Abstract
Aims/Introduction
Impaired growth and premature death of β‐cells are implicated in the progression of islet pathology in type 2 diabetes. It remains unclear, however, how aging affects islet cells, or whether the islet change in diabetes is an augmented process of aging. We studied age‐related changes of the islet structure in Japanese non‐diabetic subjects and explored the underlying mechanism of the changes.
Materials and Methods
A total of 115 non‐diabetic autopsy cases were subjected to morphometric analysis for volume densities of islets, β‐ and non‐β‐cells, as well as their masses. Proliferation activity identified by Ki67, and expressions of pancreatic and duodenal homeobox (PDX)‐1, cell cycle inhibitor P16, and oxidative stress marker γH2AX were also examined.
Results
There was a gradual and marginal decline of volume densities of islets, β‐ and non‐β‐cells with aging, while masses of these components were increased during maturation and slowly decreased after the 40s. Islet density was high in the young, but reduced after maturation. There was only a minimal influence of increased body mass index (BMI) on the increase in β‐cell mass, but not on the other variables. Ki67 positivity and PDX‐1 expressions were high in the young, but low after maturation, whereas expressions of P16 and γH2AX were elevated in the aged.
Conclusions
Age‐associated decline of β‐cell mass is marginal after maturation, and the reduction of β‐cell mass could be a specific process in diabetes. The impact of BMI on the islet structure is limited in Japanese with normal glucose tolerance.
Keywords: β‐Cell turnover, Aging, Islet structure
Introduction
Type 2 diabetes has become a recent epidemic. With prolongation of life expectancy, the prevalence of diabetes is increased with aging1. Recent studies disclosed selective reduction of β‐cell mass or its volume density in type 2 diabetic patients and subjects with impaired fasting glucose3. Thus, there is a progressive decline of β‐cells during development of type 2 diabetes. Nevertheless, the question why aging is associated with an increased incidence of diabetes is not fully answered. Whether the β‐cell decrease in older diabetic patients is ascribed to increased insulin resistance in peripheral tissues or direct gluco‐ or lipotoxicity to senescent β‐cells is not clear. It could also be speculated that the aged β‐cells become more fragile to environmental insults, resulting in premature death. The phenotypic alterations of islet endocrine cells with aging are, however, yet to be addressed.
In previous studies, β‐cell mass was shown to increase until young adulthood7, and gradually decreased thereafter6. It was recently found that obesity was associated with expansion of the β‐cell mass4. It is still open to question, however, whether the change in obesity is dependent on the increased pancreas volume or reflection of compensatory hyperplasia related to insulin resistance. Butler et al.4 found more robust β‐cell depletion in obese diabetic patients than lean diabetic patients when compared with respective weight‐matched non‐diabetic subjects. The reason for the difference in the extent of β‐cell loss between obese and lean diabetic patients was not well explained in that study. There is a possibility that the plasticity of β‐cells or genesis of diabetes is different between obese and lean subjects. Alternatively, other factors, such as ethnicity or lifestyle, could be involved in β‐cell dynamic changes. As the average body mass index (BMI) of Japanese type 2 diabetes (22–25) is much different from that of American or European diabetic patients (mostly >30), information on the changes of β‐cells in Japanese subjects is valuable for comparison with Westerners.
There is an increasing demand to know the value of islet or β‐cell mass for the prediction of diabetes severity and for the validation of new diabetes treatment. To this end, body imaging of the islet is also being explored10. Consequently, background data on the islet distribution, its density, size, endocrine cell turnover, and proliferation capacity are essential to the comparison between diabetes and their controls. In the present study, we therefore carried out precise morphometric analysis on the islet structure with special interest in the effect of aging and BMI, and explored the phenotypic differences in the islets between young and old subjects.
Materials and Methods
Participants
A total of 115 non‐diabetic subjects were collected and the pancreatic specimens were obtained from the subjects who did not have an apparent history of diabetes themselves, or their family history, or previous evidence of hyperglycemia (Table S1). The cases with a history of parenteral alimentation or continuous therapies with potential influences on glucose intolerance, such as steroid or cyclosporine, were also excluded. For inclusion in the present study, the cases were required to have: (i) had a full autopsy within 5 h of death; and (ii) pancreatic tissues stored at full size and a good quality. The samples with lesions of strong inflammation of acute pancreatitis or neoplasms were excluded. Samples undergoing autolysis were also excluded. The use of paraffin blocks was approved by the ethics committee of the Hirosaki University Graduate School of Medicine, and the study conforms to the provision of the Declaration of Helsinki.
Pathological and Immunohistochemical Evaluation
After resection, the pancreas was carefully trimmed of adherent fat and mesenchymal tissue, and weighed. Entire blocks of the body and the tail were fixed in 4% formaldehyde solution, and embedded in paraffin. From the paraffin blocks, several consecutive cross 5‐μm thick sections were obtained. The first section was stained with hematoxylin–eosin (HE) to assess the quality or integrity of the pancreatic tissue. The following serial sections were immunostained by classical streptavidin–biotin–peroxidase system (Nichirei Co., Tokyo, Japan), first incubated overnight at 4°C with monoclonal antibody to chromogranin A (1:1000; Dako Cytomation, Glostrup, Denmark) for the determination of islet volume density, monoclonal anti‐insulin antibody (1:2000; Santa Cruz Biotech, Inc., Santa Cruz, CA, USA) for volume density of β‐cells, and cocktail polyantibodies of glucagon (1:3000; Dako), somatostatin (1:2000; Dako) and pancreatic polypeptide (1:3000; Life Tech. Corp., Carlsbad, CA, USA) for volume density of non‐β‐cells. The sections were lightly counterstained with nuclear hematoxylin to count the number of nuclei of endocrine cells.
