturbance and stress—via climate, herbivory and competition—across the growing season. Because climate change may alter the timing and severity of stress and disturbance in many systems, it could provide novel opportunities for invasion—depending upon the dominant climate controller of the system, the projected climate change, and the traits of native and non-native species. Based on our current understanding of plant phenological and growth strategies—especially rapid growing, early-flowering species versus later-flowering species that make slower-return investments in growth—we project optimal periods for invasions across three distinct systems under current climate change scenarios. Research on plant invasions and phenology within this predictive framework would provide a more rigorous test of what drives invader success, while at the same time testing basic plant ecological theory. Additionally, extensions could provide the basis to model how ecosystem processes may shift in the future with continued climate change.
Introduction
Understanding the forces that allow species to invade established communities is a central goal of ecology (Elton 1958) and critical to mitigating impacts of invasive species (Levine et al. 2003). Mechanistic models of community assembly have helped develop frameworks for predicting when and where invasions are likely to occur (e.g. Shea and Chesson 2002); however, numerous factors may influence invasion success, including competition with established species for limiting resources (MacArthur 1970; Tilman 1982, 1988), interactions with higher trophic levels (Keane and Crawley 2002; Colautti et al. 2004) and processes associated with environmental variability (Chesson and Warner 1981; Chesson 1986). Further, climate change may facilitate invasion by non-native species (Dukes 2011). While many studies have focused mechanistically on direct positive effects of warming or resource enhancement on invasive species (Bradley et al. 2010), there is growing recognition that climate change could facilitate invasions because of the distinct phenology or phenological sensitivity of non-native species (Willis et al. 2010; Wolkovich et al. 2013).
Theories regarding fluctuating resources (Davis et al. 2000) and ‘windows of invasion opportunity’ (Drake et al. 2006; Caplat et al. 2010) suggest that seasonal phenology—the timing of life history events—may play a critical role in invasions (Wolkovich and Cleland 2011). Models of invasion success that hinge on phenology build from the concept of a temporal niche (Fig. 1)—that time is a fundamental axis by which species may partition resource use (Gotelli and Graves 1996), reduce interspecific competition and thus promote coexistence (Chesson and Huntly 1997). Extensions of this basic niche theory have suggested how such distinct phenologies may result in a competitive advantage for non-native species (Godoy et al. 2009), especially in areas with shifting growing seasons due to climate change. If native species do not accurately and rapidly track shifting climate, then climate change may produce phenological vacant niches. In brief, such vacant niches may then promote invader success (i) directly (i.e. an invader occupies the open niche space) or in concert with (ii) early-season priority effects, via (iii) invader plasticity, where non-native species track climate shifts more closely than native species, or (iv) greater niche breadth (see Fig. 1 and Wolkovich and Cleland 2011). Alongside these theoretical developments, a growing body of research focused on plants has found phenological differences, especially in leafing and flowering times, between non-native and native species. Several studies have found that especially early (McEwan et al. 2009; Wilsey et al. 2011; Throop et al. 2012; Wainwright et al. 2012) or late (Godoy et al. 2009; Fridley 2012; Paquette et al. 2012; Pearson et al. 2012) phenologies may aid the success of non-native species, while more recent work suggests that non-native species may be the major drivers of longer growing seasons in North America (Wolkovich et al. 2013). Here we build on current theoretical perspectives and empirical work to develop predictions of how phenology may enhance plant invasions with climate change. Our review begins first with the role of phenology in avoiding or mitigating disturbance, stress and competition. Next, we review recent literature on plant functional traits to highlight evidence for a fundamental trade-off between flowering phenology and the return rate of growth investments, which may impact how climate change and phenology affect invasions. Considering projected scenarios of climate change, we make mechanistic predictions for when during the growing season across three major ecosystem types vacant phenological niche space may promote invasions, and consider current evidence for our predictions. We close by reviewing major questions whose answers would improve predictions of future invasions via phenology.
Figure 1.
Basic invasion theory, built on limiting similarity theory, suggests that species should invade during times when most other species are inactive (vacant phenological niche; see Wolkovich and Cleland 2011). Here we show idealized niche diagrams for four non-native species (purple, dashed-line distributions) and seven native species (grey distributions) in a hypothesized simple mesic temperate system where temperature limits viable periods for plant growth. Across the growing season, variation in stress, disturbance and competition may dictate the optimal phenological strategy, with early-active and late-active species experiencing lower competition but also more variable temperatures, in the mid-season community flowering peaks (see Fig. 2), and thus we expect that mid-season active species may be strong competitors for many resources. With climate change extending viable periods for plant growth (dark blue lines), non-native species with highly plastic phenologies may have an increased opportunity for invasion at the start and end of the growing seasons in temperate mesic systems. As reviewed in Wolkovich and Cleland (2011), there are several major ways in which species may exploit such vacant phenological niches. Species that can track the start of the season closely may exploit even very small vacant niches in the early season via priority effects. Additionally, climate change—by extending growing seasons in many systems—may increase vacant niche space at the start and end of the growing season, possibly allowing for invasions early and late in the season. Non-native species with early phenology and rapid growth strategies may succeed either early or late in the season, while species with greater phenological niche breadth (e.g. longer flowering period) may succeed late in the season.
A note on terminology
Given the debate over terminology in invasion biology (Colautti and Richardson 2009), we wish to be clear about our definitions. We use ‘non-native’ to refer to any species established outside of its home range; such a distinction between native and non-native is important because non-native species have evolved in a different community than the one into which they have been introduced. Thus, they may exhibit differing strategies and trade-offs than native species. We use the term ‘invasive’ for non-native species with a detrimental impact in their introduced community (following Mack et al. 2000). Finally, ‘invade’ and ‘invasion’ refer to the introduction of species, whether they are eventually invasive or not.
Phenological Strategies
Phenology within plant life history theory
Phenology is an important component of plant life history theory (Al-Mufti et al. 1977; Grime 1977; Stanton et al. 2000) affecting both biotic constraints (e.g. competition, herbivory, pollination) and abiotic constraints (e.g. frost, drought) on plant performance. Extensive work over the past several decades has focused on how biotic interactions are informed by phenology, including competition (e.g. Rathcke 1988; van Schaik et al. 1993) and mutualisms (e.g. Brody 1997), while recently the balance of studies has shifted towards a more abiotic focus with climate change (e.g. Inouye 2008; Miller-Rushing and Primack 2008).
Plant strategy theory has generally focused on how both abiotic and biotic factors affect acquisition, allocation and loss, often extrapolating into a focus on how well plants handle competition, stress and disturbance (Grime 1977). Stress, as generally defined, does not lead to major tissue loss while disturbance does—thus the best definition of stress versus disturbance is often species and location specific (Craine 2009). For example, in temperate systems, both frost (abiotic) and herbivores (biotic) may act as a disturbance around which plants must balance their leafout timing. The major difference between the plant's ability to adapt to these abiotic and biotic constraints arises via the feedbacks possible with biotic factors (e.g. herbivores may adjust, within their own set of climatic limits, to match earlier leafout). In contrast, abiotic constraints on plant growth (e.g. frost) are unlikely to be impacted by plant phenology. Thus, in most systems where abiotic factors have been relatively stationary across years—in timing and variability especially—we expect plants to have adjusted their strategies to these system properties. Further, given temporal variation in the abiotic and biotic environment (i.e. across the growing season and across years), we expect phenology to be a major axis along which plants structure their overall life history strategies (Grime 1977). Indeed, recent research in the expanding field of functional plant traits suggests that phenology may be tied to a suite of other traits producing several major phenological strategies.
Trait correlations with phenology
A review of the functional traits literature (Table 1) highlights a strong axis for flowering phenology where earlier flowering is associated with a suite of traits related to rapid return on investment, while later flowering is often associated with the reverse. This axis makes sense when considering how stress, disturbance and competition vary across the growing season in many systems (Fig. 1): early in the season when abiotic stress and disturbance are high, but competition low, an early-flowering, rapid-growth and comparatively low-investment strategy allows species to grow and reproduce quickly before periods of strong competition begin. Such a strategy may also make some loss of tissue to environmental disturbance early in the season less detrimental if rapid growth allows rapid replacement of tissue. While it may seem obvious that earlier flowering would require a quicker return on investment, many perennial species use resources from previous years for the current-year's flowering, and thus this correlation is not automatic (Muller 1978). Further, such a trade-off is seen across both herbaceous and woody species (Table 1). In contrast to early-flowering species, species that flower later in the season must survive high competition throughout the mid-season and thus traits that allow more efficient access to, transport and use of resources would be key. Loss of tissue to disturbance in such a strategy, however, may impart a relatively higher cost, as regrowth would be much slower. This major phenological trait axis—of early and fast versus later and slower—has been noted by many researchers (e.g. Lechowicz 1984; Sun and Frelich 2011), but an additional strategy may be viable in the late season. It seems that some species may also exhibit a rapid-growth and low-investment strategy in the late season (Sun and Frelich 2011); however, as this has been noted less often, we do not consider it extensively.
Table 1.
Current research suggests one major axis by which phenology co-varies with other traits: earlier flowering (and in some cases, earlier leafing) is often associated with traits related to quicker returns on investments (faster growth rates, shallower roots, etc.) while later-flowering species show traits associated with slower returns on investments (slower growth rates, greater heights, deeper roots, etc.). Studies characterizing this trade-off are presented above the double line, while additional studies are shown below. We reviewed the literature using ISI Web of Science and the following search: Topic=(phenolog*) AND Topic=(plant*) AND (functional trait*) Refined by: Document Types=(ARTICLE) AND Web of Science Categories=(ECOLOGY), which returned 79 papers. Of these we included studies that documented phenology and at least one other trait for multiple species. Studies were excluded if they only studied animal guilds or if they focused on selection within a single plant species. Additionally, leaf lifespan was omitted as a measure of phenology if it was simple evergreen/deciduous (as this represents more leaf lifespan than phenology). We included several additional studies that we encountered in the process of writing the manuscript. ‘Flowering date’ includes flowering date, peak flowering date and flowering onset date; SLA=specific leaf area.
| Phenological trait | Other trait(s) studied | Relationship | Plant functional group(s) | Location(s) and reference(s) | ||
|---|---|---|---|---|---|---|
| Flowering date | Max height | Positive (earlier, smaller) | Herbaceous species | Mediterranean old field in Israel (Hadar et al. 1999); semi-natural grasslands in France (Louault et al. 2005; Vile et al. 2006); southeastern Sweden (Bolmgren and Cowan 2008); Tibetan plateau (Du and Qi 2010); eastern North America (Sun and Frelich 2011); mountain meadows in Italy (Catorci et al. 2012) | ||
| Flowering date | Max height | Positive (later, taller) | Mixed woody and herbaceous | Ponderosa pine forest (Laughlin et al. 2010) | ||
| Flowering date | Seed size | Positive (later, larger) | Herbaceous species | Semi-natural grassland in France (Vile et al. 2006) | ||
| Flowering date | Seed size | Negative (earlier, smaller) | Mixed woody and herbaceous | Indiana (USA) dunes (Mazer 1989) | ||
| Flowering date | Growth rate | Negative (earlier, faster) | Herbaceous species | Eastern North America (Sun and Frelich 2011) | ||
| Flowering season | Rooting depth | Positive (later, deeper) | Herbaceous species | Patagonian Steppe (Golluscio and Sala 1993); Mediterranean annual grassland, CA, USA (Gulmon et al. 1983); Tibetan plateau (Dorji et al. 2013) | ||
| Length of growing season | Rooting depth | Positive (later, deeper) | Mixed | Semi-arid woodland in Australia (Campanella and Bertiller 2008) | ||
| Flowering date | Generation time | Positive (later, longer) | Herbaceous species | Semi-natural grassland in France (Vile et al. 2006) | ||
| Flowering date | SLA | Negative (earlier, thinner) | Herbaceous species | Semi-natural grassland in France (Vile et al. 2006) | ||
| Length of growing season | SLA | Negative (longer, thicker) | Mixed | Semi-arid woodland in Australia (Campanella and Bertiller 2008) | ||
| Leafout date | Diameter of spring vessels and/or greater numbers of narrow-diameter vessels | Positive (later, larger) | Trees | Northern North American forests (Lechowicz 1984) | ||
| Flowering date | Leaf tissue density | Positive (later, greater) | Herbaceous species | Tallgrass prairie in Kansas, USA (Craine et al. 2012b) | ||
| Flowering date | Grazing tolerance | Negative (later, tolerant) | Herbaceous species | Mediterranean old field in Israel (Hadar et al. 1999) | ||
| Leaf flushing date | SLA | Positive (later, thinner) | Trees | Savannah/Cerrado in Brazil (Rossatto et al. 2009) | ||
| Length of leaf season | Leaf size | Positive (later, larger) | Mixed woody and long-lived perennial species | High-elevation Mediterranean woodland, Morocco (Navarro et al. 2010) | ||
| Flowering date | Seed size | Negative (later, smaller) | Herbaceous species | Southeastern Sweden (Bolmgren and Cowan 2008) | ||
| Flowering date | Seed size | Bimodal (early and late flowering had small seeds, mid-season was mixed) | Herbaceous species | Mountain meadows in Italy (Catorci et al. 2012) | ||
| Mixed | Morphology, leaf thickness, photosynthetic pathway, life history, seed biology | Complex (multivariate) | Mixed woody and herbaceous | Semi-arid woodland, Australia (Leishman and Westoby 1992); northeast China (Wang and Ni 2005) | ||
| Length of growing season | Origin | Invading species had longer, later-growing seasons | Mixed woody and herbaceous | Germany (Kuester et al. 2010) | ||
Examining phenology as one trait within a complex network of correlated traits raises an important issue of considering when phenology is a major trait on which selection acts, versus only linked to a more critical trait. For example, flowering time is often associated with seed size (Table 1), and teasing out how much phenology or seed size is constrained by the other remains a puzzle, with correlations varying by clade and study (Mazer 1989; Bolmgren and Cowan 2008). Thus, phenology may be structured strongly by selection on it directly, via links with other traits, or shaped by evolutionary history (see Lechowicz 1984; Ollerton and Lack 1992, and see below). If, however, phenology is a major trait structuring life history strategies, then given a relatively stationary abiotic and biotic environment we would expect each species to optimize its phenology for that environment. Given sufficient time and species dispersal, we would also predict that communities would contain a suite of phenological strategies that take up most available resources across the growing season.
Climate Change, Phenology and Invasions: Predictions
Climate change has altered the climate of most ecosystems globally such that they cannot be considered stationary (that is, to have consistent climate means with some stochastic variation around those means; see Betancourt 2012). Non-stationarity could change the optimal phenological strategy—both in absolute timing and in flexibility in this timing—leaving native species less well-adapted to their current environment and providing an opportunity for invasions. Understanding how phenology may intersect with plant invasions requires an explicit temporal model of how competition, stress and disturbance vary across growing seasons and with climate change. Such a model should make basic but testable predictions about when (within a growing season and over longer timescales), in which systems and how phenology may contribute to invader success. We lay out general predictions below, but focus on how temperature increases and precipitation change may affect species invasions. Given this focus, we consider predominantly three types of systems that differ in the dominant abiotic controls over phenology: temperate mesic systems (temperature control), temperate grasslands (temperature and precipitation control) and semi-arid systems (precipitation control). These systems provide useful contrasting examples of how competition, stress and disturbance may interact with phenology to influence community assembly and invasions. We base our predictions on recent climate change projections (Knutti and Sedlacek 2013), trait correlations and plant strategies related to phenology (Table 1 and above), and more generally to invasion. Specifically, we assume (i) that across space and time non-native species may invade environments of relatively high stress and disturbance, but low competition (e.g. Rejmánek 1996; Gelbard and Belnap 2003), up to a point, (ii) as there is also evidence that non-native plant species rarely occupy the most climatically stressful environments (Rejmánek 1989). Additionally, (iii) invaders are often more plastic and thus may adjust to new conditions quickly (Funk 2008; Hierro et al. 2009; Davidson et al. 2011; Wainwright and Cleland 2013). We thus predict temporal opportunities for invasions in periods of relatively high stress and disturbance, but low competition, and discuss how and when climate change may alter these opportunities.
Predictions: early season
Across systems with distinct growing seasons, the early season often represents a period of relatively high stress and disturbance but low competition (Fig. 2)—as most species slowly begin to reactivate tissues and grow. Climatically, the early portion of a temperate growing season is signalled by a rise in temperature. This rise, however, is correlated strongly with increased variability in temperatures (Fig. 2), resulting in high stress and possible disturbance for plants active during this period, and—relatedly—low competition. Plant species active in the early season may risk tissue loss to frost damage or other extreme temperature swings present in the spring (Linkosalo et al. 2000; Augspurger 2009), or to greater herbivore damage (Lechowicz 1984), as species may be more apparent to herbivores in the early season (Brody 1997) and have less-defended tissues (van Asch and Visser 2007). Early-season phenologies, however, also benefit from reduced competition. Across habitats, few species leaf and flower early (Fig. 2) yielding lower competition for soil and light resources and for pollinators (Mosquin 1971). Additionally, competition with microbes for soil nutrients may be especially low as in many systems the soil microbial community turns over with warm spring weather, producing a flush of soil nutrients (Zak et al. 1990).
Figure 2.
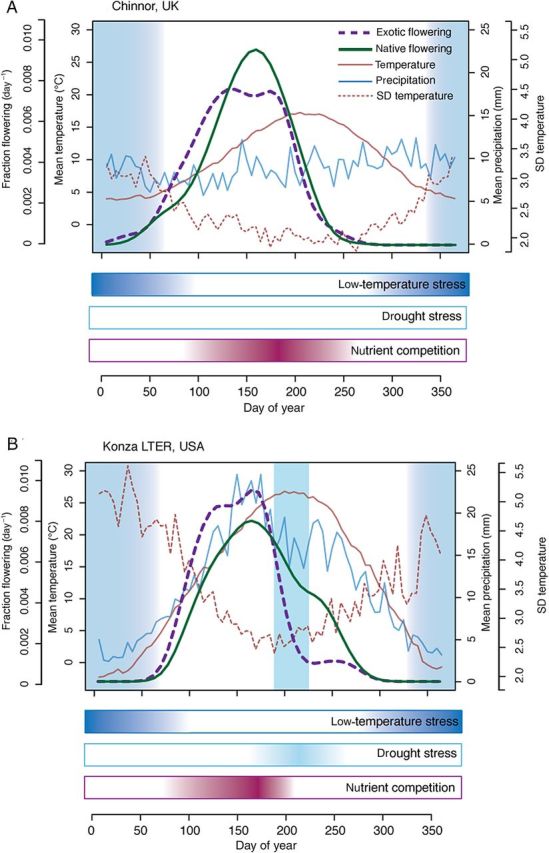
Flowering of non-native and native species within a community varies across habitats. This variation in flowering patterns may be strongly driven by climate differences between systems, which impact the various flavours of stress, disturbance and competition that plants experience. Mesic temperate systems such as Chinnor (UK, A) are often defined by temperature (darker blue shading), while other systems such as the tallgrass prairie of Konza (Kansas, USA, B) may have variable drivers across the growing season. In both systems, temperature sets the beginning and end of the season and, as such, early-season species show strong sensitivity to temperature (Cook et al. 2012; Craine et al. 2012b). In Konza, a consistent mid-season drought, however, coincides with a decrease in the number of species flowering at that time (Craine et al. 2012b). We assume that temperature <5 °C limits development, as this is the temperature at which many cell processes slow down dramatically or stop (Larcher 2003) and, further, is the suggested lower threshold temperature for tissue growth and development globally for alpine trees (Korner 1998). Standard deviation (SD) of temperature and the coefficient of variation (CV) of precipitation are given as pentad (5-day) averages. Flowering data are species averages from NECTAR (Wolkovich et al. 2012b), climate data for Chinnor were taken from GHCN UK000056225 and cover 1853–2001, while climate data for Konza were taken from GHCN USC00144972 and cover 1893–2010.
Early phenologies of non-native species may thus succeed through two major mechanisms independent of climate change. First, non-native species that are active early in the growing season may be particularly successful because they have the opportunity to preempt space and soil resources, grow quickly and shade later-active species (Weiner 1990; Wilsey et al. 2011; Wolkovich and Cleland 2011). This type of asymmetric competition could create ‘seasonal priority effects,’ one mechanism by which non-native species could establish and rise to dominance in a new community (Dickson et al. 2012; Wainwright et al. 2012). Second, invaders may succeed via an early-season enemy release mechanism. In invasion biology the enemy release hypothesis suggests that non-native species could be less susceptible to herbivory than native species due to a lack of specialist herbivores (Keane and Crawley 2002; Liu and Stiling 2006). Thus, if the early season is a critical period of susceptibility to herbivory (Feeny 1970; Barbehenn et al. 2013), this may also be the critical time for invaders to benefit from herbivore release. This suggests a mechanism by which non-native species could break the risk–benefit trade-off of early phenology experienced by native species, thereby giving them a special advantage from early-season phenology.
Climate change may additionally promote early-season phenologies and provide a mechanism whereby non-native species with early-season phenologies are especially successful. Recent increases in spring temperatures in the temperate biome (at least partially associated with increases in greenhouse gases; see Trenberth and Josey 2007) have been studied extensively. Most studies find that spring temperatures have increased as much or more than temperatures in other seasons (Cohen et al. 2012), meaning this is a season of especially high non-stationarity in climate (Fig. 1). Predictions for precipitation-limited systems are more variable but include options for increased total and increased variability in early-season rainfall (Trenberth and Josey 2007; Knutti and Sedlacek 2013). Such high variability may make it an optimum period for invasion of other species for several reasons. First, such high non-stationarity should mean native species are being pushed far away from the previous long-term climate means to which their phenology should be adapted. Next, if non-native species have higher phenological plasticity (e.g. Wainwright and Cleland 2013), they may more closely track shifting climate than native species. Early flowering is also often correlated with plant traits related to rapid growth. Non-native species exploiting an early-season vacant niche may thus grow rapidly and take up much of their needed resources to complete reproduction before competition with native species effectively sets in (Fig. 3).
Figure 3.

Predictions for how climate change may promote invasions vary across the growing season, across systems with differing dominant climate regimes, and by how climate shifts (red arrows refer to temperature increases, while blue arrows refer to precipitation increases or decreases). Here we consider three major systems and how dominant climate drivers are projected to shift with climate change, based on recent models (Knutti and Sedlacek 2013). Because models of precipitation shifts are often divergent (Knutti and Sedlacek 2013), for systems with precipitation control we consider increases or decreases in precipitation. In all systems an increase in the dominant climate factor that controls the start of the season may increase invasions by species that can track this shift closely (invader plasticity). Because we suggest that successful invasions are rare in times of very high resource competition and extremely high climatic stress and disturbance, we do not predict invasions during periods when competition is already high (mid-season of many systems) or when climate change increases drought stress (declines in precipitation in semi-arid systems or during mid-season drought in grasslands). When climate change pushes systems far beyond their historic climate regimes, however, native species may be pushed well away from their optimal climate, and we may see an increase in invasions. See the main text for further details, including background assumptions leading to predictions.
Predictions: mid-season
In most systems, the mid-season represents the period when the greatest number of species flower (e.g. Fitter et al. 1995; Aldridge et al. 2011), and work to date using community datasets suggests that this is true for both native and non-native species (Wolkovich et al. 2013). This is perhaps not surprising as in most systems the mid-season represents a period of relatively low stress due to the physical environment and high abundance of pollinators. This high abundance of species in flower, however, means it is also the period of high competition for resources (Fig. 2) and thus we generally predict few invasions driven by phenology mid-season (Fig. 3).
In some systems with precipitation control, however, the mid-season often has a reduced period of plant competition associated with a mid-season drought (Fig. 2). Temperate grasslands, for example, are generally characterized by mid-season droughts when the highest temperatures coincide with low precipitation, drying soils and fewer species that initiate flowering during this period (e.g. Craine et al. 2012b). This reduced competition could result in an opportunity for invasion. However, because it also generally represents a period of extremely high drought stress, we do not predict invasions mid-season generally. Increases in mid-season precipitation—especially those outside historical ranges—may, however, provide a novel vacant niche. Many early-flowering species appear to end flowering well before drought onset and, depending on the phenological cues they use for flowering onset and end, may thus not be able to exploit greater mid-season precipitation because they have adapted primarily to avoid the mid-season drought (Craine et al. 2012b, 2013). Native species flowering during the mid-season may have trade-offs between drought tolerance and competitive abilities for other resources (Craine et al. 2013), which may make them less successful at exploiting increased mid-season precipitation. We speculate that, under this scenario, increased mid-season precipitation (that is not offset by higher temperatures) may promote mid-season invaders with climate change.
Predictions: late season
Climatically, in many systems the end of the growing season mirrors the beginning (Fig. 2), but plant phenology differs strongly. For example, while in both temperate mesic and grassland systems plant leafing and flowering (Fig. 2) generally tracks closely the rise in spring temperatures around 5 °C (Larcher 2003), community flowering curves generally end at least one month before temperatures return to 5 °C. The reasons for this may be simply related to physiological constraints: species must flower well before the end of the season in order to have enough time to fruit and set seed. A balance between risk and investment may also be important: as mean temperatures drop, temperature variability climbs (Fig. 2), just as low spring temperatures are also correlated with higher temperature variability. In contrast to the spring, however, later in the season almost all species have made a heavy investment in growth and reproductive tissues and loss to frost may pose an even greater threat than frosts in the spring; thus plants may be especially conservative, even those with a ‘late and fast’ phenological-growth strategy. This risk/investment balance may explain why many species often have plastic spring phenologies based on temperatures that are flexible across habitats, but static fall phenologies based mainly on photoperiod cues (Howe et al. 2003). The result is that, in many systems, the end of the growing season is a period of generally climatically high stress and disturbance, but low competition. Without climate change we expect that most native species have adapted towards the optimal time to senesce based on their risk–investment strategy and there may be little opportunity for invasion.
With climate change, however, we expect pronounced opportunities for invasion late in the growing season when climate change extends the end of the season (Fig. 3), as native species may be constrained by their evolutionary history, and cues associated with the end of the growing season (i.e. photoperiod). Thus, non-native species with greater phenological niche breadth (either via a generally static longer growth or flowering period, or via greater flexibility to extend their phenology resulting in greater niche breadth) may be able to exploit this late-season vacant niche. This should apply to all systems where the late season is a period of relatively high stress and disturbance but low competition—up to a point. In many systems where both temperature and precipitation shape growing season dynamics, the late season can have high drought stress; we do not expect significant invasion during this window because non-native species may not be as adapted to high drought stress compared with the native species in these systems (Alpert et al. 2000).
Role of Climate Change in Phenologically Mediated Invasions: Evidence to Date
Results: early season
Work to date supports evidence for early-season invasions, which are linked to climate change in several temperate mesic systems (Wolkovich et al. 2013) and linked to seasonal priority effects in semi-arid systems (Dickson et al. 2012; Wainwright et al. 2012). Additionally, research on one non-native understorey shrub species (Xu et al. 2007) suggests a role for seasonal priority effects in temperate forest systems. Results in temperate grasslands, however, do not show a strong link between early phenology and invasion (Wolkovich et al. 2013). Across temperature-controlled systems, however, early-season species, whether native or non-native, also tend to be the most sensitive to temperature (Cook et al. 2012; Wolkovich et al. 2012a). Relatedly, multiple studies using various methods now show that in many mesic temperate biomes, invaders are highly sensitive to temperature—tending to advance their phenology significantly more than native species (Willis et al. 2010; Wolkovich et al. 2013), though, again, this does not appear to be the case in temperate grasslands (Wolkovich et al. 2013). Moving forward, accurate predictions of phenologically mediated invasions will require teasing out exact mechanisms. Thus, future research with climate change in temperate biomes will need to quantify how much invasion success occurs because of flexibility in phenology (i.e. the trait of the invader leads to success) versus via open niche opportunities present in the early season due to non-stationarity with climate change (i.e. the system is open to invasion in the early season), or a mix of the two scenarios.
Results: mid-season
Several North American studies have documented declines in native species alongside shifts with climate change in mid-season drought periods, generally showing that the drought period may be extending or becoming more pronounced (Aldridge et al. 2011), and multiple authors have postulated that this period will result in a vacant niche for invaders (Sherry et al. 2007; Aldridge et al. 2011). To date, however, no studies (of which we are aware) have shown invaders occupying these mid-season drought periods, while in contrast two studies of phenology at Konza Prairie LTER have found a decline in non-native flowering coinciding with the mid-season drought (Craine et al. 2012b; Wolkovich et al. 2013). Further, work on previous extreme droughts has generally documented a shift in native species composition, but not invasions (Weaver and Albertson 1936). These patterns are based on findings in North American prairie systems; however, far more work is needed additionally to understand if this period is occupied by invaders in other systems or is possibly too stressful. This represents an area where predictions are difficult for several reasons: (i) understanding and modelling how species respond to moisture has proven far more difficult than modelling temperature responses (Crimmins et al. 2011; Wolkovich et al. 2013), (ii) work to date suggests that phenological responses to drought are highly variable between different species (Jentsch et al. 2009; Prieto et al. 2009), and (iii) projections of how precipitation and droughts will shift in the future are some of the most uncertain of all climate change forecasts (Knutti and Sedlacek 2013).
Results: late season
Recent studies of plant invasions, especially in eastern North American forests, suggest that non-native and invasive species may successfully exploit a late-season vacant niche via greater niche breadth in temperate biomes—which may be linked to climate change. A pervasive non-native understorey species in the Ohio River Valley, Lonicera maacki, stays green later than any native understorey species (Becker et al. 2013). Similarly, the invasive tree Acer platanoides also stays green later than one studied North American congener (Paquette et al. 2012). Further, recent work suggests that these non-native species may not play by the same risk–investment rules as native species in the fall. A study of several dozen North American non-native species from the understorey confirms that these species consistently senesce in the fall later than native species (Fridley 2012). Fridley (2012) also found that many of these species remain green until the first major frost and thus lose their leaves to frost versus plant-induced senescence. This should be a major cost to the plant—as most species resorb nutrients well before the first frost (Lambers et al. 2008). Further work showed that the longer leaf lifespan of the non-native species enabled a greater time-integrated nutrient-use efficiency (Heberling and Fridley 2013), and, coupled with high rates of nutrient uptake the following spring, provides a mechanism by which the unique phenology of non-native species, compared with the native community, could promote invasion.
If these non-native species in eastern North America do gain a large benefit from occupying an open late-season temporal niche, even while losing tissue to frost, then climate change could further increase their success. In many habitats, fall temperatures are rising more quickly than even spring temperatures (Cohen et al. 2012), and are expected to continue to rise—further extending the open niche space at the end of the season, which already appears temporally much greater than the early season (Fig. 2). Thus, the late season appears to be a period of very low plant competition and is often also when microbial communities turn over (Bardgett et al. 2005), suggesting that species which can remain active until the end of the season may have access to a large available resource pool.
Across systems, however, autumnal shifts in climate and phenology are still relatively unstudied compared with spring. More work is needed to understand where, and by how much, mean fall temperatures are shifting in comparison to other seasonal temperature shifts and how species are adjusting their late-season phenological events. In particular, there is very little work from the late season in grasslands, where the combination of shifts in mid-season droughts alongside shifted fall temperatures may create novel climates and, possibly, novel opportunities for invasion. Alternatively, systems with mid-season droughts may have reduced opportunities for invasion if such droughts—over the long term—have favoured more variable phenological strategies (e.g. species flower very late to avoid stress of mid-season drought; see Craine et al. 2012a) compared with simple temperature-controlled systems, which have been noted to have far greater late-season empty phenological space compared with grasslands (Craine et al. 2012a).
Major Questions
Recent research connecting phenology and invasions has clearly provided support for the idea that invaders may benefit from phenological vacant niches during periods of relatively high stress and disturbance but low competition. Further work is needed, however, to mechanistically link phenology to plant invasions and to build towards more accurate and specific predictions of how climate change may promote invaders in the future. Below we outline what we consider the major questions impeding robust predictions in this area.
How do longer-term properties of a system's climate affect phenological invasions?
Climate change represents a long-term climate trend overlaid onto already complex climates of most ecosystems, including a climate's mean, cyclical variation (e.g. seasons and multi-year cycles often driven by large-scale oscillations) and extremes. Thus, a coherent understanding of how species and communities will shift with climate change requires consideration of more than shifts in the timing and magnitude of temperature and precipitation.
Furthermore, such a coherent picture of how species respond to shifts in temperatures and precipitation will include a focus not just on mean or aggregated climate metrics, but also on extreme events. An example of the importance of this comes from attempts to understand the correlation between phenology and performance with climate change. Several studies show that species that tend to advance with warming also tend to increase in abundance or performance (Cleland et al. 2012), including invasive species (Willis et al. 2010; Chuine et al. 2012); yet other work shows that the early-season (native) species most sensitive to climate are those that suffer the greatest performance losses with climate change (Inouye 2008). Such conflicting results can be better understood when considering the role of extreme events. In the latter study, a shift in earlier springs that did not coincide with a shift in frost dates produced the performance declines (Inouye 2008). Accurate predictions of which systems may have viable early-season open niches for invasions will require examining how much temperatures have shifted on average while also considering important spring climate events related to plant stress and disturbance, such as frost. To date, increases in frost risk with spring warming have been documented in parts of North America (Gu et al. 2008; Inouye 2008; Augspurger 2012), but not much of North America (Easterling et al. 2000), nor in Europe (Menzel et al. 2003; Scheifinger et al. 2003) or China (Dai et al. 2013), where shifts in freeze and frost days have occurred in step with the climate shifts driving earlier spring onset (Dai et al. 2013). Currently, there is little known about how early-season non-native versus native species cope with frost and frost risks. Additionally, most work on phenology has focused on temperature and related events such as frost, with little work on precipitation events.
Studies of precipitation-controlled systems must also deal with large-scale, longer-term cycles in precipitation that often dominate in such systems (e.g. El Niño in many semi-arid systems in western North America). Such longer-term cycles may be a critical consideration because native species may be adapted to these cycles. Relevant studies of population dynamics will, therefore, need to work across the relevant climate oscillation timescales and predictions will need to consider whether the oscillation may shift with climate change, as projected (IPCC 2007). Such oscillations may also be directly important to non-native species as they may dictate invasion lags and jumps (Salo 2005).
Do invaders share the same strategies and trade-offs with phenology as native species?
Our predictions here are based on the plant traits literature that suggests several major options for species' phenological strategies, and thus how phenological traits may trade off with other traits. Thus, a critical assumption of our hypotheses is that these strategies are consistent across non-native and native species. It is possible, however, that non-native species may exhibit novel strategies, similar strategies that involve additional traits, or that they may exhibit trade-offs of different magnitudes.
Understanding these trade-offs more fully has additional predictive benefits. In particular, it would advance efforts to integrate phenology within a more holistic trait framework and enable scaling to ecosystem predictions more easily. If certain phenological strategies are more common in invaders, it would suggest a suite of plant traits that would change in concert with plant invasions and climate change. Recent work shows that temperate non-native species are more phenologically responsive to temperature than native species at some sites (Wolkovich et al. 2013) and that species that advance with warming also tend to increase in abundance and performance (Cleland et al. 2012). Together these findings suggest that future temperate ecosystems may be dominated by more phenologically plastic species. If such flexibility correlates with other traits (e.g. lower leaf lifespan or lower nutrient content in leaves), we would then predict cascading shifts in ecosystem properties such as decomposition and nutrient cycling.
When and how does high phenological flexibility yield a competitive advantage?
One commonality across climatically diverse systems is evidence linking non-native species with high phenological flexibility. Across systems where the start of the growing season is determined by temperature (Wolkovich et al. 2013) or by precipitation (Wainwright et al. 2012), species that track the start of the season most closely are highly successful invaders. Understanding the benefits and trade-offs of high phenological tracking of environmental variables would address a fundamental question in invasion biology: if high plasticity yields a competitive advantage, why do species differ in their plasticity? One hypothesis is that species that track climate change well over the timescales for which we have data (or focus our analyses) may suffer major population losses during years of extreme climate. Alternatively, the current non-stationarity of climate may have shifted the playing field; this may mean that species that evolved in more variable environments are now often successful invaders. These hypotheses have not been tested to our knowledge. However, adaptations of bet-hedging models (e.g. Donaldson-Matasci et al. 2012), combined with currently available climate data, should allow basic vetting.
Studies examining the plasticity of phenology in non-native species may also want to consider how much evolutionary change following introduction has contributed to this plasticity (e.g. Sultan et al. 2013), and how quickly species can genotypically adjust to more static phenological cues post-invasion. Many of the studies mentioned here examine phenological shifts that are attributable to phenotypic plasticity (e.g. they are of marked perennial individuals or come from woody species known to shift leafing and flowering plastically between years with different climates), but recent studies have documented rapid evolutionary shifts in invaders, especially in phenology (e.g. Colautti and Barrett 2011; Konarzewski et al. 2012; Novy et al. 2013). Understanding how much phenological flexibility is driven by underlying plastic versus genetic shifts is important to projections of which species may become invaders—if much change is genotypic, it suggests then that predictions may be more difficult and will require knowing a priori how quickly phenology can evolve under new selection regimes. More studies examining invaders in their native and introduced ranges (e.g. Godoy et al. 2009; Matesanz et al. 2012) would begin to build data on how often phenologies shift with invasions and how similar or distinct invader phenologies are in their native versus introduced ranges (e.g. Wolkovich et al. 2013).
How does evolutionary history influence phenological invasions?
Research from molecular ecology has consistently shown a strong genetic basis for leafing and flowering times within species (Howe et al. 2003; Fournier-Level et al. 2011); thus it may be expected that related species would share similarities in their phenologies, and possibly their phenological responses to climate. Indeed, a growing number of studies have documented significant evolutionary structure in the distribution of flowering times and sensitivities to climate change across species (Davis et al. 2010), including invaders (Willis et al. 2010; Wolkovich et al. 2013). A recent, more comprehensive analysis across >20 sites, from temperate to tropical Northern Hemisphere zones, shows phylogenetic structure in flowering and leafing for almost all communities studied (Davies et al. 2013), such that more closely related species also tend to have more similar phenologies. This structure means studies of phenology including multiple species may want to consider how much variation in phenology and related phenological traits is explained by the evolutionary distances separating species, versus other factors.
Considering phylogenetic structure is especially important in studies attempting to link phenology to invasion success and any studies looking for correlations between phenology and other traits, because species cannot be treated as statistically independent. For example, studies finding multiple non-native species with distinct phenologies compared with the native community will need to test how much this finding is driven by the phylogenetic affinity of non-native species compared with species in the native community. If non-native species are only distantly related to the native species pool, we might expect them to differ in their phenologies, irrespective of the actual traits explaining their invasion success. In addition, when looking at correlations between phenological traits and invasion, apparent trade-offs might simply reflect phylogenetic affinities if non-native species are evolutionarily distant for the native species pool. Our mechanistic explanation for invader success might, thus, be quite different depending on whether invaders are filtered on phylogenetically conserved traits versus a scenario in which they diverge from the native community following introduction. Phylogenetic methods aid in distinguishing between these two scenarios.
Conclusions
With future climate change, invasive species are predicted to increase both in abundance and in spatial distribution (IPCC Core Writing Team et al. 2007; Bradley et al. 2010). We have outlined here a more focused framework for examining how phenology may affect plant invasions. This framework considers phenology as one factor by which plants attempt to optimally balance acquisition, allocation and loss in an environment where most systems' climates are now highly non-stationary. As increasing research builds to test and advance this framework, resource managers will in turn need to evaluate how they may use phenology in their decision making. If many species appear to gain a foothold or spread in introduced communities via phenological differences compared with the native community, it may suggest novel management practices. These could include optimally timing treatments for when only non-native species are active, or using phenological differences to identify species that may have a high potential to be invaders with climate change. Such applications will, of course, be bolstered by additional studies of phenology. In particular, further work is needed to understand how phenology correlates with and is constrained by other traits, whether this varies between different climate regimes, functional groups and clades, and whether non-native species appear to face the same constraints to their phenologies as native species.
Sources of Funding
E.M.W. was supported by the Natural Sciences and Engineering Research Council of Canada's Collaborative Research and Training Experience Program (NSERC CREATE) in biodiversity research through the Biodiversity Research Centre at the University of British Columbia (Vancouver, BC, Canada).
Contribution by the Authors
E.M.W. and E.E.C. contributed ideas and concepts and edited the manuscript. In addition E.M.W. conceived of and wrote the manuscript and designed and created all figures.
Conflicts of Interest Statement
None declared.
Acknowledgements
Comments from J. Craine, J. Dukes, B. Cook, T. J. Davies and two anonymous reviewers improved this manuscript.
Literature Cited
- Aldridge G, Inouye DW, Forrest JRK, Barr WA, Miller-Rushing AJ. Emergence of a mid-season period of low floral resources in a montane meadow ecosystem associated with climate change. Journal of Ecology. 2011;99:905–913. [Google Scholar]
- Al-Mufti MM, Sydes CL, Furness SB, Grime JP, Band SR. Quantitative analysis of shoot phenology and dominance in herbaceous vegetation. Journal of Ecology. 1977;65:759–791. [Google Scholar]
- Alpert P, Bone E, Holzapfel C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspectives in Plant Ecology, Evolution and Systematics. 2000;3:52–66. [Google Scholar]
- Augspurger CK. Spring 2007 warmth and frost: phenology, damage and refoliation in a temperate deciduous forest. Functional Ecology. 2009;23:1031–1039. [Google Scholar]
- Augspurger CK. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage risk is increasing. Ecology. 2012;94:41–50. doi: 10.1890/12-0200.1. [DOI] [PubMed] [Google Scholar]
- Barbehenn RV, Niewiadomski J, Pecci C, Salminen JP. Physiological benefits of feeding in the spring by Lymantria dispar caterpillars on red oak and sugar maple leaves: nutrition versus oxidative stress. Chemoecology. 2013;23:59–70. [Google Scholar]
- Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK. A temporal approach to linking aboveground and belowground ecology. Trends in Ecology and Evolution. 2005;20:634–641. doi: 10.1016/j.tree.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Becker RH, Zmijewski KA, Crail T. Seeing the forest for the invasives: mapping buckthorn in the Oak Openings. Biological Invasions. 2013;15:315–326. [Google Scholar]
- Betancourt JL. Reflections on the relevance of history in a nonstationary world. In: Wiens JA, Hayward GD, Safford HD, Giffen C, editors. Historical environmental variation in conservation and natural resource management. West Sussex, UK: Wiley-Blackwell; 2012. pp. 307–318. [Google Scholar]
- Bolmgren K, Cowan PD. Time–size tradeoffs: a phylogenetic comparative study of flowering time, plant height and seed mass in a north-temperate flora. Oikos. 2008;117:424–429. [Google Scholar]
- Bradley BA, Blumenthal DM, Wilcove DS, Ziska LH. Predicting plant invasions in an era of global change. Trends in Ecology and Evolution. 2010;25:310–318. doi: 10.1016/j.tree.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Brody AK. Effects of pollinators, herbivores, and seed predators on flowering phenology. Ecology. 1997;78:1624–1631. [Google Scholar]
- Campanella MV, Bertiller MB. Plant phenology, leaf traits and leaf litterfall of contrasting life forms in the arid Patagonian Monte, Argentina. Journal of Vegetation Science. 2008;19:75–85. [Google Scholar]
- Caplat P, Anand M, Bauch C. Modelling invasibility in endogenously oscillating tree populations: timing of invasion matters. Biological Invasions. 2010;12:219–231. [Google Scholar]
- Catorci A, Cesaretti S, Gatti R, Tardella FM. Trait-related flowering patterns in submediterranean mountain meadows. Plant Ecology. 2012;213:1315–1328. [Google Scholar]
- Chesson P. Environmental variation and the coexistence of species. In: Diamond JT, Case TJ, editors. Community ecology. New York: Harper & Row; 1986. pp. 240–256. [Google Scholar]
- Chesson P, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. The American Naturalist. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- Chesson PL, Warner RR. Environmental variability promotes coexistence in lottery competitive-systems. The American Naturalist. 1981;117:923–943. [Google Scholar]
- Chuine I, Morin X, Sonie L, Collin C, Fabreguettes J, Degueldre D, Salager JL, Roy J. Climate change might increase the invasion potential of the alien C4 grass Setaria parviflora (Poaceae) in the Mediterranean Basin. Diversity and Distributions. 2012;18:661–672. [Google Scholar]
- Cleland EE, Allen JM, Crimmins TM, Dunne JA, Pau S, Travers SE, Zavaleta ES, Wolkovich EM. Phenological tracking enables positive species responses to climate change. Ecology. 2012;93:1765–1771. doi: 10.1890/11-1912.1. [DOI] [PubMed] [Google Scholar]
- Cohen JL, Furtado JC, Barlow M, Alexeev VA, Cherry JE. Asymmetric seasonal temperature trends. Geophysical Research Letters. 2012;39 L04705. doi:10.1029/2011GL050582. [Google Scholar]
- Colautti RI, Barrett SCH. Population divergence along lines of genetic variance and covariance in the invasive plant Lythrum salicaria in Eastern North America. Evolution. 2011;65:2514–2529. doi: 10.1111/j.1558-5646.2011.01313.x. [DOI] [PubMed] [Google Scholar]
- Colautti RI, Richardson DM. Subjectivity and flexibility in invasion terminology: too much of a good thing? Biological Invasions. 2009;11:1225–1229. [Google Scholar]
- Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. Is invasion success explained by the enemy release hypothesis? Ecology Letters. 2004;7:721–733. [Google Scholar]
- Cook BI, Wolkovich EM, Davies TJ, Ault TR, Betancourt JL, Allen JM, Bolmgren K, Cleland EE, Crimmins TM, Kraft NJB, Lancaster LT, Mazer SJ, McCabe GJ, McGill BJ, Parmesan C, Pau S, Regetz J, Salamin N, Schwartz MD, Travers SE. Sensitivity of spring phenology to warming across temporal and spatial climate gradients in two independent databases. Ecosystems. 2012;15:1283–1294. [Google Scholar]
- Craine JM. Resource strategies of wild plants. Princeton, NJ: Princeton University Press; 2009. [Google Scholar]
- Craine JM, Wolkovich EM, Towne EG. The roles of shifting and filtering in generating community-level flowering phenology. Ecography. 2012a;35:1033–1038. [Google Scholar]
- Craine JM, Wolkovich EM, Towne EG, Kembel SW. Flowering phenology as a functional trait in a tallgrass prairie. New Phytologist. 2012b;193:673–682. doi: 10.1111/j.1469-8137.2011.03953.x. [DOI] [PubMed] [Google Scholar]
- Craine JM, Ocheltree TW, Nippert JB, Towne EG, Skibbe AM, Kembel SW, Fargione JE. Global diversity of drought tolerance and grassland climate-change resilience. Nature Climate Change. 2013;3:63–67. [Google Scholar]
- Crimmins TM, Crimmins MA, Bertelsen CD. Onset of summer flowering in a ‘Sky Island’ is driven by monsoon moisture. New Phytologist. 2011;191:468–479. doi: 10.1111/j.1469-8137.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- Dai J, Wang H, Ge Q. The decreasing spring frost risks during the flowering period for woody plants in temperate area of eastern China over past 50 years. Journal of Geographical Sciences. 2013;23:641–652. [Google Scholar]
- Davidson AM, Jennions M, Nicotra AB. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecology Letters. 2011;14:419–431. doi: 10.1111/j.1461-0248.2011.01596.x. [DOI] [PubMed] [Google Scholar]
- Davies TJ, Wolkovich EM, Kraft NJB, Salamin N, Allen JM, Ault TR, Betancourt JL, Bolmgren K, Cleland EE, Cook BI, Crimmins TM, Mazer SJ, McCabe GJ, Pau S, Regetz J, Schwartz MD, Travers SE. Phylogenetic conservatism in plant phenology. Journal of Ecology. 2013;101:1520–1530. [Google Scholar]
- Davis CC, Willis CG, Primack RB, Miller-Rushing AJ. The importance of phylogeny to the study of phenological response to global climate change. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:3201–3213. doi: 10.1098/rstb.2010.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: a general theory of invasibility. Journal of Ecology. 2000;88:528–534. [Google Scholar]
- Dickson TL, Hopwood JL, Wilsey BJ. Do priority effects benefit invasive plants more than native plants? An experiment with six grassland species. Biological Invasions. 2012;14:2617–2624. [Google Scholar]
- Donaldson-Matasci MC, Bergstrom CT, Lachmann M. When unreliable cues are good enough. The American Naturalist. 2012;182:313–327. doi: 10.1086/671161. [DOI] [PubMed] [Google Scholar]
- Dorji T, Totland O, Moe SR, Hopping KA, Pan J, Klein JA. Plant functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Global Change Biology. 2013;19:459–472. doi: 10.1111/gcb.12059. [DOI] [PubMed] [Google Scholar]
- Drake JM, Drury KLS, Lodge DM, Blukacz A, Yan ND, Dwyer G. Demographic stochasticity, environmental variability, and windows of invasion risk for Bythotrephes longimanus in North America. Biological Invasions. 2006;8:843–861. [Google Scholar]
- Du G, Qi W. Trade-offs between flowering time, plant height, and seed size within and across 11 communities of a QingHai-Tibetan flora. Plant Ecology. 2010;209:321–333. [Google Scholar]
- Dukes JS. Responses of invasive species to a changing climate and atmosphere. In: Richardson DM, editor. Fifty years of invasion ecology: the legacy of Charles Elton. Oxford, UK: Blackwell Publishing; 2011. pp. 345–357. [Google Scholar]
- Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. Climate extremes: observations, modeling, and impacts. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- Elton CS. The ecology of invasions by animals and plants. Chicago: University of Chicago Press; 1958. [Google Scholar]
- Feeny P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology. 1970;51:565–581. [Google Scholar]
- Fitter AH, Fitter RSR, Harris ITB, Williamson MH. Relationships between first flowering date and temperature in the flora of a locality in central England. Functional Ecology. 1995;16:55–60. [Google Scholar]
- Fournier-Level A, Korte A, Cooper MD, Nordborg M, Schmitt J, Wilczek AM. A map of local adaptation in Arabidopsis thaliana. Science. 2011;333:86–89. doi: 10.1126/science.1209271. [DOI] [PubMed] [Google Scholar]
- Fridley JD. Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature. 2012;485:359–362. doi: 10.1038/nature11056. [DOI] [PubMed] [Google Scholar]
- Funk JL. Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology. 2008;96:1162–1173. [Google Scholar]
- Gelbard J, Belnap J. Roads as conduits for exotic plant invasions in a semiarid landscape. Conservation Biology. 2003;17:420–432. [Google Scholar]
- Godoy O, Castro-Diez P, Valladares F, Costa-Tenorio M. Different flowering phenology of alien invasive species in Spain: evidence for the use of an empty temporal niche? Plant Biology. 2009;11:803–811. doi: 10.1111/j.1438-8677.2008.00185.x. [DOI] [PubMed] [Google Scholar]
- Golluscio RA, Sala OE. Plant functional types and ecological strategies in Patagonian forbs. Journal of Vegetation Science. 1993;4:839–846. [Google Scholar]
- Gotelli NJ, Graves GR. The temporal niche. In: Gotelli NJ, Graves GR, editors. Null models in ecology. Washington, DC: Smithsonian Institution; 1996. pp. 95–111. [Google Scholar]
- Grime JP. Evidence for existence of 3 primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Gu L, Hanson PJ, Mac Post W, Kaiser DP, Yang B, Nemani R, Pallardy SG, Meyers T. The 2007 eastern US spring freezes: increased cold damage in a warming world? Bioscience. 2008;58:253–262. [Google Scholar]
- Gulmon S, Chiarello N, Mooney HA, Chu C. Penology and resource use in three co-occurring grassland annuals. Oecologia. 1983;58:33–42. doi: 10.1007/BF00384539. [DOI] [PubMed] [Google Scholar]
- Hadar L, Noy-Meir I, Perevolotsky A. The effect of shrub clearing and grazing on the composition of a Mediterranean plant community: functional groups versus species. Journal of Vegetation Science. 1999;10:673–682. [Google Scholar]
- Heberling JM, Fridley JD. Resource-use strategies of native and invasive plants in Eastern North American forests. New Phytologist. 2013;200:523–533. doi: 10.1111/nph.12388. doi:10.1111/nph.12388. [DOI] [PubMed] [Google Scholar]
- Hierro JL, Eren O, Khetsuriani L, Diaconu A, Torok K, Montesinos D, Andonian K, Kikodze D, Janoian L, Villarreal D, Estanga-Mollica ME, Callaway RM. Germination responses of an invasive species in native and non-native ranges. Oikos. 2009;118:529–538. [Google Scholar]
- Howe GT, Aitken SN, Neale DB, Jermstad KD, Wheeler NC, Chen THH. From genotype to phenotype: unraveling the complexities of cold adaptation in forest trees. Canadian Journal of Botany. 2003;81:1247–1266. [Google Scholar]
- Inouye DW. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology. 2008;89:353–362. doi: 10.1890/06-2128.1. [DOI] [PubMed] [Google Scholar]
- IPCC. New York, NY: Cambridge University Press; 2007. Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. [Google Scholar]
- Pachauri RK, Reisinger A IPCC Core Writing Team. Geneva, Switzerland: IPCC; 2007. IPCC, 2007: Climate change 2007: synthesis report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. [Google Scholar]
- Jentsch A, Kreyling J, Boettcher-Treschkow J, Beierkuhnlein C. Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Global Change Biology. 2009;15:837–849. [Google Scholar]
- Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology and Evolution. 2002;17:164–170. [Google Scholar]
- Knutti R, Sedlacek J. Robustness and uncertainties in the new CMIP5 climate model projections. Nature Climate Change. 2013;3:369–373. [Google Scholar]
- Konarzewski TK, Murray BR, Godfree RC. Rapid development of adaptive, climate-driven clinal variation in seed mass in the invasive annual forb Echium plantagineum L. PloS ONE. 2012;7:e49000. doi: 10.1371/journal.pone.0049000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner C. A re-assessment of high elevation treeline positions and their explanation. Oecologia. 1998;115:445–459. doi: 10.1007/s004420050540. [DOI] [PubMed] [Google Scholar]
- Kuester EC, Durka W, Kuehn I, Klotz S. Differences in the trait compositions of non-indigenous and native plants across Germany. Biological Invasions. 2010;12:2001–2012. [Google Scholar]
- Lambers H, Chapin FS, III, Pons TL. Life cycles: environmental influences and adaptations. In: Lambers H, Chapin FS III, Pons TL, editors. Plant physiological ecology. 2nd edn. New York: Springer; 2008. pp. 375–402. [Google Scholar]
- Larcher W. Physiological plant ecology: ecophysiology and stress physiology of functional groups. 4th edn. Heidelberg: Springer; 2003. Plants under stress. Chapter 6; pp. 345–450. [Google Scholar]
- Laughlin DC, Leppert JJ, Moore MM, Sieg CH. A multi-trait test of the leaf–height–seed plant strategy scheme with 133 species from a pine forest flora. Functional Ecology. 2010;24:493–501. [Google Scholar]
- Lechowicz MJ. Why do temperate deciduous trees leaf out at different times—adaptation and ecology of forest communities. The American Naturalist. 1984;124:821–842. [Google Scholar]
- Leishman MR, Westoby M. Classifying plants into groups on the basis of associations of individual traits evidence from Australian semiarid woodlands. Journal of Ecology. 1992;80:417–424. [Google Scholar]
- Levine JM, Vila M, D'Antonio CM, Dukes JS, Grigulis K, Lavorel S. Mechanisms underlying the impacts of exotic plant invasions. Proceedings of the Royal Society of London Series B Biological Sciences. 2003;270:775–781. doi: 10.1098/rspb.2003.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkosalo T, Carter T, Hakkinen R, Hari P. Predicting spring phenology and frost damage risk of Betula spp. under climatic warming: a comparison of two models. Tree Physiology. 2000;20:1175–1182. doi: 10.1093/treephys/20.17.1175. [DOI] [PubMed] [Google Scholar]
- Liu H, Stiling P. Testing the enemy release hypothesis: a review and meta-analysis. Biological Invasions. 2006;8:1535–1545. [Google Scholar]
- Louault F, Pillar V, Aufrere J, Garnier E, Soussana J. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. Journal of Vegetation Science. 2005;16:151–160. [Google Scholar]
- MacArthur RH. Species packing and competitive equilibria for many species. Theoretical Population Biology. 1970;1:1–11. doi: 10.1016/0040-5809(70)90039-0. [DOI] [PubMed] [Google Scholar]
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications. 2000;10:689–710. [Google Scholar]
- Matesanz S, Horgan-Kobelski T, Sultan SE. Phenotypic plasticity and population differentiation in an ongoing species invasion. PLoS ONE. 2012;7:e44955. doi: 10.1371/journal.pone.0044955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazer SJ. Ecological, taxonomic, and life-history correlates of seed mass among Indiana dune angiosperms. Ecological Monographs. 1989;59:153–175. [Google Scholar]
- McEwan RW, Birchfield MK, Schoergendorfer A, Arthur MA. Leaf phenology and freeze tolerance of the invasive shrub Amur honeysuckle and potential native competitors. Journal of the Torrey Botanical Society. 2009;136:212–220. [Google Scholar]
- Menzel A, Jakobi G, Ahas R, Scheifinger H, Estrella N. Variations of the climatological growing season (1951–2000) in Germany compared with other countries. International Journal of Climatology. 2003;23:793–812. [Google Scholar]
- Miller-Rushing AJ, Primack RB. Global warming and flowering times in Thoreau's Concord: a community perspective. Ecology. 2008;89:332–341. doi: 10.1890/07-0068.1. [DOI] [PubMed] [Google Scholar]
- Mosquin T. Competition for pollinators as a stimulus for evolution of flowering time. Oikos. 1971;22:398–402. [Google Scholar]
- Muller RN. Phenology, growth and ecosystem dynamics of Erythronium americanum in northern hardwood forest. Ecological Monographs. 1978;48:1–20. [Google Scholar]
- Navarro T, El Oualidi J, Taleb MS, Pascual V, Cabezudo B, Milla R. Leaf patterns, leaf size and ecologically related traits in high Mediterranean mountain on the Moroccan High Atlas. Plant Ecology. 2010;210:275–290. [Google Scholar]
- Novy A, Flory SL, Hartman JM. Evidence for rapid evolution of phenology in an invasive grass. Journal of Evolutionary Biology. 2013;26:443–450. doi: 10.1111/jeb.12047. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Lack AJ. Flowering phenology—an example of relaxation of natural-selection. Trends in Ecology and Evolution. 1992;7:274–276. doi: 10.1016/0169-5347(92)90175-B. [DOI] [PubMed] [Google Scholar]
- Paquette A, Fontaine B, Berninger F, Dubois K, Lechowicz MJ, Messier C, Posada JM, Valladares F, Brisson J. Norway maple displays greater seasonal growth and phenotypic plasticity to light than native sugar maple. Tree Physiology. 2012;32:1339–1347. doi: 10.1093/treephys/tps092. [DOI] [PubMed] [Google Scholar]
- Pearson DE, Ortega YK, Sears SJ. Darwin's naturalization hypothesis up-close: intermountain grassland invaders differ morphologically and phenologically from native community dominants. Biological Invasions. 2012;14:901–913. [Google Scholar]
- Prieto P, Penuelas J, Niinemets U, Ogaya R, Schmidt IK, Beier C, Tietema A, Sowerby A, Emmett BA, Lang EK, Kroel-Dulay G, Lhotsky B, Cesaraccio C, Pellizzaro G, de Dato G, Sirca C, Estiarte M. Changes in the onset of spring growth in shrubland species in response to experimental warming along a north-south gradient in Europe. Global Ecology and Biogeography. 2009;18:473–484. [Google Scholar]
- Rathcke B. Flowering phenologies in a shrub community—competition and constraints. Journal of Ecology. 1988;76:975–994. [Google Scholar]
- Rejmánek M. Invasibility of plant communities. In: Drake JA, Mooney HA, di Castri F, Groves R, Kruger F, Rejmánek M, Williamson M, editors. Biological invasions, a global perspective. Chichester: Wiley; 1989. pp. 369–388. [Google Scholar]
- Rejmánek M. Species richness and resistance to invasions. In: Orians R, Dirzo R, Cushman JH, editors. Diversity and processes in tropical forest ecosystems. New York: Springer; 1996. pp. 153–172. [Google Scholar]
- Rossatto DR, Hoffmann WA, Franco AC. Differences in growth patterns between co-occurring forest and savanna trees affect the forest-savanna boundary. Functional Ecology. 2009;23:689–698. [Google Scholar]
- Salo LF. Red brome (Bromus rubens subsp. madritensis) in North America: possible modes for early introductions, subsequent spread. Biological Invasions. 2005;7:165–180. [Google Scholar]
- Scheifinger H, Menzel A, Koch E, Peter C. Trends of spring time frost events and phenological dates in Central Europe. Theoretical and Applied Climatology. 2003;74:41–51. [Google Scholar]
- Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends in Ecology and Evolution. 2002;17:170–176. [Google Scholar]
- Sherry RA, Zhou XH, Gu SL, Arnone JA, Schimel DS, Verburg PS, Wallace LL, Luo YQ. Divergence of reproductive phenology under climate warming. Proceedings of the National Academy of Sciences of the USA. 2007;104:198–202. doi: 10.1073/pnas.0605642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ML, Roy BA, Thiede DA. Evolution in stressful environments. I. Phenotypic variability, phenotypic selection, and response to selection in five distinct environmental stresses. Evolution. 2000;54:93–111. doi: 10.1111/j.0014-3820.2000.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Sultan SE, Horgan-Kobelski T, Nichols LM, Riggs CE, Waples RK. A resurrection study reveals rapid adaptive evolution within populations of an invasive plant. Evolutionary Applications. 2013;6:266–278. doi: 10.1111/j.1752-4571.2012.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Frelich LE. Flowering phenology and height growth pattern are associated with maximum plant height, relative growth rate and stem tissue mass density in herbaceous grassland species. Journal of Ecology. 2011;99:991–1000. [Google Scholar]
- Throop HL, Reichmann LG, Sala OE, Archer SR. Response of dominant grass and shrub species to water manipulation: an ecophysiological basis for shrub invasion in a Chihuahuan Desert Grassland. Oecologia. 2012;169:373–383. doi: 10.1007/s00442-011-2217-4. [DOI] [PubMed] [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton, NJ: Princeton University Press; 1982. Monographs in population biology. [PubMed] [Google Scholar]
- Tilman D. Plant strategies and the dynamics and structure of plant communities. Princeton: Princeton University Press; 1988. Monographs in population biology. [Google Scholar]
- Trenberth KE, Josey SA. Observations: surface and atmospheric climate change. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate change 2007: the physical science basis: contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge, UK: IPCC; 2007. pp. 235–336. [Google Scholar]
- van Asch M, Visser ME. Phenology of forest caterpillars and their host trees: the importance of synchrony. Annual Review of Entomology. 2007;52:37–55. doi: 10.1146/annurev.ento.52.110405.091418. [DOI] [PubMed] [Google Scholar]
- van Schaik CP, Terborgh JW, Wright SJ. The phenology of tropical forests—adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics. 1993;24:353–377. [Google Scholar]
- Vile D, Shipley B, Garnier E. A structural equation model to integrate changes in functional strategies during old-field succession. Ecology. 2006;87:504–517. doi: 10.1890/05-0822. [DOI] [PubMed] [Google Scholar]
- Wainwright CE, Cleland EE. Exotic species display greater germination plasticity and higher germination rates than native species across multiple cues. Biological Invasions. 2013;15:2253–2264. [Google Scholar]
- Wainwright CE, Wolkovich EM, Cleland EE. Seasonal priority effects: implications for invasion and restoration in a semi-arid system. Journal of Applied Ecology. 2012;49:234–241. [Google Scholar]
- Wang G, Ni J. Plant traits and environmental conditions along the Northeast China Transect. Ekologia Bratislava. 2005;24:170–185. [Google Scholar]
- Weaver JE, Albertson FW. Effects on the great drought on the prairies of Iowa, Nebraska, and Kansas. Ecology. 1936;17:567–639. [Google Scholar]
- Weiner J. Asymmetric competition in plant-populations. Trends in Ecology and Evolution. 1990;5:360–364. doi: 10.1016/0169-5347(90)90095-U. [DOI] [PubMed] [Google Scholar]
- Willis CG, Ruhfel BR, Primack RB, Miller-Rushing AJ, Losos JB, Davis CC. Favorable climate change response explains non-native species’ success in Thoreau's woods. PLoS ONE. 2010;5:e8878. doi: 10.1371/journal.pone.0008878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey BJ, Daneshgar PP, Polley HW. Biodiversity, phenology and temporal niche differences between native- and novel exotic-dominated grasslands. Perspectives in Plant Ecology Evolution and Systematics. 2011;13:265–276. [Google Scholar]
- Wolkovich EM, Cleland EE. The phenology of plant invasions: a community ecology perspective. Frontiers in Ecology and the Environment. 2011;9:287–294. [Google Scholar]
- Wolkovich EM, Cook BI, Allen JM, Crimmins TM, Betancourt JL, Travers SE, Pau S, Regetz J, Davies TJ, Kraft NJB, Ault TR, Bolmgren K, Mazer SJ, McCabe GJ, McGill BJ, Parmesan C, Salamin N, Schwartz MD, Cleland EE. Warming experiments underpredict plant phenological responses to climate change. Nature. 2012a;485:494–497. doi: 10.1038/nature11014. [DOI] [PubMed] [Google Scholar]
- Wolkovich EM, Cook BI, Regetz J. 2012b. NECTAR: Network of Ecological and Climatological Timings Across Regions http://knb.ecoinformatics.org/knb/metacat/nceas.988/knb .
- Wolkovich EM, Davies TJ, Schaefer H, Cleland EE, Cook BI, Travers SE, Willis CG, Davis C. Temperature-dependent shifts in phenology contribute to the success of exotic species with climate change. American Journal of Botany. 2013;100:1407–1421. doi: 10.3732/ajb.1200478. [DOI] [PubMed] [Google Scholar]
- Xu CY, Griffin KL, Schuster WSF. Leaf phenology and seasonal variation of photosynthesis of invasive Berberis thunbergii (Japanese barberry) and two co-occurring native understory shrubs in a northeastern United States deciduous forest. Oecologia. 2007;154:11–21. doi: 10.1007/s00442-007-0807-y. [DOI] [PubMed] [Google Scholar]
- Zak DR, Groffman PM, Pregitzer KS, Christensen S, Tiedje JM. The vernal dam—plant microbe competition for nitrogen in northern hardwood forests. Ecology. 1990;71:651–656. [Google Scholar]



