Abstract
Aims/Introduction
Admission hyperglycemia is associated with poor outcome in patients with myocardial infarction. The present study evaluated the relationship between admission glucose level and other clinical variables in patients with ST‐elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI).
Materials and Methods
The 959 consecutive STEMI patients undergoing primary PCI were divided into five groups based on admission glucose levels of <100, 100–139, 140–189, 190–249 and ≥250 mg/dL. Their short‐ and long‐term outcomes were compared.
Results
Higher admission glucose levels were associated with significantly higher in‐hospital morbidity and mortality, the overall mortality rate at follow up, and the incidence of reinfarction or heart failure requiring admission or leading to mortality at follow up. The odds ratios (95% confidence interval) for in‐hospital morbidity, in‐hospital mortality, mortality at follow up and re‐infarction or heart failure or mortality at follow up of patients with admission glucose levels ≥190 mg/dL, compared with those with admission glucose levels <190 mg/dL, were 2.12 (1.3–3.4, P = 0.001), 2.74 (1.4–5.5, P = 0.004), 2.52 (1.2–5.1, P = 0.01) and 1.70 (1.03–2.8, P = 0.04), respectively. Previously non‐diabetic patients with admission glucose levels ≥250 mg/dL had significantly higher in‐hospital morbidity or mortality (44 vs 70%, P = 0.03). Known diabetic patients had higher rates of reinfarction, heart failure or mortality at follow up in the 100–139 mg/dL (8 vs 27%, P = 0.04) and 140–189 mg/dL (11 vs 26%, P = 0.02) groups.
Conclusions
Admission hyperglycemia, especially at glucose levels ≥190 mg/dL, is a predictor of poor prognosis in STEMI patients undergoing primary PCI.
Keywords: Acute coronary syndrome, Admission glucose, Diabetes
Introduction
Elevated glucose levels on admission are associated with poor outcomes in patients with acute myocardial infarction (MI), regardless of comorbid diabetes1. Most studies include patients diagnosed with both ST‐elevation myocardial infarction (STEMI) and non‐STEMI1, and fibrinolysis is usually the initial treatment3. Two studies showed that admission hyperglycemia is related to in‐hospital mortality in MI patients10, approximately 90% of whom are diagnosed as STEMI and primary percutaneous coronary intervention (PCI) is carried out in 72%. However, coronary angiographic features and long‐term outcomes are not available for these studies.
Several randomized trials have shown better outcomes from primary PCI and stent implantation compared with fibrinolysis for MI patients12, so we focused on the MI patients undergoing primary PCI in the present study. However, in the era of PCI, few papers have discussed the effects of admission glucose levels in patients with STEMI undergoing PCI. Lazzeri et al.15 have shown that admission hyperglycemia is an independent predictor of mortality among elderly patients (age ≥75 years) undergoing primary PCI in the intensive cardiac care unit. Pres et al.16 showed that elevated admission glucose levels result in increased in‐hospital and long‐term mortality in STEMI patients complicated by cardiogenic shock, and treated with primary PCI. In two other studies, admission hyperglycemia is associated with larger infarct sizes and more severely impaired epicardial coronary flow when compared with normoglycemic patients17.
However, relevant data on admission glucose levels in STEMI patients undergoing primary PCI remains limited. The present study aimed to investigate the predictive value of admission glucose levels for the clinical features at short‐ and long‐term outcomes in STEMI patients undergoing PCI.
Methods
Patients
Between November 1992 and December 2008, 1,035 consecutive patients underwent primary PCI for STEMI, which was defined using the criteria of the time19: (i) characteristic chest pain lasting at least 20 min; (ii) elevated levels of serum cardiac biomarkers at least twofold higher than the upper limit of normal; (iii) ST‐segment elevation ≥1 mm, with subsequent evolution of negative T‐waves with a depth of ≥1 mm and the development of new Q‐waves for at least ≥0.04 s or deeper than one‐quarter of the following R wave in voltage. Coronary angiography confirmed the complete occlusion or critical stenosis of the infarct‐related arteries in all of the patients. The admission glucose values of these patients were reviewed. Patients who received fibrinolysis therapy or had no available data on glucose levels were excluded. A total of 959 patients were included in the present study. The local ethics committee approved this observational study and all patients provided written informed consent.
Definitions
Admission glucose levels were determined by the first venous blood samples routinely drawn at the emergency room and analyzed at the central laboratory of the hospital. The patients were categorized into five groups based on admission glucose levels of <100, 100–139, 140–189, 190–249 and ≥250 mg/dL8. Patients were defined as having previously recognized diabetes if they were under antidiabetic therapy at the time of admission.
To evaluate peak serum cardiac biomarkers, blood samples were obtained every 6 h for 48 h, or until activity returned to normal. Blood samples for lipid profile were drawn after an 8‐h fast during the index hospitalization.