To explore cell death, proliferation rate, insulin synthetic activity or cellular senescence, double immunostainings with anti‐insulin with terminal deoxynucleotidyl transferase dUTP nick endlabeling (TUNEL) of an ApopTag® (Millipore, Bellerica, MA, USA), Ki67 (MIB1) (1:2500; Dako), β‐cell transcription factor (pancreatic and duodenal homeobox 1 (PDX‐1), 1:2500, EPR3358; Epitomics, Burlingame, CA, USA)12, or cyclin‐dependent kinase inhibitor P16 (1:500, F‐12; Santa Cruz)14 were carried out, respectively. To estimate the degree of oxidative stress‐related cell damage as a sign of preapoptotic state, the expression of immunoreactive γH2AX (Ser139, 1:100, JBW301; Millipore)16 were examined. Briefly, the sections were first incubated with TUNEL agents (ApopTag®) or antibodies against Ki67, PDX‐1, P16, H2AX and then antibody to insulin. After incubation with secondary antibody and enhancing agents, the reaction products were visualized with diaminobenzidine18.
Morphometric Analysis
For determination of islet area, the fractional β‐cell area and non‐β‐cell area, the entire pancreatic sections were imaged at ×5 and ×40 magnification (×4 objective), respectively. The ratio of the islet area (islet volume density [Vi]), β‐cell area (β‐cell volume density [Vβ]) and non‐β‐cell area (volume density of non‐β‐cell [Vnβ]) to total parenchymal area including exocrine pancreas, interstitial stromal and fatty tissues was digitally quantified at a point count basis as previously described using Image J (version 1.56; NIH, Bethesda, MD, USA)18. The masses of islets (Mi), β‐cell (Mβ) and non‐β‐cell (Mnβ) were obtained by multiplication of Vi, Vβ, and Vnβ by pancreas weight. The islet density per unit area (population of the islet) was obtained by division of the number of chromogranin A‐positive area by total parenchymal area. Average islet size and β‐cell size were obtained by division of the total islet area or β‐cell area by the number of islets or the number of β‐cells, respectively9.
To determine β‐cell growth, double‐positive cells with insulin and Ki67 (among over 2,000 β‐cells) in all the islets in the section were examined, and the number of Ki67‐positive cells expressed in terms of cells per respective β‐cells. For determination of apoptotic cells, we used slides double‐stained for insulin and TUNEL. As apoptotic cells were rarely found, we did not quantify the number because of unfeasibility to statistics. Intensities of immunoreactions of PDX‐1, P16 and γH2AX were semiquantified in each case using at least 100 islets larger than 200 μm in size. Each islet's reaction was determined to be negative (no reaction), 1+ positive (weakly positive with negative reaction in exocrine areas), 2+ (clearly positive) and 3+ (strongly positive). Average scores represented the value of individual subjects.
Statistical Analysis
Data are presented as mean ± standard error of the mean. Statistical comparisons were carried out using one‐way anova with Bonnferroni post‐hoc corrections. A simple regression was carried out for the correlation analysis. P‐values <0.05 were taken as significant (StatView version 5.0.1; SAS Institute, Mountain View, CA, USA).
Results
Subject Demography
A total of 115 cases (49 male, 66 female) among 670 autopsy cases procured from our autopsy files at Hirosaki University Hospital, Hirosaki, Japan, and related hospitals fulfilled our inclusion criteria. Other cases excluded were late autopsies showing autolysis, signs of pancreatitis, history of diabetes, glucose intolerance, steroid use, parenteral alimentation or cachexia as a result of malignancy (Table S1). The number of cases in each decade of age was 11 in 0–9 years, 17 in 10–19 years, 22 in 20–29 years, ten in 30–39 years, 21 in 40–49 years, ten in 50–59 years, 16 in 60–69 years and eight in 70–79 years. The average age was 58 years in males and 59 years in females. Original diseases were cardiovascular, liver, digestive diseases, amyloidosis, renal disease and psychiatric disorders. BMI was increased with maturation and reduced after 60 year‐of‐age. Average BMI was 13.7 until 20 years‐of‐age, and 24.6 in the 40s and 50s. The overall average BMI was 20.0 ± 3.8. As the BMI values were much lower in the young than those in the adults and aged, the effects of BMI on the morphometric parameters of the islets were evaluated in the subjects aged more than 20 years in the following text.
The mean pancreas weight rapidly increased until the third decade of life (Figure 1a). Thereafter, it was constant until the 50s, and then slightly reduced in the 60s and 70s. There was a strong correlation of increased pancreas weight with BMI (r = 0.46, P < 0.001; Figure 1b).
Figure 1.
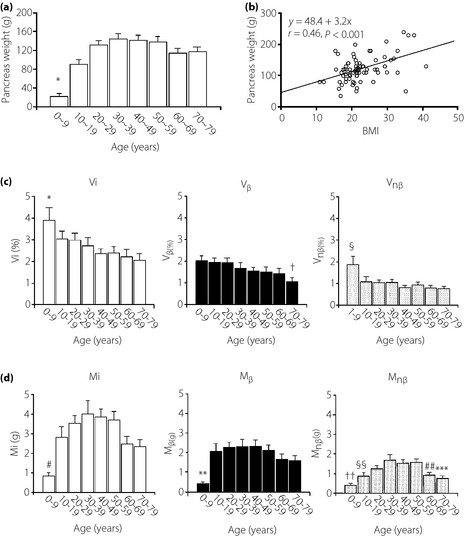
Age‐related changes of pancreas weight and the effect of body mass index (BMI) on the weight. (a) Pancreas weight increases with maturation until the third decade of life and remains constant until the 50s. Thereafter, the weight decreases slightly (P < 0.01 vs 10–70). (b) With increasing BMI, pancreas weight also increases. There is a strong correlation between pancreas weight and BMI. (c) By morphometric analysis, average volume densities of islets (Vi), β‐cells (Vβ) and non‐β‐cells (Vnβ) in each decade of life are shown. Islet volume density (Vi) of the first decade of life is significantly greater compared with those in later life (*P < 0.05), suggesting late development of the exocrine pancreas. Thereafter, Vi is preserved, but slowly and minimally declines with aging (see also Figure S2). Similarly, Vβ gradually and marginally decreases with aging, but is relatively well preserved (†P < 0.05 vs first and second decade). In the young, Vnβ is relatively greater compared with those aged older than 10 years (§P < 0.01 vs 10–79). (d) In contrast to volume density, total islet mass (Mi) increases with maturation until the 30s, and thereafter gradually decreases (#P < 0.01 vs 10–59, P < 0.05 vs 60–79). The peak was found from the 20s to the 50s. β‐Cell mass (Mβ) also increases with maturation until the 20s, and is thereafter maintained until the 50s and then gradually declines, but is still preserved (**P < 0.01 vs 10–69, P < 0.05 vs 70–79). Non‐β‐cell mass (Mnβ) also increases with maturation, but slightly later than β‐cells and after the 50s it declines slightly (††P < 0.01 vs 20–50 years, §§P < 0.01 vs 30 years, P < 0.05 vs 40–50 years, ##P < 0.05 vs 30–49 years and 70–79 years, ***P < 0.01 vs 30–39 years, P < 0.05 vs 40–59 years).
Although small to large islets were randomly and densely distributed in pancreatic parenchyma where the exocrine pancreas was not fully developed in the young, large and relatively uniform islets were sparsely distributed in the adult pancreas (Figure S1).
Vi was markedly high in the first decade, and there was a mild and gradual decline of Vi with aging (Figure 1c). This alteration was in parallel with the age‐associated changes of Vnβ. Vβ also slowly and progressively declined with aging, and reduction was marked in the 70s. Correlation analysis confirmed statistical significance (Figure S2). Mi and Mnβ were stepwisely increased until the fourth decade of life, and then there appeared to show a gradual decline. Mβ was low in the first decade of life, and rapidly expanded in the second decade and was well preserved thereafter (Figure 1d). The high values of Vi, Vβ and Vnβ in the young reflect the slower development of the exocrine pancreas during maturation. There were no differences in these variables between males and females.
Islet density was high in the first decade, but after maturation it was stable (Table 1). Islets composed of less than four cells (small islets termed neogenetic islets) followed a similar trend, whereas large islets were well preserved even in the aged subjects. Conversely, β‐cell occupancy in the islet was low in the first decade, but thereafter constantly exceeded 60%. Average β‐cell size was increased with maturation and further increased with aging. In contrast, β‐cell density was high in the young, and gradually declined with aging. A size frequency histogram of the islets showed left‐sided distribution, indicating a greater population of small islets (<250 μm2) in the young and a gradual reduction of small islets with aging (Figure 2a). Large islets exceeding 10,000 μm2 were most prevalent in the fourth decade. The density of neogenetic islets correlated with Vi (r = 0.27, P < 0.01; Figure 2b).
Table 1. Numerical data of islet density, occupancy of β‐cells in the islet, β‐cell size and β‐cell density.
| Age (years) | 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 |
|---|---|---|---|---|---|---|---|---|
| Islet density, n (/mm2) | 9.0 ± 1.6* | 4.4 ± 0.4 | 4.2 ± 0.5 | 3.6 ± 0.5 | 3.4 ± 0.4 | 3.2 ± 0.5 | 3.5 ± 0.3 | 3.4 ± 0.5 |
| Small islet density (/mm2) | 5.3 ± 1.3* | 2.7 ± 0.3 | 2.5 ± 0.4 | 2.1 ± 0.4 | 2.0 ± 0.3 | 2.0 ± 0.4 | 1.9 ± 0.2 | 1.9 ± 0.2 |
| Large islet density (/mm2) | 3.7 ± 0.6* | 1.7 ± 0.2 | 1.7 ± 0.1 | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.3 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.3 |
| β‐Cell occupancy in islet (%) | 54.4 ± 3.5 | 65.8 ± 3.1† | 65.6 ± 1.7† | 64.9 ± 2.6** | 66.0 ± 2.5† | 63.1 ± 2.3** | 63.0 ± 2.1** | 60.2 ± 3.9 |
| β‐Cell size (μm2) | 83.3 ± 11.2 | 100.5 ± 7.3 | 111.6 ± 5.8** | 120.1 ± 6.4** | 119.4 ± 11.4** | 125.1 ± 11.1† | 126.0 ± 8.5** | 129.4 ± 12.0† |
| β‐Cell number (/mm2) | 152.3 ± 24.8 | 101.2 ± 7.6** | 91.0 ± 4.4† | 83.7 ± 4.3† | 86.2 ± 8.8† | 85.9 ± 6.8† | 82.9 ± 6.7† | 79.3 ± 8.9† |
*P < 0.01 vs 10 –79 years, †P < 0.01 vs 0–9 years, **P < 0.05 vs 0–9 years.
Figure 2.
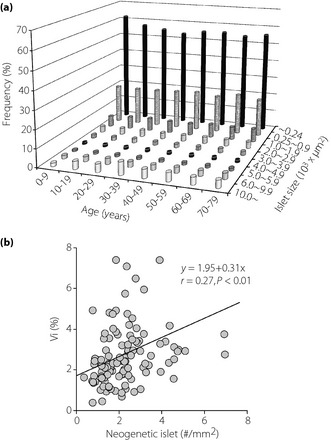
Frequency histogram of the islet size and number, and changes with aging. (a) Small islets (<1,000 μm2) occupy more than 80% of all islets. Larger islets (>10,000 μm2) were relatively marked during the 30s to 40s. Middle‐sized islets (3,000–5,999 μm2) were constant throughout life. In juveniles, most of the islets were small, mainly <2,000 μm2. During maturation, the number of islets per unit area was much reduced until the 20s and remained constant thereafter. With aging, the number of small islets was decreased and there was a gradual increase in larger islets with maturation. (b) The number of small neogenetic islets per unit area is well correlated with islet volume density (Vi).
In contrast to the positive correlation of pancreas weight with BMI, there was no significant effect of BMI on the value of Vi, Vβ and Vnβ (Figure 3a). Similarly, BMI did not significantly influence the values of Mi, Mβ and Mnβ, despite near significant correlation between BMI and Mβ (r = 0.23, P = 0.056; Figure 3b). The separation of groups into an obese group (BMI ≥ 25) and lean group (BMI < 25) yielded a marginally significant difference in the Mi and Mβ (P < 0.05 for both), but not Vi, Vβ, Vnβ or Mnβ between these two groups (Figure 3c). In addition, there was no significant effect of BMI on the density of neogenetic islets (small islets <4 cells; data not shown).
Figure 3.
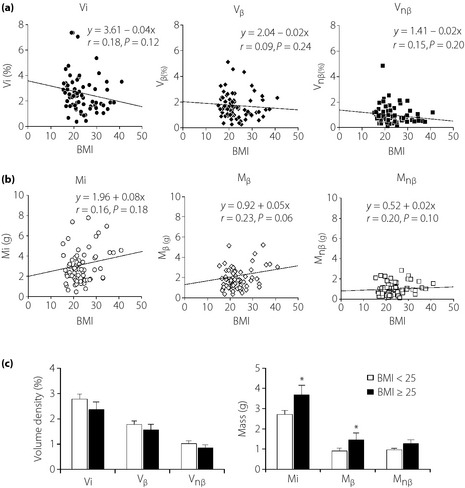
The relationship between body mass index (BMI) and volume densities of islets (Vi), β‐cells (Vβ) and non‐β‐cells (Vnβ), and their masses (M). (a) There is no significant correlation between BMI and Vi, Vβ or Vnβ. (b) Similarly, BMI does not have a significant impact on the islet mass (Mi), β‐cell mass (Mβ) and non‐β‐cell mass (Mnβ), although the effect on Mβ was nearly significant (P = 0.06). (c) The comparison of volume densities of islets (Vi), β‐cells (Vβ), non‐β‐cells (Vnβ), and masses of islets (Mi), β‐cells (Mβ) and non‐β‐cells (Mnβ) between lean and obese subjects. Lean subjects had a BMI < 25 and obese subjects had a BMI ≥ 25. There were no significant differences in Vi, Vnβ, Vβ and Mnβ, whereas there were marginal but significant increases in Mi and Mβ in obese subjects. *P < 0.05 vs BMI < 25.
During maturation until the late 20s, the Ki67‐positive rate (index) of islet endocrine cells including both β‐ and non‐β‐cells was relatively high (Figure 4a). Proliferation of non‐β‐cells exceeded that of β‐cells in the young. Thereafter, the index of these cells was rapidly decreased to below 0.7%. The levels remained consistently low at 0.2–0.5% thereafter. The Ki67 index correlated with the increases in Vi, Vβ and Vnβ (Figure 4b), and was also associated with increases in Mi, Mβ and Mnβ. There was no significant association, however, between Ki67 index of islet cells, β‐cells or non‐β‐cells and BMI (Figure 4c). Apoptotic cells were rarely found in the islets in all groups, although there were some acinar or stromal cells positive for TUNEL (figure not shown).
Figure 4.
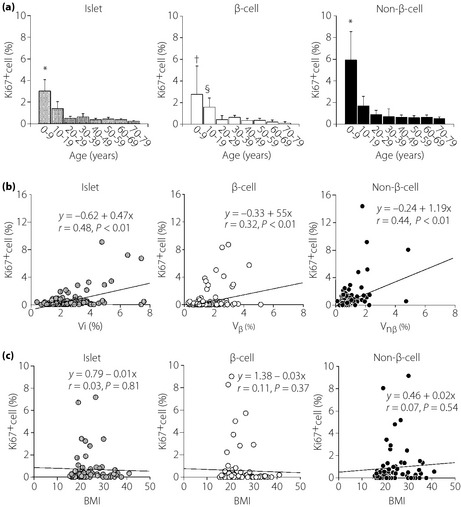
Proliferation activity (Ki67 index) of endocrine cells and their changes with aging. (a) During maturation, Ki67 indices of islets, β‐cells and non‐β‐cells are relatively high compared with those in later life. They are negatively correlated with aging. However, if comparison is limited to the subjects aged older than 10 years, the correlation is not significant and the index is consistently low (<0.1%), indicating low replicating activity of islet endocrine cells in the adult pancreas (*P < 0.01 vs 10–79 years, †P < 0.01 vs 20–79 years, §P < 0.05 vs 20–79 years). (b) Ki67 index is positively correlated with volume densities of islet (Vi), β‐cell (Vβ) and non‐β‐cell (Vnβ). (c) Body mass index (BMI) does not influence the values of Ki67 index in β‐cells or non‐β‐cells.
There was strongly positive expression of PDX‐1 in the nuclei of β‐cells in the young (Figure 5a). Ductal cells, scattered other endocrine and exocrine cells were also positive. The reaction of β‐cells appeared to be weakened in the aged. In contrast, P16 expressions, as an inhibitor of cell cycle‐dependent kinase, were equivocal in the nuclei of islet cells in the young, whereas they were clearly positive in the adult or older subjects. Concurrently, γH2AX reactions, as a marker of oxidative stress‐induced DNA damage, were weak in the nuclei of islet cells in the young and middle‐aged adults, whereas they were robust in the aged.
Figure 5.
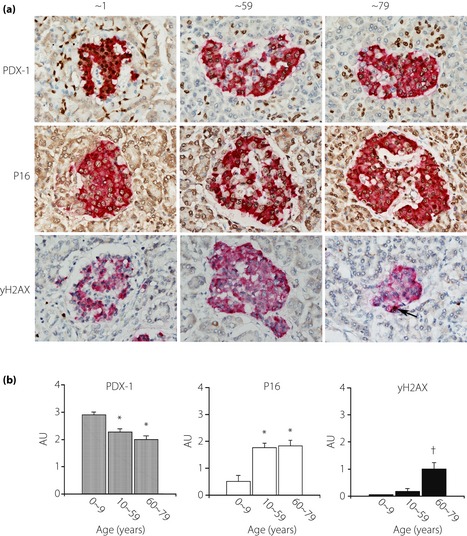
Immunohistochemical expression of pancreatic and duodenal homeobox‐1 (PDX‐1), cyclin‐dependent kinase inhibitor (P16) and oxidative‐stress‐related DNA damage (γH2AX) in the islet and their alterations in aging. (a) PDX‐1 expression is clearly identified in the nuclei of islet β‐cells and intensity is strong in young subjects (upper left panel), whereas reactions in older subjects subsided (upper center and right panels). Some acinar cells and ductular cells are also positively stained. In contrast, P16 expression is not so apparent in the young (middle left), whereas older subjects show stronger reactions in the islets (middle center and right). γH2AX expressions are faint in the young and adult until middle age (lower left and right), but intensified in older subjects (>60 years; lower right). (b) Semiquantification of immunoexpressions of PDX‐1, P16 and γH2AX showed that PDX‐1 expression is greater in the young compared with older subjects. In contrast, P16 expression is low in the young subjects compared with adult and older adult subjects. Expression of γH2AX is increased in aged subjects (*P < 0.01 vs 0–9 years, †P < 0.01 vs 0–59 years).
Semiquantitative analysis showed that the expression of PDX‐1 was marked in the young (<10 years) compared with adults (Figure 5b). The expression was further decreased in older subjects (>60 years). In contrast, the expression of P16 was much lower in the young (<10 years) compared with that in the older groups (>10 years). γH2AX expression was faint in the young and middle‐aged (<59 years), whereas it was enhanced in the aged (>60 years). The scores of PDX‐1 expression correlated with Vβ (Figure S3). In contrast, there was an inverse correlation between P16 expression and Vβ. However, there was no significant correlation between γH2AX expression and Vi.
Discussion
The present study is the first to comprehensively show the age‐associated changes of islet endocrine cells, and provide differences in β‐cell features between young and aged subjects. Our findings are in accord with recent results on the β‐cell expansion with enlargement of islet size and the exocrine pancreas during development after birth until the 20s in American non‐diabetic subjects7. The gradual age‐associated decline of Mβ after adulthood was common in Europeans and Japanese, despite the differences in analytical methods6, but not with Americans9. Indeed, the present study confirmed that the decrease in Mβ after the 60s was minimal, just 0.1–0.2% levels and more than 80% masses were well preserved even in the 70s. The findings could indicate that the 30–50% reduction of Mβ or Vβ that we encountered in type 2 diabetic subjects either in Japan or Western countries is not merely the consequence of enhanced dropout of aged β‐cells, but due to a specific injury to β‐cells as well as poor ability of β‐cell replication3.
The mechanism of a gradual decline in Mβ with aging is yet to be made clear, and previous studies have not addressed this issue well. The present study disclosed several possibilities by detection of: (i) a gradual decrease in Ki67 index and PDX‐1 expressions in adults; (ii) an enhanced expression of P16; and (iii) accumulation of γH2AX in the aged. The reduced PDX‐1 expression in aging β‐cells was established in animal models20, and we found it was also the case in humans. As we showed an increased expression of γH2AX in the aged, DNA injury as a result of oxidative stress could interfere with the signals of β‐cell transcription factors, resulting in a lowered expression of PDX‐1. In fact, enhanced oxidative stress is known to promote translocation of PDX‐1 from the nucleus to cytoplasm in rodent islets22. Interestingly, P16 expression was inversely correlated with Ki67 index. Such opposite expression of P16 against PDX‐1 and Ki67 was recently shown in the islets during β‐cell maturation in the prenatal and postnatal human pancreas15. Collectively, it is likely that the gradual decline of Vi or Vβ was a result of the low replicating activity of cells, not by increased apoptosis, as apoptotic β‐cells were hardly detected, as described in the human pancreas3. Nevertheless, the absence of TUNEL‐positive cells might not directly indicate the lack of apoptosis, as the cell cycle of apoptosis is fairly rapid, and the cells undergoing apoptosis can rapidly be phagocytosed. In addition, there might be other mechanisms of β‐cell decline; autophagy deficit24, telomere shortening26, as well as necrosis27. Recent substructural demonstration of lipofuscin accumulation suggested an extremely long lifespan of islet endocrine cells29. In concert with this contention, the present findings of enhanced expression of γH2AX in aged β‐cells could indicate that aged β‐cells are more or less damaged by long‐term exposure to environmental insults and susceptible to cell death.
In contrast to previous investigators, who found that Vβ in human obesity was increased by ~50% in American and European subjects4, we could not obtain a significant impact of BMI on Vi or Vβ, but only marginal effects on Mi and Mβ (Figure 3). A comparison between the lean group (BMI < 25) and obese group (BMI ≥ 25) yielded a significant increase only in Mi and Mβ in the latter, but not in Vi or Vβ. Thus, the impact of obesity on the β‐cell mass is not so robust in Japanese people compared with Americans. In addition, there was no significant correlation of BMI with Ki67 index (Figure 4c). Collectively, distinct from Western people, cellular adaptation to obesity or insulin resistance in β‐cells appears to be limited in Japanese non‐diabetic subjects. The reason for this discrepancy is not clear, but the ethnicity or different lifestyles might have yielded the different results. Alternatively, the increase in BMI in Japanese subjects compared with Americans might have been too small to yield an influence of BMI on the islet structure. In fact, an average BMI was much lower, and its range was smaller in Japanese subjects (mean 20.8, range 19–28) than those in Americans (mean 25–26, range 18–42)31. It should be of note, however, that there was a significant correlation of BMI with Vβ even in a small number of Korean subjects (just nine cases)5. The difference between the Korean study and ours could be ascribed to the limited younger autopsy cases and the inclusion of more obese subjects in the Korean study compared with ours. In addition, the difference in cultural habits or diet could have influenced the results among the same Asian people. Notwithstanding, the present results might suggest that diabetes develops differently between obese and lean subjects, as the comparison of reduced Vβ showed a large difference between obese and lean diabetic subjects in one American study4. This contention is purely speculative, however, requiring critical investigation in future.
There is a caution that the interpretation of the morphometric data could mislead, because the increased pancreatic weight is affected by fatty changes in obese or aged subjects33. Exclusion of peripancreatic fat tissues infiltrating into pre‐existing exocrine pancreatic parenchyma in obese or aged subjects might erroneously result in a false increase in Vβ and Mβ. Indeed, it is often difficult to make a border of pancreatic parenchyma and quantify the exocrine pancreatic area where fatty tissues are involved in the pancreas of obese or aged subjects. Standardization of morphometric analysis should, therefore, be established for the comparison of the data reported from different institutions.
The present study had a number of limitations that could make interpretation difficult. Our data might have been influenced by unavoidable factors encountered at the agonal stage of the patients: circulatory insufficiency, blood pressure changes, rapid glucose imbalance and metabolic catabolism. It was not feasible to find a correlation of blood glucose elevations with the structural data, because of inadequate information at the time of autopsy. We searched the clinical records of the patients extensively and excluded the subjects who had a possibility of glucose intolerance in their clinical history. In a few studies, the use of samples obtained at the time of surgery was attempted5. In such studies, however, there are again factors that might affect the values, such as blood changes or underlying diseases for surgery. β‐Cells are known to respond to environmental factors, resulting in islet hyperplasia in pregnancy35 or gastrectomy37. There is a possibility that we might also have included some cases in which normal age‐associated changes of the islet structure were influenced by hitherto unknown mediators. We hope that a sufficient number of subjects using 115 cases, which is the largest cohort in human islet structural studies, could have reduced the influence of confounding factors in the present study.
The main purpose of diabetes treatment has become to prevent a progressive decline of β‐cells and to restore the β‐cell deficit. To this end, development of in vivo imaging of β‐cells to monitor the efficacy of treatment is expected10. The data obtained from the current study can therefore be hoped to serve non‐diabetic standards for the comparison with diabetic subjects. Quantitative analysis on the islet might not be so straightforward because of variation of the data caused by different methodology39. Critical comparison of the data between isolated islets, and freshly obtained sections by immunohistochemistry and ultrastructure found −30 + 23% differences in β‐cell occupancy and size39. Future investigations might be warranted to confirm the data on the age‐associated dynamic changes by addition of molecular and biochemical alterations of the islets.
Supplementary Material
Table S1 | Age distribution and number of cases investigated in each decade and mean body mass index (BMI)
Figure S1 | Islet distribution and contour from youth to old age in Japanese non‐diabetic subjects immunostained with chromogranin A
Figure S2 | Correlation of analysis on the relationship between age and volume densities of islet (Vi), β‐cell (Vβ) and non‐β‐cell (Vnβ)
Figure S3 | Scoring of immunohistochemical expression of pancreatic and duodenal homeobox‐1 (PDX‐1), and P16 and γH2AX and their relationships to β‐cell volume density, and Ki67 index
Acknowledgements
The present study was supported by a grant in aid to SY and HM from the Japanese Ministry of Education, Science, Culture, and Sports, Diabetes Masters Conference, Japan and the Japan Diabetes Foundation. A part of the study was presented at the annual meeting of 72nd American Diabetes Association held in Philadelphia, on 8–12 June 2012. The authors are grateful for expert technical assistance from Ms Hiroko Mori, Saori Ogasawara and Misato Sakamoto in our department. Staining of γH2AX was owed to Professor A Kurose and Mr N Kumagai in the Department of Diagnostic Pathology, Hirosaki University Hospital. There is no conflict of interest in all listed authors. The authors declare no conflict of interest in this study.
J Diabetes Invest 2014; 5: 38–47
References
- 1.Wareham NJ. Epidemiology of type 2 diabetes. Endocrinol Nutr 2009; 56(Suppl 4): 60–62 [PubMed] [Google Scholar]
- 2.Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev 2010; 6: 134–143 [DOI] [PubMed] [Google Scholar]
- 3.Sakuraba H, Mizukami H, Yagihashi N, et al Reduced beta‐cell mass and expression of oxidative stress‐related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002; 45: 85–96 [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner‐Weir S, et al β‐cell deficit and increased β‐cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102–110 [DOI] [PubMed] [Google Scholar]
- 5.Yoon KH, Ko SH, Cho JH, et al Selective β‐cell loss and α‐cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003; 88: 2300–2308 [DOI] [PubMed] [Google Scholar]
- 6.Rahier J, Guiot Y, Goebbels RM, et al Pancreatic β‐cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008; 10(Suppl 4): 32–42 [DOI] [PubMed] [Google Scholar]
- 7.Meier JJ, Butler AE, Saisho Y, et al β‐cell replication is the primary mechanism subserving the postnatal expansion of β‐cell mass in humans. Diabetes 2008; 57: 1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregg BE, Moore PC, Demozay D, et al Formation of a human β‐cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 2012; 97: 3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saisho Y, Elashoff D, Butler AE, et al β‐Cell mass and turnover in humans. Diabetes Care 2013; 36: 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malaisse WJ, Louchami K, Sener A. Noninvasive imaging of pancreatic β‐cells. Nat Rev Endocrinol 2009; 5: 394–400 [DOI] [PubMed] [Google Scholar]
- 11.Yagihashi S. Imaging of insulin factory: is it just imagination or approaching reality? J Diabetes Invest 2012; 3: 429–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maedler K, Schumann DM, Schulthess F, et al Aging correlates with decreased β‐cell proliferative capacity and enhanced sensitivity to apoptosis. A potential role of Fas and pancreatic duodenal homeobox‐1. Diabetes 2006; 55: 2455–2462 [DOI] [PubMed] [Google Scholar]
- 13.Gannon M, Ables ET, Crawford L, et al pdx‐1 function is specifically required in embryonic β cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol 2008; 314: 406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elghazi L, Balcazar N, Blandino‐Rosano M, et al Decreased IRS signaling impairs β‐cell cycle progression and survival in transgenic mice overexpressing S6K in beta‐cells. Diabetes 2010; 59: 2390–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kőhler CU, Olewinski M, Tannapfel A, et al Cell cycle control of β‐cell replication in the prenatal and postnatal human pancreas. Am J Physiol 2011; 300: E221–E230 [DOI] [PubMed] [Google Scholar]
- 16.Soliman MA, Berardi P, Pastyryeva S, et al ING1a expression increases during replicative senescence and induces a senescent phenotype. Aging Cell 2008; 7: 783–794 [DOI] [PubMed] [Google Scholar]
- 17.Mah LJ, El‐Osta A, Karagiannis TC. γH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 2010; 24: 679–686 [DOI] [PubMed] [Google Scholar]
- 18.Mizukami H, Inaba W, Takahashi K, et al Augmented reduction of islet β‐cell mass in Goto‐Kakizaki rats fed high‐fat diet and its suppression by pitavastatin treatment. J Diabetes Invest 2012; 3: 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inaba W, Mizukami H, Kamata K, et al Effects of long‐term treatment with the dipeptidyl peptidase‐4 inhibitor vildagliptin on islet endocrine cells in non‐obese type 2 diabetic Goto‐Kakizaki rats. Eur J Pharmacol 2012; 691: 297–306 [DOI] [PubMed] [Google Scholar]
- 20.Maedler K, Schumann DM, Sauter N, et al Low concentration of interleukin‐1β induces FLICE‐inhibitory protein‐mediated β‐cell proliferation in human pancreatic islets. Diabetes 2006; 55: 2713–2722 [DOI] [PubMed] [Google Scholar]
- 21.Reers C, Erbel S, Esposito I, et al Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol 2009; 160: 185–191 [DOI] [PubMed] [Google Scholar]
- 22.Kaneto H, Xu G, Fujii N, et al Involvement of c‐Jun N‐terminal kinase in oxidative stress‐mediated suppression of insulin gene expression. J Biol Chem 2002; 277: 30010–30018 [DOI] [PubMed] [Google Scholar]
- 23.Kawamori D, Kajimoto Y, Kaneto H, et al Oxidative stress induces nucleo‐cytoplasmic translocation of pancreatic transcription factor PDX‐1 through activation of c‐Jun NH(2)‐terminal kinase. Diabetes 2003; 52: 2896–2904 [DOI] [PubMed] [Google Scholar]
- 24.Ebato C, Uchida T, Arakawa M, et al Autophagy is important in islet homeostasis and compensatory increase of β cell mass in response to high‐fat diet. Cell Metab 2008; 8: 325–332 [DOI] [PubMed] [Google Scholar]
- 25.Ishii A, Nakamura K, Kishimoto H, et al Telomere shortening with aging in the human pancreas. Exp Gerontol 2006; 41: 882–886 [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res 2009; 104: 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des 2008; 14: 3565–3573 [DOI] [PubMed] [Google Scholar]
- 28.Vanlangenakker N, Vanden Berghe T, Krysko DV, et al Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med 2008; 8: 207–220 [DOI] [PubMed] [Google Scholar]
- 29.Cnop M, Hughes SJ, Igoillo‐Esteve M, et al The long lifespan and low turnover of human islet β cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 2010; 53: 321–330 [DOI] [PubMed] [Google Scholar]
- 30.Maclean N, Ogilvie RF. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes 1955; 4: 367–376 [DOI] [PubMed] [Google Scholar]
- 31.Funakoshi S, Fujimoto S, Hamasaki A, et al Analysis of factors influencing pancreatic β‐cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract 2008; 82: 353–358 [DOI] [PubMed] [Google Scholar]
- 32.Oka R, Yagi K, Sakurai M, et al Insulin secretion and insulin sensitivity on the oral glucose tolerance test (OGTT) in middle‐aged Japanese. Endocr J 2012; 59: 55–64 [DOI] [PubMed] [Google Scholar]
- 33.Saisho Y, Butler AE, Meier JJ, et al Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type‐2 diabetes. Clin Anat 2007; 20: 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szczepaniak LS, Victor RG, Mathur R, et al Pancreatic steatosis and its relationship to β‐cell dysfunction in humans: racial and ethnic variations. Diabetes Care 2012; 35: 2377–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Toyofuku Y, Lynn FC, et al Serotonin regulates pancreatic β cell mass during pregnancy. Nat Med 2010; 16: 804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler AE, Cao‐Minh L, Galasso R, et al Adaptive changes in pancreatic β cell fractional area and β cell turnover in human pregnancy. Diabetologia 2010; 53: 2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh H, Takei K. Immunohistochemical and statistical studies on the islets of Langerhans pancreas in autopsied patients after gastrectomy. Hum Pathol 2000; 31: 1368–1376 [PubMed] [Google Scholar]
- 38.De Paula AL, Stival AR, Halpern A, et al Improvement in insulin sensitivity and β‐cell function following ileal interposition with sleeve gastrectomy in type 2 diabetic patients: potential mechanisms. J Gastrointest Surg 2011; 15: 1344–1353 [DOI] [PubMed] [Google Scholar]
- 39.Pisania A, Weir GC, O'Neil JJ, et al Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest 2010; 90: 1661–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonner‐Weir S, Li W‐C, Ouziel‐Yahalom L, et al β‐cell growth and regeneration: replication is only part of story. Diabetes 2010; 59: 2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Age distribution and number of cases investigated in each decade and mean body mass index (BMI)
Figure S1 | Islet distribution and contour from youth to old age in Japanese non‐diabetic subjects immunostained with chromogranin A
Figure S2 | Correlation of analysis on the relationship between age and volume densities of islet (Vi), β‐cell (Vβ) and non‐β‐cell (Vnβ)
Figure S3 | Scoring of immunohistochemical expression of pancreatic and duodenal homeobox‐1 (PDX‐1), and P16 and γH2AX and their relationships to β‐cell volume density, and Ki67 index


