Abstract
Cardiovascular disease (CVD) remains the main cause of death in diabetic patients, and once it has developed, diabetic patients have a worse outcome as compared with non‐diabetic patients. One reason for this is the difficulty of early diagnosis of atherosclerotic change in these patients. Although cardiovascular risk assessment based on conventional risk factors is recommended for predicting cardiovascular risk, validation studies showed only moderate performance. In contrast, it is unrealistic to screen for subclinical or silent atherosclerosis by sophisticated modalities, such as myocardial perfusion scintigraphy, coronary computed tomography angiography or conventional angiography in all diabetic patients, as these tests are limited by the potential of significant adverse effects, technical difficulty, availability and high cost. Therefore, a non‐invasive and inexpensive tool for risk prediction of subclinical atherosclerosis is required for identifying individuals at high risk of CVD. Recently, a number of studies have shown close associations between carotid atherosclerosis and cerebrovascular or coronary artery disease. Carotid ultrasonography has allowed clinicians to visualize the characteristics of the carotid wall and lumen surfaces to quantify the severity of atherosclerosis. Carotid intima‐media thickness (IMT) is an especially useful marker of the progression of atherosclerosis throughout the body, and is an excellent predictor of cardiovascular events. As a simple and non‐invasive procedure, measurement of carotid IMT is one of the most appropriate screening methods to specify high‐risk individuals in subjects with and without diabetes. Therefore, it is expected that carotid ultrasonography will become a potent tool for better clinical practice of atherosclerosis in diabetic patients.
Keywords: Cardiovascular diseases, Carotid ultrasound, Diabetes mellitus
Introduction
Cardiovascular disease (CVD) remains the main cause of death in diabetic patients, and once it has developed, diabetic patients have a worse outcome as compared with non‐diabetic patients. One reason for this is the difficulty of early diagnosis of atherosclerotic change in major arteries, including coronary, cerebral, renal and peripheral arteries. For example, many cases of coronary atherosclerosis develop without symptoms, especially in diabetic patients. Indeed, it is reported that, in patients with ischemic heart disease, the frequency of silent myocardial ischemia is approximately three to sixfold higher in diabetic patients than in non‐diabetic patients1. According to the Detection of Ischemia in Asymptomatic Diabetes (DIAD) study, as much as 22% of asymptomatic type 2 diabetic patients aged 50–70 years were shown to have silent myocardial ischemia identified by myocardial perfusion scintigraphy2. Therefore, early detection of asymptomatic severe coronary artery disease (CAD), as well as cerebrovascular disease, and subsequent rapid intervention, are important to reduce mortality in the management of diabetes.
Atherosclerosis is a multifactorial disease, and the development of atherosclerotic disease involves the interaction of many genetic and environmental factors through conventional risk factors, such as diabetes, dyslipidemia, hypertension and obesity (Figure 1). Therefore, cardiovascular risk assessment based on such conventional risk factors is recommended for predicting cardiovascular risk. However, validation studies showed that this approach had only moderate performance3. In contrast, sophisticated cardiac studies, such as exercise electrocardiogram (ECG), myocardial perfusion scintigraphy, coronary computed tomography angiography (coronary CT angiography) and conventional coronary angiography can determine disease severity with a high degree of sensitivity and specificity. However, it is unrealistic to screen for silent myocardial ischemia with these tools in all diabetic patients, as these tests are limited by the potential of significant adverse effects, technical difficulty, availability and high cost. The same is true for cerebrovascular diseases. Therefore, a non‐invasive and inexpensive tool for prediction of risk of subclinical or silent atherosclerosis with more than moderate predictive ability is required for identifying individuals at high risk of CVD (Figure 2).
Figure 1.
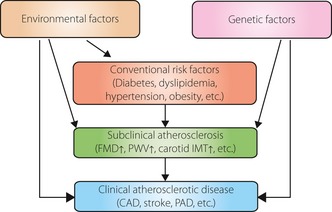
Atherosclerosis is a multifactorial disease, and the development of atherosclerotic disease involves the interaction of many genetic and environmental factors, as well as conventional risk factors, such as diabetes, dyslipidemia, hypertension and obesity. CAD, coronary artery disease; FMD, flow‐mediated vasodilation; IMT, intima‐media thickness; PAD, peripheral artery disease; PWV, pulse wave velocity.
Figure 2.
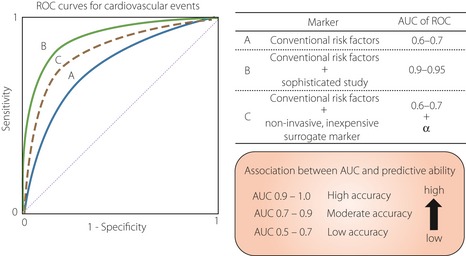
Previous studies reported that a conventional risk assessment approach showed limited performance (blue solid line). In contrast, sophisticated studies, such as exercise electrocardiogram, myocardial perfusion scintigraphy, computed tomography, magnetic resonance imaging and coronary angiography, can determine disease severity with a high degree of sensitivity and specificity (green solid line). However, it is unrealistic to screen for cardiovascular disease (CVD) with these tools in all diabetic patients, as these tests are limited by the potential of significant adverse effects, technical difficulty, availability and high cost. Therefore, a non‐invasive and inexpensive risk prediction tool with more than moderate predictive ability (dot line) is required for identifying individuals at high‐risk of CVD. AUC, area under curve; ROC, receiver operator characteristic.
Recently, a number of studies have shown close associations between carotid atherosclerosis and cerebrovascular or coronary artery disease. Carotid ultrasonography has allowed clinicians to visualize the characteristics of the carotid wall and lumen surfaces to quantify the severity of atherosclerosis. Carotid intima‐media thickness (IMT) measured with B‐mode ultrasound correlates well with that obtained by pathological measurements, and has been confirmed to be a quantitative and reproducible measure of carotid arteriosclerosis6. Carotid IMT measured by ultrasonography also correlates well with aortic IMT measured by transesophageal echocardiography, and is believed to reflect the severity of systemic atherosclerosis. As it is a non‐invasive, convenient and economical procedure, carotid ultrasonography could be useful in the diagnosis and screening of atherosclerosis.