Coronary Angiography
All patients underwent coronary artery angiography. Judgment of vessel flow was based on thrombolysis in myocardial infarction (TIMI) flow grade, ranging from 0 to 3, whereby TIMI 0 flow indicated the absence of any antegrade flow beyond a coronary occlusion, whereas TIMI 3 flow indicated normal flow completely filling the distal coronary bed21.
Outcomes
The clinical outcomes analyzed were short‐term outcomes, including length of hospital stay, length of intensive care unit (ICU) stay, and in‐hospital mortality and morbidity, as well as long‐term outcomes, such as incidence of reinfarction, heart failure requiring hospital admission and mortality at follow up. Clinical follow‐up variables, including reinfarction, heart failure requiring admission and mortality data, were obtained from clinic visits, telephone conversations and chart reviews.
Statistical Analysis
Quantitative data were expressed as mean ± standard deviation. The χ2‐test with Yate's correction or Fisher's exact test was used to analyze non‐parametric data. Cox regression analysis was used to identify baseline variables independently associated with short‐ and long‐term outcomes. These variables were presented as odds ratios (OR) followed by 95% confidence interval (CI). Statistical significance was set at P < 0.05 and significant OR was defined as 95% CI >1. Event‐free survival curves (defined as free of reinfarction, heart failure requiring admission, or mortality) were constructed using the Kaplan–Meier method. The significance of differences between curves was assessed by log–rank test.
Results
Risk Factors and Presentations
The patients were predominantly male. Compared with those with lower admission glucose levels, patients with higher admission glucose levels were significantly older, had higher likelihood of previously recognized diabetes and had a previous history of heart failure. Patients with higher admission glucose levels also presented with significantly less typical angina and higher Killip class (III and IV). They also had significantly higher peak creatine kinase levels. However, there were no differences in creatine kinase‐MB, cholesterol, high‐density lipoprotein, low‐density lipoprotein or platelet levels.
The proportion of patients receiving antihypertensive or antidyslipidemic therapy did not differ significantly among the five groups. Nonetheless, the proportion of patients receiving antidiabetic therapy was greater in patients with higher admission glucose levels (Table 1).
Table 1. Patient characteristics of the admission glucose groups.
| Admission glucose level (mg/dL) | <100 (n = 72) | 100 – 139 (n = 345) | 140 – 189 (n = 237) | 190 – 249 (n = 114) | ≥250 (n = 191) | P‐value |
|---|---|---|---|---|---|---|
| Age (years) | 61.2 ± 12.9 | 58.0 ± 12.6 | 61.7 ± 12.7 | 62.2 ± 12.2 | 63.0 ± 12.0 | <0.001 |
| Male | 64 (88.9) | 306 (88.7) | 197 (83.1) | 90 (78.9) | 130 (68.1) | <0.001 |
| Previously recognized diabetes | 13 (18.1) | 22 (6.4) | 50 (21.1) | 63 (55.3) | 158 (82.7) | <0.001 |
| History of heart failure | 4 (5.6) | 6 (1.7) | 2 (0.8) | 7 (6.1) | 9 (4.7) | <0.001 |
| Presentation | ||||||
| Typical angina | 63 (87.5) | 321 (93.0) | 214 (90.3) | 100 (87.7) | 155 (81.2) | 0.001 |
| Killip III/IV | 14 (19.4) | 64 (18.6) | 61 (25.7) | 36 (31.6) | 89 (46.6) | <0.001 |
| Laboratory analysis | ||||||
| Creatine kinase (IU/L)† | 1864.3 ± 106.0 | 2910.0 ± 136.4 | 2995.2 ± 155.4 | 2734.2 ± 227.5 | 3374.8 ± 239.8 | 0.002 |
| Creatine kinase‐MB (IU/L)† | 185.9 ± 19.6 | 269.8 ± 14.1 | 260.6 ± 12.8 | 250.5 ± 24.5 | 278.0 ± 22.2 | 0.11 |
| Total cholesterol (mg/dL) | 177.5 ± 37.5 | 187.7 ± 41.5 | 183.4 ± 40.1 | 178.9 ± 46.2 | 189.8 ± 48.3 | 0.09 |
| Triglyceride (mg/dL) | 129.4 ± 85.6 | 132.7 ± 101.9 | 130.1 ± 95.7 | 180.2 ± 177.6 | 184.5 ± 199.9 | <0.001 |
| High‐density lipoprotein (mg/dL) | 42.3 ± 16.0 | 43.4 ± 12.7 | 43.1 ± 13.8 | 40.4 ± 12.9 | 40.3 ± 13.5 | 0.22 |
| Low‐density lipoprotein (mg/dL) | 111.1 ± 34.1 | 123.0 ± 35.5 | 117.8 ± 37.7 | 110.5 ± 39.2 | 116.7 ± 40.4 | 0.06 |
| Platelets (K/uL) | 235.9 ± 123.8 | 223.7 ± 68.0 | 215.0 ± 53.3 | 226.8 ± 65.6 | 227.7 ± 82.0 | 0.24 |
| