The flexibility of plant cell walls is characterized by bulk modulus of elasticity (ϵ); which is an important component of how plants maintain adequate water continent. For example, plants with rigid tissues (high ϵ) that accumulate solutes may better tolerate drought or saline soils. This concept is termed the ‘cell water conservation hypothesis.’ While it is generally held that marine plants have higher ϵ, no study has considered that notion across a number of species residing in marine and coastal habitats. The finding from this study show that aquatic marine plants do maintain rigid tissues with lower osmotic potentials (relative to freshwater plants), and support the tenets of the cell water conservation hypothesis
Keywords: Bulk elastic modulus, halophytes, hydrophytes, salinity, solute potential, symplastic water content.
Abstract
Bulk modulus of elasticity (ɛ), depicting the flexibility of plant tissues, is recognized as an important component in maintaining internal water balance. Elevated ɛ and comparatively low osmotic potential (Ψπ) may work in concert to effectively maintain vital cellular water content. This concept, termed the ‘cell water conservation hypothesis’, may foster tolerance for lower soil-water potentials in plants while minimizing cell dehydration and shrinkage. Therefore, the accumulation of solutes in marine plants, causing decreases in Ψπ, play an important role in plant–water relations and likely works with higher ɛ to achieve favourable cell volumes. While it is generally held that plants residing in marine systems have higher leaf tissue ɛ, to our knowledge no study has specifically addressed this notion in aquatic and wetland plants residing in marine and freshwater systems. Therefore, we compared ɛ and Ψπ in leaf tissues of 38 freshwater, coastal and marine plant species using data collected in our laboratory, with additional values from the literature. Overall, 8 of the 10 highest ɛ values were observed in marine plants, and 20 of the lowest 25 ɛ values were recorded in freshwater plants. As expected, marine plants often had lower Ψπ, wherein the majority of marine plants were below −1.0 MPa and the majority of freshwater plants were above −1.0 MPa. While there were no differences among habitat type and symplastic water content (θsym), we did observe higher θsym in shrubs when compared with graminoids, and believe that the comparatively low θsym observed in aquatic grasses may be attributed to their tendency to develop aerenchyma that hold apoplastic water. These results, with few exceptions, support the premise that leaf tissues of plants acclimated to marine environments tend to have higher ɛ and lower Ψπ, and agree with the general tenets of the cell water conservation hypothesis.
Introduction
The flexibility of plant tissues, as characterized by bulk modulus of elasticity (ɛ), is an important component of plant–water relations (Steudle et al. 1977; Joly and Zaerr 1987). Plants with relatively flexible cell walls (low ɛ) can maintain adequate turgor during periods of tissue-water decline (Clifford et al. 1998). In contrast, rigid tissues restrict appreciable changes in cell size, and it has been suggested that limiting cellular water flux could help maintain intermolecular distances within cell fluids supporting optimal metabolic function (e.g. maintaining appropriate cellular pH or preventing ‘salting out’ of important metabolites; Jones 1973; Jones and Corlett 1992; Clifford et al. 1998). Cell wall elasticity and osmotic potentials (Ψπ) are closely linked to changes in cell volume (e.g. ΔV/ΔΨ = V/(ɛ + Ψπ); Tyerman 1982), and it is possible that rigid tissues can limit dehydration as both low Ψπ and high ɛ together are effective means of maintaining vital cellular water content (Cheung et al. 1975; Bartlett et al. 2012). This concept, termed the ‘cell water conservation hypothesis’, likely plays an important role in marine and coastal plants that accumulate ions or other solutes as an osmoregulatory response to elevated environmental salts.
Although plants with comparatively rigid tissues tend to lose turgor faster than plants with more flexible tissues (given comparable decreases in cell volume), loss in turgor may promote other important water-stress responses including stomatal closure, wilting and leaf rolling (Barker et al. 1993). Changes in ɛ may be induced by developmental and/or environmental factors including plant or tissue age (Bowman and Roberts 1985; Moore and Cosgrove 1991; Merchant et al. 2007), hysteresis from water stress altering cell wall viscoelastic properties (Nolte and Schopfer 1997; Schopfer 2006), drought (Joly and Zaerr 1987; Clifford et al. 1998; Touchette et al. 2007) and salinity (Bolaños and Longstreth 1984; Nabil and Coudret 1995; Touchette et al. 2009a). Indeed, these factors often work in concert, wherein the specific response of ɛ to water stress may depend on, for example, the age of the tissue during drought (Saito and Terashima 2004). While salinity- and drought-strain are physiologically similar in plants (both fostering cellular dehydration, ion accumulation, formation of reactive oxygen species and diminished photosynthetic capacity), the ratio of accumulated ions will be markedly different due to selective ion uptake during salinity stress (Kirst 1989; Bartels and Sunkar 2005; Touchette 2007). Moreover, it would appear that salt stress promotes a disproportionally higher ɛ and lower Ψπ in coastal–marine plants in comparison to drought stress which fosters smaller increases or even decreases in tissue elasticity (Touchette et al. 2007, 2009a). In some plant tissues, higher salinities can promote up to a 30-fold increase in ɛ along with a concomitant accumulation of compatible osmolytes that lower Ψπ (Bolaños and Longstreth 1984). In contrast, one study found that three of five freshwater perennials receiving drought had decreased ɛ and/or increased Ψπ (Touchette et al. 2007).
As mentioned, several studies have shown strong associations between elevated environmental salinities and increased tissue rigidities (Bolaños and Longstreth 1984; Salpeter et al. 2012). These findings, in general, suggest that marine halophytes should have higher ɛ values compared with freshwater or terrestrial plant species. To our knowledge, however, no study has been conducted to fully consider this relationship in hydrophytic angiosperms. Therefore, the purpose of this investigation was to test the hypothesis that coastal or marine aquatic and wetland plants (exposed to natural and/or artificial salinities) will have higher ɛ relative to plants residing in freshwater systems. We also wanted to consider how Ψπ and symplastic water content (θsym) interact with ɛ in promoting optimal water relations in plants from different aquatic systems. We intentionally focused on aquatic and wetland plants residing in relatively stable hydrologies as plants previously exposed to low soil moistures may have altered physiological properties (e.g. tissue elasticity and solute content) that would promote greater tolerances towards future soil-water deficits (Morgan 1984; Touchette et al. 2007). Therefore, as prevailing water conditions may be an important determinant for ɛ, we wanted to avoid using plants from natural systems with unknown water histories (e.g. terrestrial or upland environments) and perhaps prior drought exposure.
Methods
Plant selection and classification
This study combined laboratory and/or field evaluations on selected aquatic and wetland plants along with a meta-analysis of reported physiological values from other published studies. For the purposes of this investigation, a species is considered hydrophytic if its probability of occurring in an aquatic and/or wetland system is 67 % or greater (i.e. designated as either a facultative- or an obligate-wetland plant by the United States Fish and Wildlife Service; Reed 1988). Within this context, we focused on values obtained from pressure–volume curves on vascular plants from three habitat types: freshwater, coastal and marine (Table 1). Freshwater plants included aquatic species that were emergent and/or grew just above the water table (e.g. along stream- or lake-banks). Coastal plants included species that were living in principally freshwater systems, but were in locations where periodic salt exposure was likely. For this study, coastal plants were typically <0.5 km from seawater and could occasionally be exposed to environmental salts from storm tides and/or seawater aerosols (Touchette et al. 2009b). Marine plants were those that resided directly in marine or brackish waters and were known to have some degree of salt tolerance.
Table 1.
List of plant species use in this assessment. Data include species, taxonomic family, habitat (freshwater, coastal or marine), growth form (forb-herb, graminoid or shrub), environmental conditions at the time of collection (including salinity for marine plants; in practical salinity units [psu]) and the reference or source of the data.
| Species | Family | Habitat | Growth/habit | Environ. conditions/salinity | Source |
|---|---|---|---|---|---|
| Acorus americanus | Acoraceae | Freshwater | Forb-herb | Bioretention basin (flooded) | This study |
| Acorus americanus | Acoraceae | Freshwater | Forb-herb | Greenhouse (flooded) | Romanello et al. (2008) |
| Arundinaria gigantean | Poaceae | Coastal | Graminoid | Damp soils 100 m from seawater | This study |
| Atriplex hortensis | Chenopodiaceae | Freshwater | Forb-herb | Greenhouse | Kachout et al. (2011) |
| Bidens sp. | Asteraceae | Freshwater | Forb-herb | Greenhouse (saturated soils) | This study |
| Borrichia frutescens | Asteraceae | Marine | Shrub | Upper salt marsh/32 psu | This study |
| Carex alata | Cyperaceae | Freshwater | Graminoid | Greenhouse (flooded) | Touchette et al. (2007) |
| Carex alata | Cyperaceae | Freshwater | Graminoid | Greenhouse (saturated soils) | This study |
| Cephalanthus occidentalis | Rubiaceae | Freshwater | Shrub | Lacustrine wet shoreline | This study |
| Eleocharis sp. | Cyperaceae | Freshwater | Graminoid | Greenhouse (flooded) | This study |
| Halodule wrightii | Cymodoceaceae | Marine | Forb-herb | Seawater (submerged)/32 psu | Wilson et al. (2010) |
| Halodule wrightii | Cymodoceaceae | Marine | Forb-herb | Seawater (submerged)/32 psu | Touchette (2007) |
| Halophila ovalis | Hydrocharitaceae | Marine | Forb-herb | Seawater (submerged)/32 psu | Tyerman (1982) |
| Heteranthera limosa | Pontederiaceae | Freshwater | Forb-herb | Lotic wet shoreline | This study |
| Hibiscus moscheutos | Malvaceae | Freshwater | Shrub | Lacustrine wet shoreline | This study |
| Juncus effusus | Juncaceae | Freshwater | Graminoid | Greenhouse (flooded) | Touchette et al. (2007) |
| Juncus roemerianus | Juncaceae | Marine | Graminoid | Lower salt marsh/18 psu | Touchette (2006) |
| Juncus roemerianus | Juncaceae | Marine | Graminoid | Greenhouse (flooded)/30 psu | This study |
| Juncus roemerianus | Juncaceae | Marine | Graminoid | Greenhouse (flooded)/30 psu | Touchette et al. (2009a) |
| Juncus roemerianus | Juncaceae | Marine | Graminoid | Greenhouse (flooded)/0 psu | Touchette et al. (2009a) |
| Juncus sp. | Juncaceae | Freshwater | Graminoid | Freshwater (flooded) | This study |
| Justicia americana | Acanthaceae | Freshwater | Forb-herb | Lacustrine emergent | This study |
| Justicia americana | Acanthaceae | Freshwater | Forb-herb | Greenhouse (flooded) | Touchette et al. (2007) |
| Microstegium vimineum | Poaceae | Freshwater | Graminoid | Greenhouse (flooded) | Touchette and Romanello (2010) |
| Peltandra virginica | Araceae | Freshwater | Forb-herb | Greenhouse (flooded) | Touchette et al. (2007) |
| Polygonum amphibium | Polygonaceae | Freshwater | Forb-herb | Lotic emergent | This study |
| Pontederia cordata | Pontederiaceae | Freshwater | Forb-herb | Greenhouse (flooded) | This study |
| Posidonia australis | Posidoniaceae | Marine | Forb-herb | Seawater (submerged)/32 psu | Tyerman (1982) |
| Rosa palustris | Rosaceae | Freshwater | Shrub | Lacustrine wet shoreline | This study |
| Sabal minor | Arecaceae | Coastal | Shrub | Damp soils 200 m from seawater | This study |
| Sabal minor | Arecaceae | Coastal | Shrub | Coastal maritime swamp | Touchette et al. (2012) |
| Saururus cernuus | Saururaceae | Freshwater | Forb-herb | Lacustrine wet shoreline | This study |
| Saururus cernuus | Saururaceae | Freshwater | Forb-herb | Greenhouse (flooded) | Touchette et al. (2007) |
| Schoenoplectus pungens | Cyperaceae | Freshwater | Graminoid | Lacustrine emergent | This study |
| Schoenoplectus robustus | Cyperaceae | Marine | Graminoid | Upper salt marsh/15 psu | This study |
| Schoenoplectus tabernaemontani | Cyperaceae | Freshwater | Graminoid | Lacustrine emergent | This study |
| Sorghastrum sp. | Poaceae | Freshwater | Graminoid | Greenhouse (flooded) | This study |
| Spartina alterniflora | Poaceae | Marine | Graminoid | Greenhouse (flooded)/0 psu | Touchette et al. (2009a) |
| Spartina alterniflora | Poaceae | Marine | Graminoid | Lower salt marsh/15 psu | This study |
| Spartina alterniflora | Poaceae | Marine | Graminoid | Greenhouse (flooded)/30 psu | Touchette et al. (2009a) |
| Spartina patens | Poaceae | Marine | Graminoid | Greenhouse (wet soil)/0–45 psu | Salpeter et al. (2012) |
| Spartina patens | Poaceae | Marine | Graminoid | Lower salt marsh/32 psu | This study |
| Suaeda calceoliformis | Chenopodiaceae | Marine | Forb-herb | Greenhouse/30 psu | Youngman and Heckathorn (1992) |
| Suaeda calceoliformis | Chenopodiaceae | Marine | Forb-herb | Greenhouse/50 psu | Youngman and Heckathorn (1992) |
| Syringodium filiforme | Cymodoceaceae | Marine | Forb-herb | Seawater (submerged)/32 psu | Wilson et al. (2010) |
| Thalassia testudinum | Hydrocharitaceae | Marine | Forb-herb | Seawater (submerged)/32 psu | Wilson et al. (2010) |
| Typha angustifolia | Typhaceae | Coastal | Forb-herb | Flooded 250 m from seawater | This study |
| Typha latifolia | Typhaceae | Freshwater | Forb-herb | Lotic emergent | This study |
| Typha latifolia | Typhaceae | Coastal | Forb-herb | Flooded 100 m from seawater | This study |
| Typha sp. | Typhaceae | Freshwater | Forb-herb | Lotic emergent | This study |
| Zostera capricorni | Zosteraceae | Marine | Forb-herb | Seawater (submerged)/32 psu | Tyerman (1982) |
Plants were selected either from natural populations where hydrology was expected to be fairly static (e.g. lake or perennial stream populations) or from greenhouse-maintained hydrophytes grown in flooded or soil-saturated containers (20-L microcosms as described in Touchette et al. 2010) for more than 12 months. For the most part, collected plants were restricted to Atlantic coastal regions of North America, including Maryland, North Carolina and Vermont. During the collection of coastal and marine plants, ambient environmental salinities were recorded in surface or soil pore-waters located near the plant. Pressure–volume measurements were restricted to young fully expanded leaves to minimize differences attributed to the tissue developmental stage.
To complement data obtained on our cultured/collected samples and to expand the number of species used in this evaluation, we also incorporated values from other studies reported in the literature. For the most part, the data used were from known aquatic species (emergent or submersed plants) that were either collected or maintained in water-saturated environments (Table 1).
Pressure–volume curves
A Scholander pressure chamber (model no. 1000; PMS Instrument Co., Albany, OR, USA; Scholander et al. 1965) was used to determine leaf-water potentials (Ψleaf) on 5–10 plants per species (samples collected from different individuals with the quantity depending on the abundance or rarity of the species being considered). Prior to conducting pressure–volume analyses, leaves were fully submerged in deionized water and allowed to reach full turgor in darkness. During analysis, water deficits were established by exposing the samples to transpirational water loss on a laboratory bench. This process was favoured by Turner et al. (1984) as it minimized possible disequilibria of Ψ between apoplastic and symplastic tissues.
Pressure–volume curves were constructed by plotting the reciprocal of Ψleaf against relative water content (θ). In this case, θ was determined as follows:
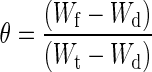 |
where Wf is the fresh weight recorded at the time of the Ψleaf measurement, Wt is the initial turgid weight and Wd is the oven dry weight (65 °C, until constant weight). First-order regressions were performed on the linear portion of the curves, which are equivalent to tissue osmotic potentials (Ψπ). By extension, this line was used to determine osmotic potential at full saturation (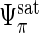 ), osmotic potential at turgor loss point (
), osmotic potential at turgor loss point (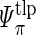 ) and symplastic water content (θsym). Following Ψπ correction, bulk elastic modulus (ɛ) was obtained from the initial part of the curve as described by the following equation:
) and symplastic water content (θsym). Following Ψπ correction, bulk elastic modulus (ɛ) was obtained from the initial part of the curve as described by the following equation:
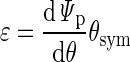 |
where changes in turgor potential (Ψp) were compared against changes in θ and symplastic water content (θsym).
Data analysis
For comparing species physiological characteristics (both data collected from our laboratory and values obtained from the literature), we used the mean value reported for each species with the exception of three seagrasses (Halophila ovalis, Posidonia australis and Zostera capricorni). For those exceptions, the reported values were maxima and ɛ were stationary volumetric elastic moduli (ɛs) and not the more commonly reported instantaneous volumetric elastic moduli (ɛi). Note, for comparative purposes, ɛi values tend to be higher than ɛs when recorded on the same plant (Tyerman 1982). Regression analyses were performed using Pearson product moment to identify correlates among physiological traits. Species-specific data were then combined into habitat types (i.e. freshwater, coastal or marine) to derive an overall grand mean. Because of the small sample sizes for coastal and marine plants, unequal replication and frequent occurrence of unequal variances, we elected to compare values classified within each habitat using a non-parametric Kruskal–Wallis one-way analysis of variance (KW one-way ANOVA) followed by Dunn's multiple comparisons test for post hoc evaluations. To further consider relationships between habitat type and physiological characteristics, we employed principal components analysis (PCA) as a reduction tool for plant physiological traits (i.e. ɛ, θsym, 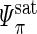 and
and 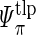 ) specific to each habitat. Initial observations also indicated that growth form (graminoid, forb and shrub) may explain some physiological traits. Therefore, grand mean values classified by plant form were also compared using KW one-way ANOVAs followed by Dunn's multiple comparisons test. All comparisons were considered significant at α = 0.05.
) specific to each habitat. Initial observations also indicated that growth form (graminoid, forb and shrub) may explain some physiological traits. Therefore, grand mean values classified by plant form were also compared using KW one-way ANOVAs followed by Dunn's multiple comparisons test. All comparisons were considered significant at α = 0.05.
Results
In all, we considered a total of 38 aquatic and wetland plants, most of which were emergent freshwater species (n = 22; Table 1). Because of the selective pressures of growing in saline environments, we had considerably fewer coastal wetland (n = 4) and marine (n = 12) plant species (Table 1). In addition, we included multiple measurements of the same species (when available) to help elucidate within-species variability. Most of the within-species values reported in this study (including values obtained from the literature) were fairly comparable. Values for Juncus roemerianus, however, were markedly different (ɛ ranging from 4.5 to 31.3 MPa). This discrepancy in J. roemerianus was most likely attributed to the prevailing environmental salinities, wherein higher salinities appeared to promote greater tissue rigidity. Pearson correlation analyses revealed significant inverse correlations between ɛ and Ψπ (at both saturation and turgor loss point; P < 0.001) and, as expected, a strong correlation between 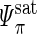 and
and 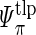 (r = 0.935; Table 2). No significant correlations were observed between θsym and the other physiological parameters.
(r = 0.935; Table 2). No significant correlations were observed between θsym and the other physiological parameters.
Table 2.
Pearson correlation matrix for plant–water relation parameters, bulk modulus of elasticity (ɛ), osmotic potential at full saturation (Ψπsat), osmotic potential at turgor loss point (Ψπtlp) and symplastic water content (θsym). Data from all species (the number of species was between 30 and 34, depending on data availability) were used regardless of habitat designation. The matrix includes correlation coefficients (r), probability values (P values) and number of comparisons made (n).
| Parameter | Correlation | 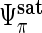 |
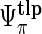 |
θsym |
|---|---|---|---|---|
| ɛ | Coefficient (r) | −0.735 | −0.458 | 0.165 |
| P value | <0.001 | <0.001 | 0.360 | |
| n | 34 | 31 | 33 | |
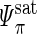 |
Coefficient (r) | – | 0.935 | 0.231 |
| P value | – | <0.001 | 0.210 | |
| n | – | 31 | 31 | |
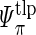 |
Coefficient (r) | – | – | 0.231 |
| P value | – | – | 0.219 | |
| n | – | – | 30 |
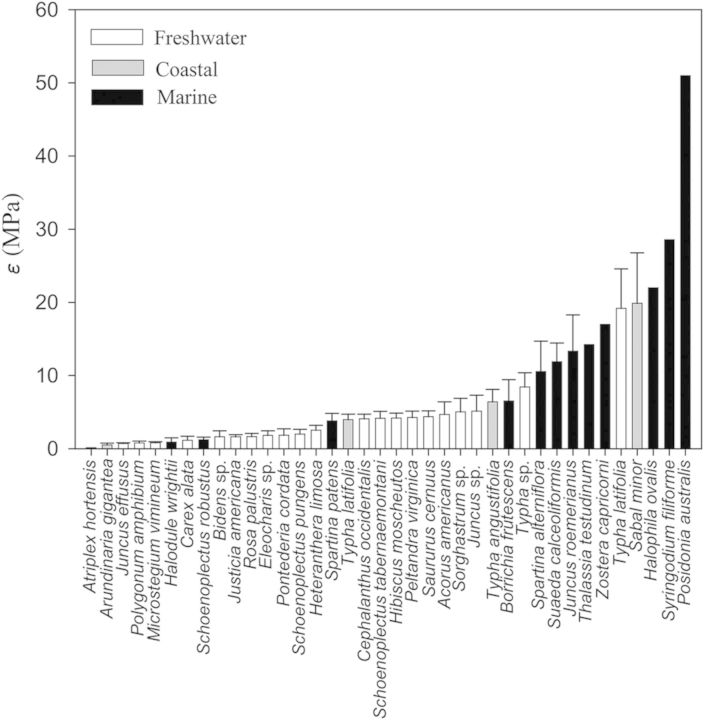
When comparing across all species it was apparent that marine plants tend to have greater ɛ values (Fig. 1). That is, 8 of the 10 most rigid tissues were found in marine plants and only one freshwater plant (Typha latifolia living as a lotic emergent) had ɛ >10 MPa. Similarly, of the 25 species with the lowest tissue rigidity, 20 species were from freshwater systems. Nevertheless, there were three marine species with surprisingly elastic tissues, including the seagrass Halodule wrightii and the salt marsh emergents Schoenoplectus robustus and Spartina patens (Fig. 1). Unlike freshwater and marine plants, there were no notable trends in coastal plants that had ɛ values ranging from 0.5 ± 0.2 to 19.8 ± 6.9 MPa. As with tissue rigidity, there appeared to be habitat-specific responses with respect to 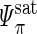 and
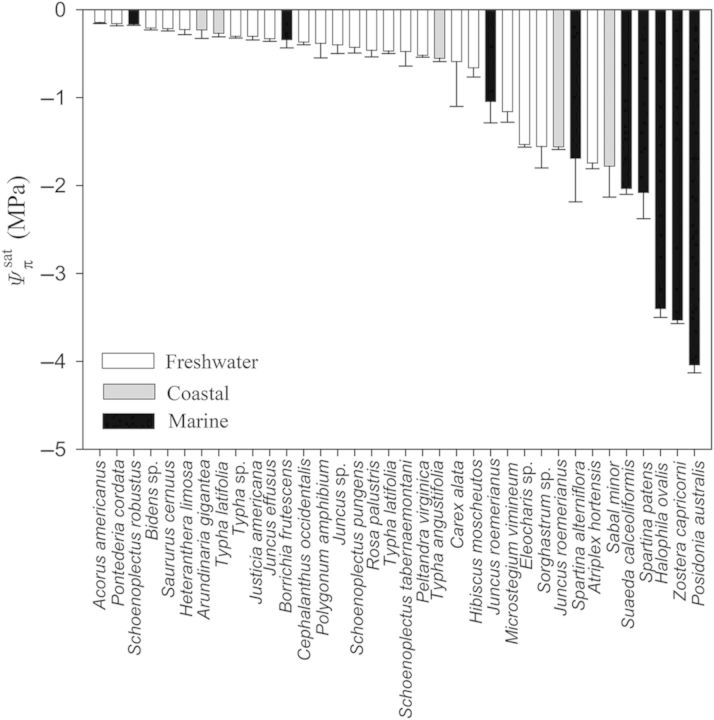
and 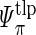 (Figs 2 and 3). Marine plants tended to have lower
(Figs 2 and 3). Marine plants tended to have lower 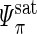 , wherein the lowest five observed values were marine species. Moreover, while most marine species had
, wherein the lowest five observed values were marine species. Moreover, while most marine species had 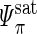 values lower than −1.0 MPa, most freshwater plants were well above −1.0 MPa (Fig. 2). As with
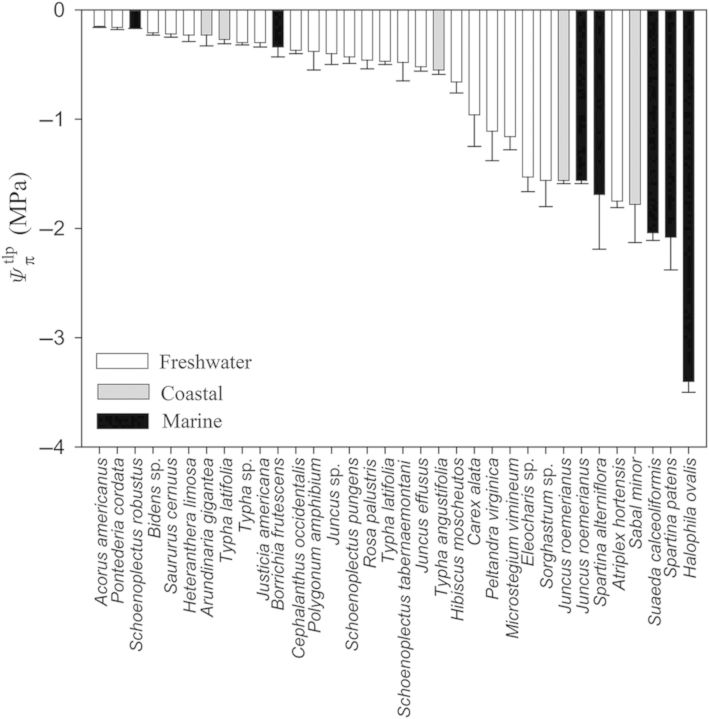
values lower than −1.0 MPa, most freshwater plants were well above −1.0 MPa (Fig. 2). As with 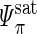 , reported values for
, reported values for 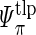 were also lower in marine plants (Fig. 3; but note fewer reported values as data were not available for some marine species).
were also lower in marine plants (Fig. 3; but note fewer reported values as data were not available for some marine species).
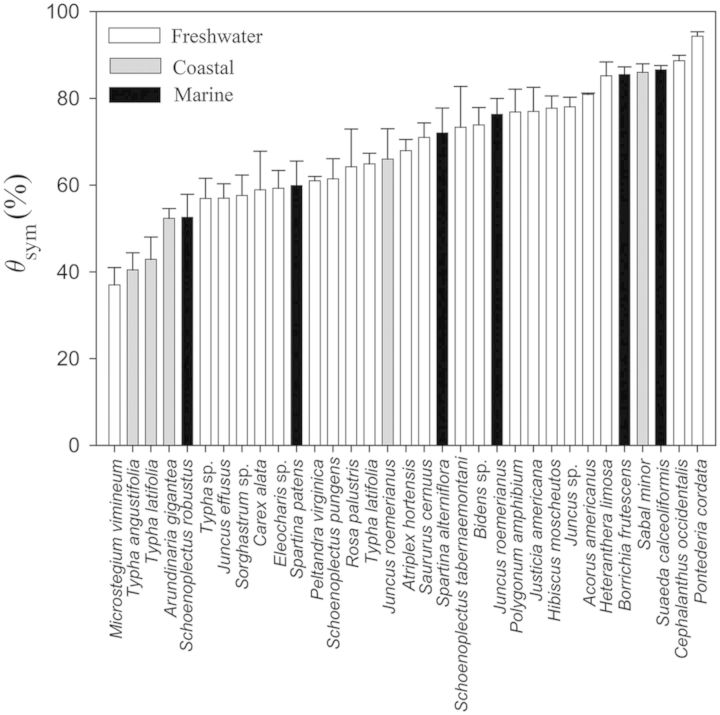
Figure 1.
Bulk elastic moduli (ɛ) of aquatic and wetland plants considered in this study. Data include values reported for different species and their respective environmental conditions including freshwater (white bars), coastal (grey bars) and marine (black bars). Data, where applicable, are presented as means ± 1 standard error (SE).
Figure 2.
Solute potentials at full saturation (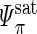 ) of aquatic and wetland plants considered in this study. Data include values reported for different species and their respective environmental conditions including freshwater (white bars), coastal (grey bars) and marine (black bars). Data, where applicable, are presented as means ± 1 SE.
) of aquatic and wetland plants considered in this study. Data include values reported for different species and their respective environmental conditions including freshwater (white bars), coastal (grey bars) and marine (black bars). Data, where applicable, are presented as means ± 1 SE.
Figure 3.
Solute potentials at turgor loss point (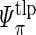 ) of aquatic and wetland plants considered in this study. Data include values reported for different species and their respective environmental conditions including freshwater (white bars), coastal (grey bars) and marine (black bars). Note that data were not available for a few marine plant species. Data, where applicable, are presented as means ± 1 SE.
) of aquatic and wetland plants considered in this study. Data include values reported for different species and their respective environmental conditions including freshwater (white bars), coastal (grey bars) and marine (black bars). Note that data were not available for a few marine plant species. Data, where applicable, are presented as means ± 1 SE.
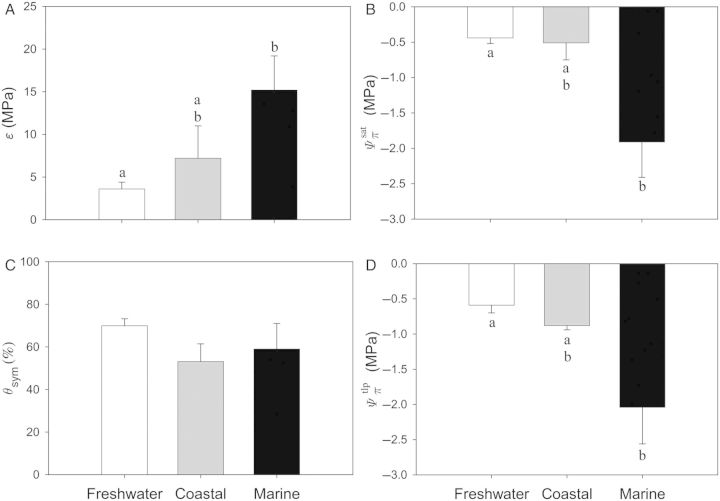
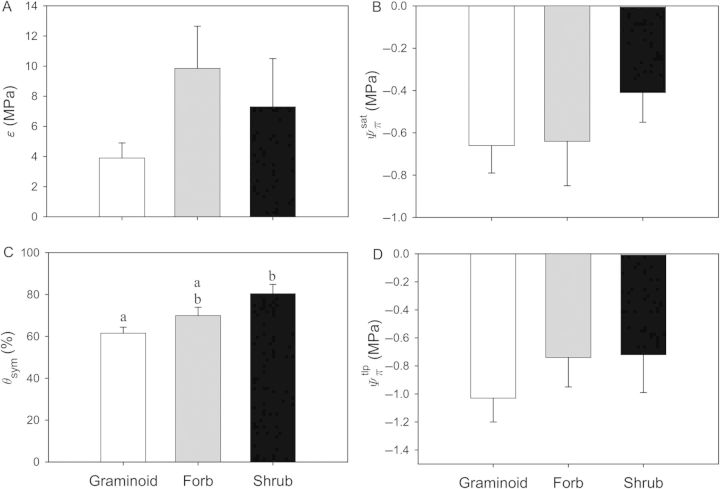
Statistical comparisons of plants residing in different habitats revealed significant differences between freshwater and marine plants (Fig. 4). In general, marine plants were 4.2 times more rigid than freshwater species (P < 0.001; Fig. 4A). Mean rigidity in coastal plants, although highly variable, was between freshwater and marine macrophytes and not significantly different from either group (P > 0.05). Solute potential was also different between marine and freshwater species (P < 0.001; Fig. 4), wherein 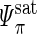 , for example, was 4.3 times lower in marine plants relative to freshwater emergent species. Moreover, while Ψπ in coastal plants were more similar to freshwater species, they were not significantly different from either freshwater or marine plants, likely attributed to high variability within this group (Fig. 4). Symplastic water content (θsym), which ranged between 53.1 ± 8.3 and 70.0 ± 3.3 %, was not significantly different among plants from different habitat types (P = 0.073; Figs 4C and 5).
, for example, was 4.3 times lower in marine plants relative to freshwater emergent species. Moreover, while Ψπ in coastal plants were more similar to freshwater species, they were not significantly different from either freshwater or marine plants, likely attributed to high variability within this group (Fig. 4). Symplastic water content (θsym), which ranged between 53.1 ± 8.3 and 70.0 ± 3.3 %, was not significantly different among plants from different habitat types (P = 0.073; Figs 4C and 5).
Figure 4.
Bulk elastic moduli (ɛ; A), solute potentials at full saturation (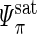 ; B), symplastic water content (θsym; C) and solute potential at turgor loss point (
; B), symplastic water content (θsym; C) and solute potential at turgor loss point (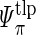 ; D) for plants categorized as freshwater (white bars), coastal (grey bars) and marine (black bars). Statistical differences are indicated by the letters above the bars, wherein different letters identify significant differences among the three habitat types. Data are presented as means (grand means for each category as described in the Methods section) ± 1 SE.
; D) for plants categorized as freshwater (white bars), coastal (grey bars) and marine (black bars). Statistical differences are indicated by the letters above the bars, wherein different letters identify significant differences among the three habitat types. Data are presented as means (grand means for each category as described in the Methods section) ± 1 SE.
Figure 5.
Symplastic water content (θsym) of aquatic and wetland plants considered in this study. Data include values reported for different species and their respective environmental conditions including freshwater (white bars), coastal (grey bars) and marine (black bars). Note that data were not available for a few marine plant species. Data, where applicable, are presented as means ± 1 SE.
While θsym appeared to be similar among plants residing in the three habitats explored in this study, there was a significant difference between θsym and plant growth form (irrespective of habitat type; Fig. 6C; P = 0.017). In this case, graminoids had significantly lower θsym than shrubs (P < 0.01). In contrast, there were no significant differences between growth form and ɛ, 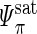 and
and 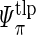 (P = 0.310, 0.597 and 0.221, respectively; Fig. 6).
(P = 0.310, 0.597 and 0.221, respectively; Fig. 6).
Figure 6.
Bulk elastic moduli (ɛ; A), solute potentials at full saturation (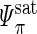 ; B), symplastic water content (θsym; C) and solute potential at turgor loss point (
; B), symplastic water content (θsym; C) and solute potential at turgor loss point (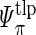 ; D) for plants categorized by form including graminoids (white bars), forbs (grey bars) and shrubs (black bars). Statistical differences are indicated by the letters above the bars, wherein different letters identify significant differences among the three habitat types. Data are presented as means (grand means for each category as described in the Methods section) ± 1 SE.
; D) for plants categorized by form including graminoids (white bars), forbs (grey bars) and shrubs (black bars). Statistical differences are indicated by the letters above the bars, wherein different letters identify significant differences among the three habitat types. Data are presented as means (grand means for each category as described in the Methods section) ± 1 SE.
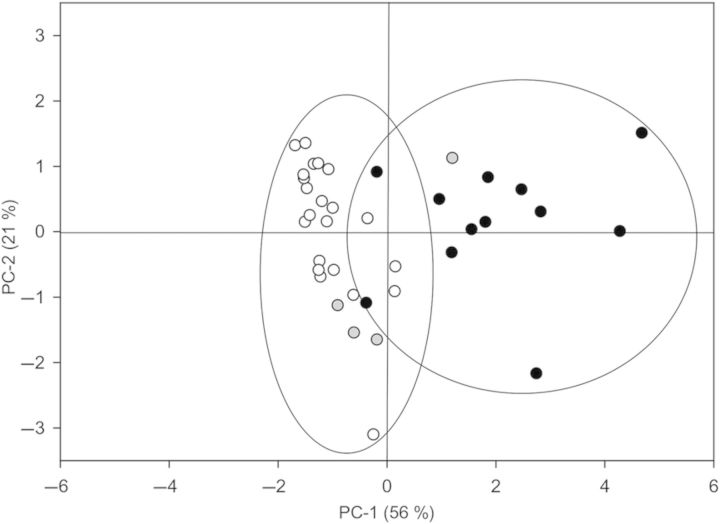
Principal components analysis comparing habitat and physiological responses among all 38 species agreed with the previous distinctions between marine and freshwater species (Fig. 7). Approximately 56 % of the variability in the data was accounted for in the first principal component. The cumulative variance was increased to 77 % when combined with the second component. Based on eigenvector loadings (Table 3), the first principal component was mostly associated with ɛ, 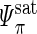 and
and 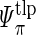 , and these three parameters contributed the most towards distinguishing between marine and freshwater plants (as habitat-group distinctions elicited strong horizontal tendencies; Fig. 7). The second principal component was overwhelmingly associated with θsym (with an eigenvector of 0.92) and was unable to provide further resolution among the three plant groups (i.e. lacking strong vertical tendencies; Fig. 7). As with other physiological characteristics, coastal plants were clustered between marine and freshwater plants and were indistinguishable from other plant habitat types.
, and these three parameters contributed the most towards distinguishing between marine and freshwater plants (as habitat-group distinctions elicited strong horizontal tendencies; Fig. 7). The second principal component was overwhelmingly associated with θsym (with an eigenvector of 0.92) and was unable to provide further resolution among the three plant groups (i.e. lacking strong vertical tendencies; Fig. 7). As with other physiological characteristics, coastal plants were clustered between marine and freshwater plants and were indistinguishable from other plant habitat types.
Figure 7.
Principal components analysis of physiological traits (ɛ, θsym, 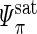 and
and 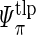 ) measured in different aquatic and wetland plant species including freshwater (white circles), coastal (grey circles) and marine (black circles). Percentages of explained variances for the first and second principal components (PC) are in parentheses. Note the pronounced horizontal tendencies between freshwater and marine species.
) measured in different aquatic and wetland plant species including freshwater (white circles), coastal (grey circles) and marine (black circles). Percentages of explained variances for the first and second principal components (PC) are in parentheses. Note the pronounced horizontal tendencies between freshwater and marine species.
Table 3.
Matrix of eigenvector loadings for physiological predictors for freshwater, coastal and marine plants. Most of the variance was explained by the first four principal components (97.1 %; 77.3 % was explained by the first two principal components).
| Variable | Principal component |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Habitat | 0.4854 | −0.0142 | −0.0723 | 0.8694 |
| ɛ | 0.4588 | 0.3445 | −0.6181 | −0.3294 |
| θsym | −0.0938 | 0.9201 | 0.3652 | 0.1001 |
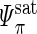 |
−0.5604 | −0.0399 | −0.0368 | 0.2584 |
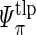 |
−0.4806 | 0.1815 | −0.6914 | 0.2427 |
Discussion
In response to sudden changes in plant-water status (e.g. water availability and/or environmental salinity), turgor pressure in tissues may increase or decrease as water moves in the direction of the osmotic gradient. The degree of water flux is largely dependent on hydraulic conductivity of cell membranes, intracellular osmotic pressure, cell volume and elastic properties of cell walls (Tyerman 1982; Kramer and Boyer 1995; Touchette 2007). Cell wall elasticity can vary greatly among plant species, with reported ranges of ɛ from 0.06 MPa in Halicystis parvula to 70 MPa in Chara corrallina (Dainty et al. 1974; Graves and Gutknecht 1976). In some instances, flexible cell walls (low ɛ) can benefit plants inhabiting systems with dynamic salinity fluctuations, as cell walls expand or contract in order to maintain osmotic equilibrium with the environment (Kirst 1989; Touchette 2006). In hyperosmotic conditions, for example, lower ɛ can foster positive turgor pressures during periods of water efflux. These physiological processes are sometimes employed in estuarine macroalgae, where changes in osmolyte levels (i.e. Ψπ) and cell volume are important mechanisms for osmotic adjustment (Kirst 1989). For these algae, sudden changes in water salinity may be balanced by expanding or shrinking of comparatively thin cell walls.
Based on observations from this study, however, only a few marine angiosperms actually have relatively flexible cell walls (ɛ < 10 MPa). This response may be explained, in part, by the saline dynamics within the particular system. Schoenoplectus robustus, for example, had the eighth lowest ɛ of the 38 species considered. Although classified as a salt marsh plant, S. robustus resides along the mid-to-upper boundaries of salt marsh systems where salinities tend to be relatively low (sometimes freshwater) with sporadic inundations of higher saline waters (Ustin et al. 1982; Crain et al. 2004). Indeed, Ustin et al. (1982) were able to demonstrate that among the different salt marsh zones, the regions dominated by S. robustus maintained the greatest seasonal fluctuations in salinity. Not only would having comparatively flexible tissues benefit S. robustus in this osmotically dynamic environment, but apparently S. robustus can lie dormant as tubers for up to 2 years to avoid extended periods of hypersalinity (Ustin et al. 1982; Crain et al. 2004). Furthermore, S. robustus had comparatively high Ψπ (the third highest among all species in this study); thus any substantial decline in cell volume may not confine existing solutes to levels where metabolic functions are compromised (e.g. salting out metabolites). Spartina patens, which also has relatively flexible tissues, is common along the upper reaches of salt marshes where it is fed by shallow freshwater systems. Unlike S. robustus, S. patens can have comparatively low Ψπ (−2.14 MPa), although highly variable with some reported potentials as high as −0.25 MPa. Apparently, S. patens can undergo extended periods of salt avoidance when exposed to hypersaline conditions, and perhaps these delays in modifying plant–water relations may contribute to a greater range in both ɛ and Ψπ (Salpeter et al. 2012). That is, S. patens will likely maintain low ɛ and high Ψπ when exposed to short periods of high salinity, but longer exposures (>5 weeks) may foster physiological alterations that include higher ɛ and lower Ψπ (Salpeter et al. 2012). The seagrass H. wrightii had the sixth most flexible tissue of the 38 species considered and, as with the other 2 species, resides in osmotically dynamic systems (Touchette 2006). That is, H. wrightii has been shown to thrive in the Guadalupe estuary where salinities can fluctuate from 5 to 25 practical salinity units (psu) over a few months, with periodic spikes in salinities up to 55 (Dunton 1996). We argue that for S. robustus, S. patens and H. wrightii to survive in these highly dynamic euryhaline environments, like estuarine macroalgae, maintaining flexible cell walls could be advantageous provided cytoplasmic ion content remains low and/or Ψπ remains high (including osmotic adjustments attributed to compatible solutes and/or vacuolar sequestration of ions; Kirst 1989; Touchette 2007).
While a few marine plants have relatively flexible cell walls, most studies indicate that plants residing in saline environments will have rigid tissues (Ike and Thurtell 1981; Melkonian et al. 1982; Bolaños and Longstreth 1984). The results from this study, with the aforementioned exceptions, support this previously untested assertion. Here 9 of the 10 species with the highest ɛ values were either marine or coastal plants. For plants with comparatively high ɛ, a corresponding reduction in osmotic potentials could help prevent dehydration and shrinkage during periods of lower Ψsoil (Cheung et al. 1975; Bartlett et al. 2012). Rigid cells may also be advantageous in marine plants that tend to sequester ions for osmotic balance, as diminishing cell volume would confine existing solutes and ions to a level that could disrupt cellular processes (Touchette 2007). This notion is supported by the tendency for plants with high ɛ to also have low Ψπ (suggesting high cellular solute content). Therefore, plants residing in more stable marine environments (including lower salt marsh areas or open coastal waters) would benefit from having thicker cell walls with relatively low elasticity (Kirst 1989; Touchette 2007; Bartlett et al. 2012).
In contrast to marine species, freshwater plants generally have lower ɛ and higher Ψπ. Although we intentionally focused on plants from areas with more stable hydrologies, freshwater wetlands are transitional areas between terrestrial and deep-water habitats and therefore many of the resident species are physiologically adapted to periodic water deprivation from water-table drawdown and/or drought (Cowardin et al. 1979; Wilcox et al. 2002). For these plants, a small ɛ can facilitate turgor maintenance in cells as tissue water declines (Kozlowski et al. 1990). Indeed water stress may promote greater turgor in some plants following water repletion (Kikuta and Richter 1986; Saito and Terashima 2004). Maintaining turgor and avoiding plasmolysis are important in plants as high turgor pressure has been shown to enhance membrane transport, plant defence responses, cytoskeleton stability and cytoplasmic streaming (Mellersh and Heath 2001; Hayashi and Takagi 2003; Heidecker et al. 2003). Therefore, with higher Ψπ limiting the likelihood of confining solutes to the point of metabolic disruption (as in marine species), freshwater species tend to maintain flexible cells that minimize the likelihood of zero turgor or plasmolysis.
Finally, although there were no significant differences in θsym among the three habitats considered in this study, we did observe that growth form may be an important component in determining the level of θsym in wetland plants. In this case, graminoids had significantly lower θsym than shrubs. One possible explanation for this difference, as well as the overall low θsym reported in aquatic grasses and forbs, is the predominance of aerenchyma that often develops in grasses and forbs in response to reduced anaerobic substratums (Jackson and Armstrong 1999). In this case, the substantial increase in apoplastic volume (due to aerenchyma development) within tissues could act, in part, as reservoirs for maintaining comparatively higher apoplastic water and consequently a proportionally lower symplastic water content (Gribble et al. 1998). The use of aerenchyma as a possible apoplastic water reservoir, along with other roles (e.g. a lacunar system that provides oxygen to roots residing in hypoxic/anoxic sediments; Grosse and Mevi-Schütz 1987), could benefit emergent wetland plants by providing water to the cells during periods of soil-water deficit or drought.
Conclusions
In this study, we were able to demonstrate that marine plants, by and large, do have higher ɛ and lower Ψπ. By maintaining rigid tissues and lower Ψπ, marine plants may be able to both prevent dangerous dehydration and achieve tolerance of lower Ψsoil (i.e. the ‘cell water conservation hypothesis’). Maintaining higher cellular water content could help minimize metabolic disruptions that may develop when existing solutes, already in high concentrations, are confined into smaller spaces during water efflux. In contrast, freshwater plants tend to have lower ɛ and higher Ψπ. Flexible tissues would allow for appreciable water loss while maintaining positive turgor pressure. In this case, because cellular solute content is low (i.e. high Ψπ) there may be greater probability that physiological disruptions will occur due to plasmolysis rather than to solute confinement.
Sources of Funding
This study was supported by the North Carolina Sea Grant (NCSG), UNC Water Resources Research Institute (WRRI) and the Japheth E. Rawls Foundation.
Contributions by the Authors
Experiments were carried out by all three authors. Compilation of data from previous published studies was conducted by B.W.T. and S.E.M. Data and statistical analyses were performed by all authors. The manuscript was written by B.W.T. and S.E.M.
Conflicts of Interest Statement
None declared.
Acknowledgements
We are grateful for the continued support from the North Carolina Sea Grant and the UNC Water Resources Research Institute. Laboratory space and consumable materials were generously provided by the Departments of Biology and Environmental Studies at Elon University. We greatly appreciate the thoughtful and constructive comments from the managing editor T. Brodribb and two anonymous reviewers.
Literature Cited
- Barker DJ, Sullivan CY, Moser LE. Water deficit effects on osmotic potential, cell wall elasticity, and proline in five forage grasses. Lincoln: Agronomy Faculty Publications, University of Nebraska; 1993. [Google Scholar]
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences. 2005;24:23–58. [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecology Letters. 2012;15:393–405. doi: 10.1111/j.1461-0248.2012.01751.x. [DOI] [PubMed] [Google Scholar]
- Bolaños JA, Longstreth DJ. Salinity effects on water potential components and bulk elastic modulus of Alternanthera philoxeroides (Mart.) Griseb. Plant Physiology. 1984;75:281–284. doi: 10.1104/pp.75.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WD, Roberts SW. Seasonal changes in tissue elasticity in chaparral shrubs. Physiologia Plantarum. 1985;65:233–236. [Google Scholar]
- Cheung YNS, Tyree MT, Dainty J. Water relations parameters on single leaves obtained in a pressure bomb and some ecological interpretations. Canadian Journal of Botany. 1975;53:1342–1346. [Google Scholar]
- Clifford SC, Arndt SK, Corlett JE, Joshi S, Sankhla N, Popp M, Jones HG. The role of solute accumulation, osmotic adjustment, and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk.) Journal of Experimental Botany. 1998;49:967–977. [Google Scholar]
- Cowardin LM, Carter V, Golet FC, LaRoe ET. Classification of wetlands and deepwater habitats of the United States. Washington, DC: US Government Printing Office; 1979. [Google Scholar]
- Crain CM, Silliman BR, Bertness SL, Bertness MD. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology. 2004;85:2539–2549. [Google Scholar]
- Dainty J, Vinters H, Tyree MT. A study of transcellular osmosis and the kinetics of swelling and shrinking in cells of Chara corallina. In: Zimmermann U, Dainty J, editors. Membrane transport in plants. Berlin: Springer; 1974. pp. 59–63. [Google Scholar]
- Dunton KH. Photosynthetic production and biomass of the subtropical seagrass Halodule wrightii along an estuarine gradient. Estuaries and Coasts. 1996;19:436–447. [Google Scholar]
- Graves JS, Gutknecht J. Ion transport studies and determination of the cell wall elastic modulus in the marine alga Halicystis parvula. Journal of General Physiology. 1976;67:579–597. doi: 10.1085/jgp.67.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble K, Tingle J, Sarafis V, Heaton A, Holford P. Position of water in vitrified plants visualised by NMR imaging. Protoplasma. 1998;201:110–114. [Google Scholar]
- Grosse W, Mevi-Schütz J. A beneficial gas transport system in Nymphoides peltata. American Journal of Botany. 1987;74:947–952. [Google Scholar]
- Hayashi T, Takagi S. Ca2+ dependent cessation of cytoplasmic streaming induced by hypertonic treatment in Vallisneria mesophyll cells: possible role of cell wall–plasma membrane adhesion. Plant and Cell Physiology. 2003;44:1027–1036. doi: 10.1093/pcp/pcg123. [DOI] [PubMed] [Google Scholar]
- Heidecker M, Wegner LH, Binder K-A, Zimmermann U. Turgor pressure changes trigger characteristics changes in the electrical conductance of the tonoplast and the plasmalemma of the marine alga Valonia utricularis. Plant, Cell and Environment. 2003;26:1035–1051. [Google Scholar]
- Ike IF, Thurtell GW. Osmotic adjustment in indoor cassava in response to water stress. Physiologia Plantarum. 1981;52:257–262. [Google Scholar]
- Jackson MB, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology. 1999;1:274–287. [Google Scholar]
- Joly RJ, Zaerr JB. Alteration of cell-wall water content and elasticity in Douglas-Fir during periods of water deficit. Plant Physiology. 1987;83:418–422. doi: 10.1104/pp.83.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG. Photosynthesis by thin leaf slices in solution II. Osmotic stress and is effects on photosynthesis. Australian Journal of Biological Sciences. 1973;26:25–34. [Google Scholar]
- Jones HG, Corlett JE. Current topics in drought physiology. Journal of Agricultural Science. 1992;119:291–296. [Google Scholar]
- Kachout SS, Mansoura AB, Hamza KJ, Leclerc JC, Rejeb MN, Ouerghi Z. Leaf–water relations and ion concentrations of the halophyte Atriplex hortensis in response to salinity and water stress. Acta Physiologiae Plantarum. 2011;33:335–342. [Google Scholar]
- Kikuta SB, Richter H. Graphical evaluation and partitioning of turgor responses to drought in leaves of durum wheat. Planta. 1986;168:36–42. doi: 10.1007/BF00407006. [DOI] [PubMed] [Google Scholar]
- Kirst GO. Salinity tolerance of eukaryotic marine algae. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:21–53. [Google Scholar]
- Kozlowski TT, Kramer PJ, Pallardy SG. The physiological ecology of woody plants. New York: Academic Press; 1990. [Google Scholar]
- Kramer PJ, Boyer JS. Water relations of plants and soils. San Diego: Academic Press; 1995. [Google Scholar]
- Melkonian JJ, Wolfe J, Steponkus PL. Determination of the volumetric modulus of elasticity of wheat leaves by pressure–volume relations and the effect of drought conditioning. Crop Science. 1982;22:116–123. [Google Scholar]
- Mellersh DG, Heath MC. Plasma membrane–cell wall adhesion is required for expression of plant defense responses during fungal penetration. The Plant Cell. 2001;13:413–424. doi: 10.1105/tpc.13.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant AW, Callister A, Arndt S, Tausz M, Adams M. Contrasting physiological responses of six Eucalyptus species to water deficit. Annals of Botany. 2007;100:1507–1515. doi: 10.1093/aob/mcm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PH, Cosgrove DJ. Developmental changes in cell and tissues water relations parameters in storage parenchyma of sugarcane. Plant Physiology. 1991;96:794–801. doi: 10.1104/pp.96.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JM. Osmoregulation and water stress in higher plants. Annual Review of Plant Physiology. 1984;35:299–319. [Google Scholar]
- Nabil M, Coudret A. Effects of sodium chloride on growth, tissue elasticity, and solute adjustment in two Acacia nilotica subspecies. Physiologia Plantarum. 1995;93:217–222. [Google Scholar]
- Nolte T, Schopfer P. Viscoelastic versus plastic cell wall extensibility in growing seedling organs: a contribution to avoid some misconceptions. Journal of Experimental Botany. 1997;48:2103–2107. [Google Scholar]
- Reed PB. Washington, DC: United States Fish and Wildlife Service; 1988. National list of plant species that occur in wetlands: national summary. Biological Report 88 (24) [Google Scholar]
- Romanello GA, Cuchra-Zbytniuk KL, Vandermer JL, Touchette BW. Morphological adjustments promote drought avoidance in the wetland plant Acorus americanus. Aquatic Botany. 2008;89:390–396. [Google Scholar]
- Saito T, Terashima I. Reversible decreases in bulk elastic modulus of mature leaves of deciduous Quercus species subjected to two drought treatments. Plant, Cell and Environment. 2004;27:863–875. [Google Scholar]
- Salpeter KE, Millemann DR, Caputo MF, White BL, Touchette BW. Delayed modifications in plant–water relations in the coastal marsh halophyte Spartina patens following sudden increases in soil salinity. Botanica Marina. 2012;55:307–310. [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Schopfer P. Biomechanics of plant growth. American Journal of Botany. 2006;93:1415–1425. doi: 10.3732/ajb.93.10.1415. [DOI] [PubMed] [Google Scholar]
- Steudle E, Zimmerman U, Lüttge U. Effect of turgor pressure and cell size on the wall elasticity of plant cells. Plant Physiology. 1977;59:285–289. doi: 10.1104/pp.59.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette BW. Salt tolerance in a Juncus roemerianus brackish marsh: spacial variations in plant water relations. Journal of Experimental Marine Biology and Ecology. 2006;337:1–12. [Google Scholar]
- Touchette BW. Seagrass–salinity interactions: physiological mechanisms used by submerged marine angiosperms for life at sea. Journal of Experimental Marine Biology and Ecology. 2007;350:194–215. [Google Scholar]
- Touchette BW, Romanello GA. Growth and water relations in a central North Carolina population of Microstegium vimineum (Trin.) A. Camus. Biological Invasions. 2010;12:893–903. [Google Scholar]
- Touchette BW, Iannacone LR, Turner GE, Frank AR. Drought tolerance versus drought avoidance: a comparison of plant–water relations in herbaceous wetland plants subjected to water withdrawal and repletion. Wetlands. 2007;27:656–667. [Google Scholar]
- Touchette BW, Smith GA, Rhodes KL, Poole M. Tolerance and avoidance: two contrasting physiological responses to salt stress in mature marsh halophytes Juncus roemerianus Scheele and Spartina alterniflora Loisel. Journal of Experimental Marine Biology and Ecology. 2009a;380:106–112. [Google Scholar]
- Touchette BW, Rhodes KL, Smith GA, Poole M. Salt spray induces osmotic adjustment and tissue rigidity in smooth cordgrass, Spartina alterniflora (Loisel.) Estuaries and Coasts. 2009b;32:917–925. [Google Scholar]
- Touchette BW, Iannacone LR, Turner G, Frank A. Ecophysiological responses of five emergent-wetland plants to diminished water supply: an experimental microcosm study. Aquatic Ecology. 2010;44:101–112. [Google Scholar]
- Touchette BW, Adams EC, Laimbeer P, Burn GA. Ridge crest versus swale: contrasting plant–water relations and performance indexes in two understory plant species in a coastal maritime forest. Journal of Plant Interactions. 2012;7:271–282. [Google Scholar]
- Turner NC, Spurway RA, Schulze ED. Comparison of water potentials measured by in situ psychrometry and pressure chamber in morphologically different species. Plant Physiology. 1984;74:316–319. doi: 10.1104/pp.74.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD. Stationary volumetric elastic modulus and osmotic pressure of the leaf cells of Halophila ovalis, Zostera capricorni, and Posidonia australis. Plant Physiology. 1982;69:957–965. doi: 10.1104/pp.69.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustin SL, Pearcy RW, Bayer DE. Plant water relations in a San Francisco Bay salt marsh. Botanical Gazette. 1982;143:268–373. [Google Scholar]
- Wilcox DA, Meeker JE, Hudson PL, Armitage BJ, Black MG, Uzarski DG. Hydrologic variability and the application of index of biotic integrity metrics to wetlands: a Great Lakes evaluation. Wetlands. 2002;22:588–615. [Google Scholar]
- Wilson CJ, Wilson PS, Greene CA, Dunton KH. Seagrass leaves in 3-D: using computed tomography and low frequency acoustics to investigate the material properties of seagrass tissue. Journal of Experimental Marine Biology and Ecology. 2010;395:128–134. [Google Scholar]
- Youngman AL, Heckathorn SA. Effect of salinity on water relations of two growth forms of Suaeda calceoliformis. Functional Ecology. 1992;6:686–692. [Google Scholar]