Measurement of Carotid IMT
Methods
Using B‐mode ultrasonography, the carotid arteries are scanned in transverse sections from the origins of the common carotid arteries, carotid sinuses, internal carotid arteries and external carotid arteries, and then examined for carotid lesions in longitudinal sections at different angles. High‐resolution ultrasound images with a range resolution of 0.1 mm can be obtained when carotid ultrasonography is carried out using a linear probe with a center frequency of 7.5 MHz or higher. Ultrasound devices equipped with automatic IMT measuring software that recently became available might reduce the interexaminer error and shorten the examination time. In clinical studies using carotid IMT as an end‐point, automatic IMT measurement is becoming essential.
Definitions of Carotid IMT and Plaques
B‐mode ultrasonography visualizes the carotid wall as three layers, consisting of a hyperechoic layer, a hypoechoic layer and another hyperechoic layer. The two layers closer to the vascular lumen are defined as the intima‐media complex, and the thickness of the intima‐media complex as the IMT. The intima‐media complex on the distal wall of the carotid artery is defined as the distance from the leading edge of the lumen‐intima interface to the leading edge of the media‐adventitia interface, which are in parallel.
There are controversies regarding the definition of plaque and whether plaque thickness should be included in IMT. In Japan, several guidelines on carotid ultrasonography have been published, and all of them include plaque thickness in IMT. For example, the Japan Academy of Neurosonology7 recommends: (i) measuring carotid IMT at the common carotid artery, carotid sinus or the bifurcation of the common carotid artery and internal carotid artery as the thickness at the thickest point, including plaque (IMT‐Cmax, IMT‐Bmax and IMT‐Imax); (ii) recording the highest value among the three carotid IMT measurements as the maximum carotid IMT (max‐IMT); (iii) calculating the mean carotid IMT (mean‐IMT) as the mean value of the IMT values at the thickest point in the common carotid artery, and 1 cm distal and proximal from the thickest point; and (iv) handling all wall hyperplasias at a thickness of ≥1.1 mm as plaques (Figure 3).
Figure 3.
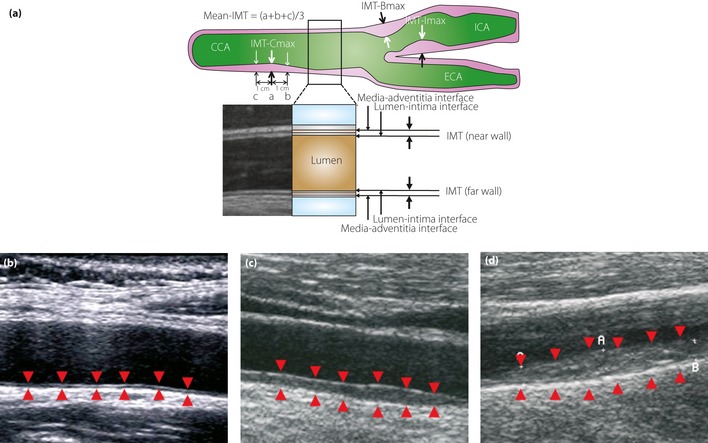
(a) The intima‐media thickness (IMT) is a double‐line pattern visualized by ultrasound on both walls of the carotid arteries. It is shown by two parallel lines that delineate the leading edges of two anatomical boundaries, the lumen‐intima and media‐adventitia interfaces. The Japan Academy of Neurosonology recommends: (i) measuring carotid IMT at the common carotid artery, carotid sinus or the bifurcation of the common carotid artery, and internal carotid artery as the thickness at the thickest point, including plaque (IMT‐Cmax, IMT‐Bmax and IMT‐Imax); (ii) recording the highest value among the three carotid IMT measurements as the maximum carotid IMT (max‐IMT); (iii) calculating the mean carotid IMT (mean‐IMT) as the mean value of the IMT values at the thickest point in the common carotid artery, and 1 cm distal and proximal from the thickest point; and (iv) handling all wall hyperplasias at a thickness of ≥1.1 mm as plaques. B‐mode views of (b) a normal carotid, (c) a carotid with moderate IMT thickening and (d) that with severe IMT thickening. Arrowheads indicate the lumen‐intima and media‐adventitia interfaces. CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery.
The guidelines proposed by the Japan Society of Ultrasonics in Medicine (JSUM)8 define plaques as ‘localized elevated lesions with maximum thickness of more than 1 mm, having a point of inflection on the surface of the intima‐media complex (IMC)', and, in cases of vascular remodeling, allow the term ‘plaques’ to be used irrespective of the presence/absence of elevation of the lesion into the vascular lumen. The JSUM recommends that plaques be included when measuring max IMT.
In a guideline document published in Europe9, plaque is defined as ‘a focal structure that encroaches into the arterial lumen by at least 0.5 mm or 50% of the surrounding IMT value or shows a thickness >1.5 mm as measured from the media‐adventitia interface to the intima‐lumen interface’. This guideline document emphasizes the importance of not including the plaque segment in the determination of IMT.
As an increase in IMT reflects not only the progression of atherosclerotic disease, but also non‐atherosclerotic compensatory enlargement of the carotid wall, it appears appropriate to assess IMT and plaque size separately. However, it is not always possible to differentiate early plaque formation from non‐atherosclerotic enlargement of the vascular wall on the basis of echographic findings alone. In addition, the maximum carotid IMT that is determined as the thickness of the thickest carotid wall including plaque is superior to the mean IMT in predicting CAD, and using IMT as the size of the carotid wall including plaque would be clinically significant.
Relationship Between Carotid IMT and Cardiovascular Diseases (Results of Cross‐Sectional Studies)
Coronary Artery Disease
In an analysis of data of 468 patients undergoing cardiac catheterization and B‐mode ultrasound of the carotid arteries, Wofford et al.10 showed that patients with more severe extracranial carotid artery atherosclerosis are several times more likely to have multiple vessel CAD. Hulthe et al.11 reported a significant correlation between the IMT of the carotid bulb and diameter stenosis of the included coronary segments (r = 0.68). Thus, the degree of carotid atherosclerosis correlates with that of coronary atherosclerosis, and the latter might be estimated to some extent by assessing the former. Recently, we carried out coronary CT angiography in 91 diabetic patients with carotid plaque (IMT ≥ 1.1 mm) to examine the relationship between the presence and extent of coronary atherosclerosis and carotid IMT, and found that more than 30% of the patients had ≥50% coronary stenosis, and max‐IMT was useful in distinguishing patients with coronary stenosis12. In another study of 241 patients with asymptomatic type 2 diabetes, we found that carotid IMT was an independent predictor of coronary stenosis, and a receiver operator characteristic (ROC) curve analysis for the prediction of the presence of coronary stenosis showed that the area under curve (AUC) increased significantly after adding max‐IMT to conventional coronary risk factors (from 0.64 to 0.74, P = 0.02013; Figure 4). These findings show that the addition of max‐IMT to conventional risk factors significantly improved the prediction ability of coronary atherosclerosis.
Figure 4.
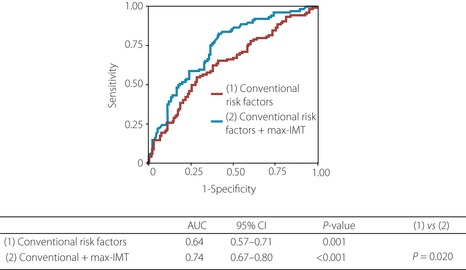
Receiver operator characteristic (ROC) curves for the prediction of the presence of coronary stenosis. ROC curves were plotted for conventional coronary risk factors, with and without maximum carotid intima‐media thickness (max‐IMT). This analysis showed that the area under curve (AUC) significantly increased after the addition of max‐IMT to conventional coronary risk factors (from 0.64 to 0.74, P = 0.020), showing that the addition of max‐IMT to conventional risk factors significantly improves the prediction ability of coronary atherosclerosis. Conventional risk factors include age, sex, smoking status, hypertension, dyslipidemia and albumin creatinine ratio. CI, confidence interval
In a study in healthy elderly individuals, Nagai et al.14 reported that those with ischemic ST‐segment depression on exercise ECG had a significant increase in carotid IMT, and each 0.1 mm increase in IMT was associated with a 1.91‐fold increased risk for concordant positive exercise tests or manifest CAD. In another study of patients with borderline glucose tolerance and those with type 2 diabetes, ischemic ECG changes were rare among patients with a carotid IMT of ≤1.0 mm, but were observed in approximately 10% of those with a carotid IMT of ≥1.1 mm15. These findings show that the risk of the presence of myocardial ischemia is higher in patients with higher carotid IMT.
In a cross‐sectional survey of 1,257 randomly selected middle‐aged men in Finland, Salonen et al.16 reported that for each 0.1 mm of common carotid IMT, the risk of acute myocardial infarction increased by 11%. O'Leary et al.17 also carried out a cross‐sectional study in which carotid ultrasound was undertaken in 5,201 men and women aged ≥65 years to determine maximum present stenosis, maximum common carotid IMT and maximum internal carotid IMT. They then assessed the relationships between these ultrasound measures, risk factors, and manifestations of coronary heart disease and stroke, and concluded that these ultrasound measures were associated with coronary heart disease and stroke. Based on a cross‐sectional study in 3,192 Japanese type 2 diabetic patients, we also confirmed that the prevalence of CAD was significantly higher in patients with a carotid IMT of ≥1.1 mm as compared with those with a carotid IMT of ≤1.0 mm (11.3% vs 5.2%, P < 0.0001). These data show a close association between carotid IMT and CAD.
We investigated the usefulness of carotid ultrasound in differentiating patients with coronary atherosclerosis or asymptomatic myocardial ischemia from those without. In our study18, 333 patients with asymptomatic type 2 diabetes with no history of CAD underwent carotid ultrasound, as well as either exercise ECG or myocardial perfusion scintigraphy to screen for CAD. Then, the patients with myocardial ischemia were subjected to coronary CT angiography or conventional coronary angiography, and were examined by cardiologists to determine whether revascularization was indicated according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines for percutaneous coronary intervention. Data were analyzed to assess the correlation between the presence of severe CAD warranting revascularization and carotid IMT, and to obtain a cut‐off level of maximum carotid IMT to predict severe CAD. The results shown that maximum carotid IMT is an independent predictor of the presence of severe CAD, and that the ability to predict severe CAD significantly increases when the maximum carotid IMT is added to conventional coronary risk factors; that is, age, sex, hypertension, dyslipidemia, smoking history and glycated hemoglobin (HbA1c; AUC of the ROC curve: 0.67 vs 0.79, P = 0.039), and that a cut‐off value of 2.45 mm for maximum carotid IMT yielded the best predictive value for severe CAD. These findings show that carotid IMT is useful in screening patients with asymptomatic type 2 diabetes for the presence of CAD.
Cerebral Infarction
In a study of 5,858 individuals aged ≥65 years without pre‐existing cardiovascular disease who lived in the local community, O'Leary et al.19 reported that an increase in carotid IMT is directly associated with an increased risk of stroke in older adults without a history of cardiovascular disease. Hougaku et al.20 assessed 117 individuals who had at least one established risk factor for stroke, and found that the prevalence of silent cerebral infarction was high in individuals with higher plaque score, more severe carotid stenosis or ulcerated carotid lesions.
Peripheral Artery Disease
In the Edinburgh Artery Study21, the presence of symptomatic or asymptomatic peripheral arterial disease was significantly associated with increased carotid IMT. In the Atherosclerosis Risk in Communities (ARIC) Study22, a prospective study in 1,651 diabetic patients, carotid IMT was an independent risk factor for developing peripheral arterial disease.
Carotid IMT as a Predictor of Cardiovascular Events
A number of longitudinal surveys have shown that carotid IMT is an independent predictor of cardiovascular events (Table 1). In a prospective study of 1,288 Finnish men to assess the relationship between ultrasonographically assessed carotid morphology and the risk of acute coronary events, Salonen et al.23 reported that the presence of any structural changes in the common carotid arteries or carotid bulbs was associated with a 3.29‐fold risk, intimal‐medial thickening (>1.0 mm) with a 2.17‐fold risk, small carotid plaques with a 4.15‐fold risk and stenotic plaques with a 6.71‐fold risk of acute coronary events. In the Rotterdam Study24 in which 1,373 individuals aged ≥55 years underwent carotid IMT measurements and were followed up for 2.7 years on average for the development of stroke and myocardial infarction, Bots et al. showed that the odds ratio for stroke per standard deviation increase (0.163 mm) in carotid IMT was 1.41 (41% increase in risk), and that for myocardial infarction the odds ratio was 1.43 (43% increase in risk). O'Leary et al.19 followed up 4,476 individuals aged ≥65 years for 6.2 years to assess the relationship between carotid IMT and the occurrence of cardiovascular events, and reported that the incidences of myocardial infarction and stroke were higher in individuals with increased carotid IMT. Chambless et al.25 and Hodis et al.26 have also reported similar results.
Table 1. Relative risk of myocardial infarction, stroke and cardiovascular disease associated with carotid intima‐media thickness in main prospective studies.
| Study [ref.] | No. participants | Sex/age | Measured IMT | Follow‐up time (event) | Relative risk (95% CI) [Hazard ratio for CIMT] |
|---|---|---|---|---|---|
| KIHD22 | 1,288 | M/42–60 | Max‐IMT (CCA) | 1.0 (MI) | 2.17 (0.70–6.74) [CIMT ≥1 vs <1 mm] |
| ROT23 | 1,373 | M/F/≥55 | Mean‐IMT (CCA) | 2.7 (MI) | 1.43 (1.16–1.78) [per 1SD (0.16 mm) CIMT]a |
| 2.7 (stroke) | 1.41 (1.25–1.82) [per 1SD (0.16 mm) CIMT]a | ||||
| CHS18 | 4,476 | M/F/≥65 | Max‐IMT (CCA) | 6.2 (MI) | 3.17 (1.96–5.12) [5th vs 1st CIMT quintile]a |
| 6.2 (stroke) | 2.76 (1.80–4.24) [5th vs 1st CIMT quintile]a | ||||
| ARIC24 | 5,552 | M/45–64 | Mean‐IMT (overall) | 5.2 (MI) | 1.85 (1.28–2.69) [>1 mm, yes vs no]b |
| 7,189 | F/45–64 | Mean‐IMT (overall) | 5.2 (MI) | 5.07 (3.08–8.36) [>1 mm, yes vs no]b | |
| 6,349 | M/45–64 | Mean‐IMT (overall) | 7.2 (stroke) | 1.98 (1.24–3.15) [>1 mm, yes vs no]b | |
| 7,865 | F/45–64 | Mean‐IMT (overall) | 7.2 (stroke) | 3.31 (1.88–5.81) [>1 mm, yes vs no]b | |
| Yoshida, et al.28 | 783 | M/F/30–75 (T2DM) | Mean‐IMT in CCA | 7.2 (CVD) | 2.39 (1.19–4.81) [per 1SD CIMT]a |
| Present study | 469 | M/F/≥25 (T2DM) | Max‐IMT (overall) | 6.1 (CVD) | 1.62 (1.32–2.00) [per 1SD CIMT]a |
ARIC, Atherosclerosis Risk in Communities; CCA, common carotid artery; CI, confidence interval; IMT; intima‐media thickness; CHS, Cardiovascular Health Study; CIMT, carotid intima‐media thickness; CVD, cardiovascular disease; F, female; KIHD, Kuopio Ischemic Heart Disease Study; M, male; MI, myocardial infarction; ROT, Rotterdam Study; SD, standard deviation; T2DM, type 2 diabetes.
Age and sex adjusted.
Age and race adjusted.
Regarding diabetic patients, Yamasaki et al.27 reported that carotid IMT at baseline was a strong predictor of the development of non‐fatal coronary heart disease in a study of 287 Japanese type 2 diabetic patients followed up for 3 years. We also confirmed that carotid IMT at baseline could be a predictor for the development of cardiovascular events in asymptomatic type 2 diabetic patients followed up for 6.1 years (Figure 5).
Figure 5.
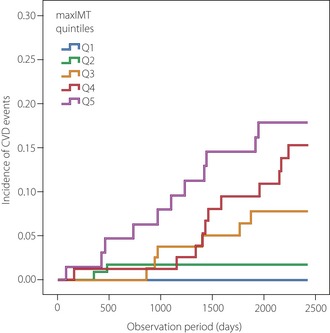
Kaplan–Meier curves showing the cumulative probability of cardiovascular events in Japanese type 2 diabetic patients. A total of 469 subjects were divided into five subgroups by quintiles (Q) according to their baseline carotid intima‐media thickness (max‐IMT) values (Q1: ≤0.8 mm, Q2: 0.9–1.1 mm, Q3: 1.2–1.4 mm, Q4: 1.5–1.9 mm, and Q5: ≥2.0 mm) and followed up for 6.1 years to assess the relationship between the baseline carotid IMT and occurrence of cardiovascular events. The risk for cardiovascular events was higher in patients with increased baseline max‐IMT (P < 0.001, log–rank test). CVD, cardiovascular disease.
Recently, Lorenz et al.28 carried out a systematic review and meta‐analysis to review data from 37,197 subjects who were followed up for a mean of 5.5 years in eight studies with general population‐based samples for which carotid IMT was measured. The age‐ and sex‐adjusted overall estimates of the relative risks of myocardial infarction and stroke were 1.26 (95% confidence interval [CI] 1.21–1.30) and 1.32 (95% CI 1.27–1.38), respectively, per 1‐standard deviation common carotid artery IMT difference, and 1.15 (1.12–1.17) and 1.18 (1.16–1.21), respectively, per 0.10‐mm common carotid artery IMT difference (Table 2). Even after adjustment for a complete set of conventional cardiovascular risk factors, carotid IMT was an independent predictor of future cardiovascular events. These results show that carotid IMT provides additional information that cannot be obtained based on the assessment of conventional cardiovascular risk factors alone. As the relative risk of stroke per IMT difference did not differ significantly from that of myocardial infarction, carotid IMT should be used as a non‐specific marker of systemic atherosclerosis rather than that of atherosclerotic complications in specific organs.
Table 2. Hazard ratio for myocardial infarction and stroke per 1 SD and 0.1 mm difference in common carotid artery intima‐media thickness adjusted for age and sex: results of a meta‐analysis.
| Myocardial infarction | Stroke | |
|---|---|---|
| IMT difference | (Hazard ratio [95% CI]) | (Hazard ratio [95% CI]) |
| +1SD IMT difference | 1.26 [1.21 –1.30] | 1.32 [1.27 –1.38] |
| +0.10 mm IMT difference | 1.15 [1.12 –1.17] | 1.18 [1.16 –1.21] |
CCA, common carotid artery; CI, confidence interval; IMT; intima‐media thickness; SD, standard deviation.
More recently, it has been reported that the combination of conventional risk factors for coronary vascular disease and carotid IMT improved the prediction of coronary vascular disease in diabetic patients, and that carotid IMT is useful in identifying patients at high risk for developing macrovascular complications of diabetes29. These results of prospective studies suggest that carotid IMT can be used to predict cardiovascular events.
Can We Use Carotid IMT as a Surrogate Marker of Cardiovascular Disease?
In a clinical study of a treatment for prevention of cardiovascular events, a large number of participants must be followed up for a long period of time when the occurrence of cardiovascular events is set as an end‐point. Such study requires a significant financial commitment. An appropriate surrogate marker might decrease the sample size and the study period. It is generally believed that the surrogate marker should have a pathophysiological relationship to the relevant clinical end‐point; should show good correlations with epidemiological evidence and endpoints; and the improvement in the surrogate marker after intervention should correlate well with the improvement in the relevant clinical end‐point after the same intervention30.
As carotid IMT is a non‐invasive, economical and quantitative measure, change over time in carotid IMT is a good candidate for a surrogate end‐point for cardiovascular events in clinical studies. A substantial quantity of evidence has been presented to show that carotid IMT satisfies the first two of the aforementioned conditions; however, sufficient evidence has not been accumulated regarding whether the progression of carotid IMT reflects an increased risk of subsequent cardiovascular events.
The following studies have provided valuable insights that should be shared for discussion of this matter. In a long‐term follow up (mean 8.8 years) of 146 male patients (age range 40–59 years) who previously had coronary artery bypass graft surgery and completed the 2‐year Cholesterol Lowering Atherosclerosis Study26, the risk for coronary events or coronary death was higher in patients with a higher progression of carotid IMT. In a meta‐analysis of seven placebo‐controlled clinical trials of statins reporting both IMT outcomes and cardiovascular events, the impact of statins on carotid IMT progression and cardiovascular end‐points were qualitatively similar31. Sabeti et al.32 followed up 1,065 patients with carotid stenosis for 3.2 years on average for the progression of carotid stenosis and the occurrence of major adverse cardiovascular events, and reported that patients with progressive carotid stenosis had a significantly increased risk for myocardial infarction, stroke and peripheral arterial events compared with patients with non‐progressive disease. These reports suggest that the progression of carotid IMT can be used as a surrogate end‐point of cardiovascular events.
There have been two meta‐analyses of clinical studies in which the relationship between the development of CAD and the progression of carotid IMT was investigated. One meta‐analysis of 28 randomized clinical trials with 15,598 patients has shown that less progression in carotid IMT over time is associated with a lower likelihood of non‐fatal myocardial infarction in selected randomized clinical trials33, whereas the other meta‐analysis of 41 randomized trials with 18,307 patients concluded that the regression or slowed progression of carotid IMT does not reflect a reduction in cardiovascular events34.
Recently, Lorenz et al.35 carried out an individual participant data meta‐analysis of participants of general population studies that assessed carotid IMT at least twice, and followed up participants for myocardial infarction, stroke or death. Of 21 eligible studies, 16 were included, with a total of 36,984 participants. During a mean follow up of 7 years, 1,519 myocardial infarctions, 1,339 strokes and 2,028 combined end‐points (myocardial infarction, stroke or vascular death) occurred. Yearly carotid IMT progression was derived from two ultrasound visits 2–7 years (median 4 years) apart. Unexpectedly, there were no associations between the progression of carotid IMT and the development of combined end‐points.
As aforementioned, the meta‐analysis of general population studies did not provide evidence supporting the usefulness of carotid IMT progression during several years as a surrogate marker of cardiovascular diseases. However, the yearly progression of carotid IMT was as small as approximately 0.01 mm/year in the general population, in which the risk of developing cardiovascular diseases is low. As carotid IMT is usually measured at a resolution of approximately 0.1 mm, it is likely that in observational studies of the general population, individual differences in the progression of carotid IMT during a few years are smaller than the measurement error. In contrast, it is expected that the progression of carotid IMT is several times higher in patients with a history of CAD and patients with diabetes or dyslipidemia than in the general population. Further studies should be carried out to investigate whether carotid IMT can be used as a surrogate end‐point of cardiovascular disease in these patient populations.
Diabetes and Carotid IMT
Diabetes as a Risk Factor of Carotid IMT Progression
Many cross‐sectional and longitudinal studies have already shown that conventional risk factors of atherosclerosis, such as aging, hypertension, hyperlipidemia, diabetes, obese, smoking and a history of atherosclerotic disorders, are risk factors of carotid IMT progression19.
Through a cross‐sectional survey, Yamasaki et al.15 have confirmed that carotid IMT in type 1 and type 2 diabetic patients was larger than that in age‐matched non‐diabetic individuals. As carotid IMT is higher in individuals with borderline glucose tolerance than in those with normal glucose tolerance, it is considered that atherosclerosis has already progressed in this population15. In a meta‐analysis of 23 studies in 24,111 participants, including 4,019 diabetic patients and 1,110 individuals with impaired glucose tolerance (IGT), the diabetic patients and individuals with IGT had greater carotid IMT than the control participants by 0.13 mm (95% CI 0.12–0.14 mm) and 0.04 mm (95% CI 0.014–0.071 mm), respectively41. We carried out a meta‐analysis of eight intervention studies in subjects with type 2 diabetes that evaluated the effect of interventions on change in carotid IMT, and found that the overall weighed rate of change in mean‐IMT based on data among control groups (i.e., type 2 diabetes without interventions) was 0.034 mm/year (95% CI 0.029–0.039)42. As it has been reported that the annual increase in carotid IMT in healthy populations is 0.007–0.008 mm, carotid IMT progression is substantial among patients with uncontrolled diabetes.
Reduction of Carotid IMT Progression by Antidiabetic Drugs
The development and progression of atherosclerosis in diabetic patients are significantly affected by: (i) metabolic abnormalities associated with chronic hyperglycemia; (ii) a complex of risk factors related to insulin resistance and visceral fat accumulation; and (iii) postprandial hyperglycemia.
It has been reported that carotid IMT correlates with various markers that reflect various metabolic abnormalities caused by chronic hyperglycemia. In the aforementioned meta‐analysis in eight studies42, a close correlation between HbA1c and carotid IMT was observed during the follow‐up periods, which suggests that carotid IMT progression is reduced when glycemic control is achieved.
In accordance with the fact that insulin resistance plays an important role in the progression of atherosclerosis in diabetic patients, a large number of reports have shown that drugs improving insulin resistance are more effective in reducing carotid IMT progression as compared with other types of anti‐diabetic drugs. Metformin, a biguanide derivative drug43, and pioglitazone, a thiazolidinedione derivative45, reduce carotid IMT progression as compared with sulfonylurea drugs.
It has also been reported that 2‐h post‐challenge plasma glucose and maximal plasma glucose levels during an oral glucose tolerance test are more strongly associated with carotid IMT than fasting plasma glucose levels48, and that carotid IMT progression was greater in patients with larger incremental glucose peaks in subgroups of all tertiles of HbA1c level49. These findings suggest that postprandial hyperglycemia plays an important role in accelerating carotid IMT progression. Indeed, it has been reported that α‐glucosidase inhibitors50 and glinides51 that ameliorate postprandial hyperglycemia can attenuate carotid IMT progression.
Thus, the drugs that ameliorate insulin resistance, as well as postprandial hyperglycemia, are more effective in reducing carotid IMT progression as compared with other types of antidiabetic drugs, suggesting that these drugs could potently inhibit the progression of atherosclerosis accelerated in type 2 diabetic patients (Figure 6).
Figure 6.

Strategy for preventing the progression of atherosclerosis in diabetic patients. The development and progression of atherosclerosis in diabetic patients are significantly affected by: (i) metabolic abnormalities associated with chronic hyperglycemia; (ii) a complex of risk factors related to insulin resistance and visceral fat accumulation; and (iii) postprandial hyperglycemia. Previous studies showed that drugs improving insulin resistance were more effective in reducing carotid intima‐media thickness (IMT) progression as compared with other types of antidiabetic drugs, and that drugs that ameliorate postprandial hyperglycemia potently attenuated carotid IMT progression. Besides being a useful marker of the progression of subclinical atherosclerosis and a good predictor of cardiovascular events, carotid IMT can be used to evaluate the efficacy of various treatments. CVD, cardiovascular disease; US; ultrasonography.
Conclusions
Carotid IMT is a useful marker of the progression of atherosclerosis throughout the body, and is an excellent predictor of cardiovascular events. As a simple and non‐invasive procedure, measurement of carotid IMT is one of the most appropriate screening methods to specify high‐risk individuals in the community (Table 3). Furthermore, carotid IMT can be used to identify unknown risk factors of atherosclerosis and to evaluate the efficacy of new treatments (Figure 7). Therefore, it is expected that carotid IMT measurement will become a more common procedure.
Table 3. Characteristics of major functional/morphological markers of atherosclerosis.
| Carotid IMT | Coronary artery calcium | FMD | PWV | |
|---|---|---|---|---|
| Predictive ability | Good | Pretty good | Relatively good | Relatively good |
| Safety | Very safe | Relatively safe | Safe | Very safe |
| Convenience | Convenient | Complicated | Complicated | Very convenient |
| Reproducibility | Good | Good | Relatively good | Relatively good |
| Cost | Low | High | Low | Low |
| AHA guideline's comments |
Benefit >> risk (class IIa) |
Benefit >> risk (class IIa) |
No benefit (class III) |
No benefit (class III) |
AHA, American Heart Association; FMD, flow‐mediated vasodilation; IMT; intima‐media thickness; PWV, pulse wave velocity.
Figure 7.
The utility of carotid intima‐media thickness (IMT) as an index of atherosclerosis.
Acknowledgements
NK is a staff member of the endowed chair (the Department of Metabolism and Atherosclerosis) donated by Kowa Pharmaceutical Co. Ltd.
J Diabetes Invest 2014; 5: 3–13
References
- 1.Nesto RW, Phillips RT. Asymptomatic myocardial ischemia in diabetic patients. Am J Med 1986; 80: 40–47 [DOI] [PubMed] [Google Scholar]
- 2.Wackers FJ, Young LH, Inzucchi SE, et al Detection of Ischemia in Asymptomatic Diabetics Investigators the DIAD study. Diabetes Care 2004; 27: 1954–1961 [DOI] [PubMed] [Google Scholar]
- 3.van Dieren S, Peelen LM, Nöthlings U, et al External validation of the UK prospective diabetes study (UKPDS) risk engine in patients with type 2 diabetes. Diabetologia 2011; 54: 264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmons RK, Coleman RL, Price HC, et al Performance of the UK prospective diabetes study risk engine and the Framingham risk equations in estimating cardiovascular disease in the EPIC‐ Norfolk cohort. Diabetes Care 2009; 32: 708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephens JW, Ambler G, Vallance P, et al Cardiovascular risk and diabetes. Are themethods of risk prediction satisfactory? Eur J Cardiovasc Prev Rehabil 2004; 11: 521–528 [DOI] [PubMed] [Google Scholar]
- 6.Pignoli P, Tremoli E, Poli A, et al Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986; 74: 1399–1406 [DOI] [PubMed] [Google Scholar]
- 7.Joint committee with the guidelines subcommittee of the Japan Academy of Neurosonology for ultrasonic assessment of carotid artery disease and the subcommittee for research into methods of screening atherosclerotic lesions . Guidelines for ultrasonic assessment of carotid artery disease: preliminary report. Neurosonology 2002; 15: 20–33 (Japanese). [Google Scholar]
- 8.Terminology and Diagnostic Criteria Committee, Japan Society of Ultrasonics in Medicine. Subcommittee for Preparing Guidelines for Ultrasound Diagnosis of Carotid Artery . Standard method for ultrasound evaluation of carotid artery lesions. Jpn J Med Ultrasonics 2009; 36: 501–518 [Google Scholar]
- 9.Touboul PJ, Hennerici MG, Meairs S, et al Mannheim carotid intima‐media thickness consensus (2004–2006). Cerebrovasc Dis 2007; 23: 75–80 [DOI] [PubMed] [Google Scholar]
- 10.Wofford JL, Kahl FR, Howard GR, et al Relation of extent of extracranial carotid artery atherosclerosis as measured by B‐mode ultrasound to the extent of coronary atherosclerosis. Arterioscler Thromb 1991; 11: 1786–1794 [DOI] [PubMed] [Google Scholar]
- 11.Hulthe J, Wikstrand J, Emanuelsson H, et al Atherosclerotic changes in the carotid artery bulb as measured by B‐mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke 1997; 28: 1189–1194 [DOI] [PubMed] [Google Scholar]
- 12.Kasami R, Kaneto H, Katakami N, et al Relationship between carotid intima‐media thickness and the presence and extent of coronary stenosis in type 2 diabetic patients with carotid atherosclerosis but without history of coronary artery disease. Diabetes Care 2011; 34: 468–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irie Y, Katakami N, Kaneto H, et al Maximum carotid intima‐media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis 2012; 221: 438–444 [DOI] [PubMed] [Google Scholar]
- 14.Nagai Y, Metter EJ, Earley CJ, et al Increased carotid artery intimal‐medial thickness in asymptomatic older subjects with exercise‐induced myocardial ischemia. Circulation 1998; 98: 1504–1509 [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki Y, Kwamori R, Matsushima H, et al Asymptomatic hyperglycaemia is associated with increased intimal plus medial thickness of the carotid artery. Diabetologia 1995; 38: 585–591 [DOI] [PubMed] [Google Scholar]
- 16.Salonen JT, Salonen R. Ultrasound B‐mode imaging in observational studies of atherosclerotic progression. Circulation 1993; 87(Suppl II): II56–II65 [PubMed] [Google Scholar]
- 17.O'Leary DH, Polak JF, Kronmal RA, et al Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke 1992; 23: 1752–1760 [DOI] [PubMed] [Google Scholar]
- 18.Irie Y, Katakami N, Kaneto H, et al The utility of carotid ultrasonography in identifying severe coronary artery disease in asymptomatic type 2 diabetes patients without history of coronary artery disease. Diabetes Care 2013; 36: 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Leary DH, Polak JF, Kronmal RA, et al Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340: 14–22 [DOI] [PubMed] [Google Scholar]
- 20.Hougaku H, Matsumoto M, Handa N, et al Asymptomatic carotid lesions and silent cerebral infarction. Stroke 1994; 25: 566–570 [DOI] [PubMed] [Google Scholar]
- 21.Allan PL, Mowbray PL, Lee AJ, et al Relationship between carotid intima‐media thickness and symptomatic and asymptomatic peripheral arterial disease. The Edinburgh Artery Study. Stroke 1997; 28: 348–353 [DOI] [PubMed] [Google Scholar]
- 22.Wattanakit K, Folsom AR, Selvin E, et al Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2005; 180: 389–397 [DOI] [PubMed] [Google Scholar]
- 23.Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Atheroscler Thromb 1991; 11: 1245–1249 [DOI] [PubMed] [Google Scholar]
- 24.Bots ML, Hoes AW, Koudstaal PJ, et al Common carotid intima‐media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997; 96: 1432–1437 [DOI] [PubMed] [Google Scholar]
- 25.Chambless LE, Heiss G, Folsom AR, et al Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol 1997; 146: 483–494 [DOI] [PubMed] [Google Scholar]
- 26.Hodis HN, Mack WJ, Labree L, et al The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med 1998; 128: 262–269 [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki Y, Kodama M, Nishizawa H, et al Carotid intima‐media thickness in Japanese type 2 diabetic subjects: predictors of progression and relationship with incident coronary heart disease. Diabetes Care 2000; 23: 1310–1315 [DOI] [PubMed] [Google Scholar]
- 28.Lorenz MW, Markus HS, Bots ML, et al Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation 2007; 115: 459–467 [DOI] [PubMed] [Google Scholar]
- 29.Yoshida M, Mita T, Yamamoto R, et al Combination of the Framingham risk score and carotid intima‐media thickness improves the prediction of cardiovascular events in patients with type 2 diabetes. Diabetes Care 2011; 35: 178–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx 2004; 1: 189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espeland MA, O'Leary DH, Terry JG, et al Carotid intimal‐media thickness as a surrogate for cardiovascular disease events in trials of HMG‐CoA reductase inhibitors. Curr Control Trials Cardiovasc Med 2005; 10: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabeti S, Schlager O, Exner M, et al Progression of carotid stenosis detected by duplex ultrasonography predicts adverse outcomes in cardiovascular high‐risk patients. Stroke 2007; 38: 2887–2894 [DOI] [PubMed] [Google Scholar]
- 33.Goldberger ZD, Valle JA, Dandekar VK, et al Are changes in carotid intima‐media thickness related to risk of nonfatal myocardial infarction? A critical review and meta‐regression analysis. Am Heart J 2010; 160: 701–714 [DOI] [PubMed] [Google Scholar]
- 34.Costanzo P, Perrone‐Filardi P, Vassallo E, et al Does carotid intima‐media thickness regression predict reduction of cardiovascular events? A meta‐analysis of 41 randomized trials. J Am Coll Cardiol 2010; 56: 2006–2020 [DOI] [PubMed] [Google Scholar]
- 35.Lorenz MW, Polak JF, Kavousi M, et al Carotid intima‐media thickness progression to predict cardiovascular events in the general population (the PROG‐IMT collaborative project): a meta‐analysis of individual participant data. Lancet 2012; 379: 2053–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salonen R, Salonen JT, et al Determinants of carotid intima‐media thickness: a population‐based ultrasonography study in eastern Finnish men. J Intern Med 1991; 229: 225–231 [DOI] [PubMed] [Google Scholar]
- 37.Heiss G, Sharrett RA, Barnes R, et al Carotid atherosclerosis measured by B‐mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC Study. Am J Epidemiol 1991; 134: 250–256 [DOI] [PubMed] [Google Scholar]
- 38.Mannnami T, Konishi M, Baba S, et al Prevalence of asymptomatic carotid atherosclerotic lesions detected by high‐resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: the Suita study. Stroke 1997; 28: 518–525 [DOI] [PubMed] [Google Scholar]
- 39.Okada M, Miida T, Hama H, et al Possible risk factors of carotid artery atherosclerosis in the Japanese population: a primary prevention study in non‐diabetic subjects. Intern Med 2000; 39: 362–368 [DOI] [PubMed] [Google Scholar]
- 40.Wagenknecht LE, D'Agostino RB, Haffner SM, et al Impaired glucose tolerance, Type 2 diabetes, and carotid wall thickness. The insulin resistance atherosclerosis study. Diabetes Care 1998; 21: 1812–1818 [DOI] [PubMed] [Google Scholar]
- 41.Brohall G, Oden A, Fagerberg B. Carotid artery intima‐media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2005; 23: 609–616 [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama H, Katakami N, Yamasaki Y. Recent advances of intervention to inhibit progression of carotid intima‐media thickness in patients with type 2 diabetes mellitus. Stroke 2006; 37: 2420–2427 [DOI] [PubMed] [Google Scholar]
- 43.Katakami N, Yamasaki Y, Hayaishi‐Okano R, et al Metformin or gliclazide, rather than glibenclamide, attenuate progression of carotid intima‐media thickness in subjects with type 2 diabetes. Diabetologia 2004; 47: 1906–1913 [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto K, Sera Y, Abe Y, et al Metformin attenuates progression of carotid arterial wall thickness in patients with type 2 diabetes. Diabetes Res Clin Pract 2004; 64: 225–228 [DOI] [PubMed] [Google Scholar]
- 45.Koshiyama H, Shimono D, Kuwamura N, et al Inhibitory effect of pioglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab 2001; 86: 3452–3456 [DOI] [PubMed] [Google Scholar]
- 46.Davidson M, Meyer PM, Haffner S, et al Increased high‐density lipoprotein cholesterol predicts the pioglitazone‐mediated reduction of carotid intima‐media thickness progression in patients with type 2 diabetes mellitus. Circulation 2008; 117: 2123–2130 [DOI] [PubMed] [Google Scholar]
- 47.Yamasaki Y, Katakami N, Furukado S, et al Long term effects of pioglitazone on carotid atherosclerosis in Japanese patients with type 2 diabetes without a recent history of macrovascular morbidity. J Atheroscler Thromb 2010; 17: 1132–1140 [DOI] [PubMed] [Google Scholar]
- 48.Temelkova‐Kurktschiev TS, Koehler C, Henkel E, et al Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000; 23: 1830–1834 [DOI] [PubMed] [Google Scholar]
- 49.Esposito K, Ciotla M, Carleo D, et al Post‐meal glucose peaks at home associate with carotid intima‐media thickness in type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 1345–1350 [DOI] [PubMed] [Google Scholar]
- 50.Yamasaki Y, Katakami N, Hayaishi‐Okano R, et al Alpha‐glucosidase inhibitor reduces the progression of carotid intima‐media thickness. Diabetes Res Crin Pract 2005; 67: 204–210 [DOI] [PubMed] [Google Scholar]
- 51.Esposito K, Giugliano D, Nappo F, et al Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004; 110: 214–219 [DOI] [PubMed] [Google Scholar]
- 52.Mita T, Watada H, Shimizu T, et al Nateglinide reduces carotid intima‐media thickening in type 2 diabetic patients under good glycemic control. Arterioscler Thromb Vasc Biol 2007; 27: 2456–2462 [DOI] [PubMed] [Google Scholar]


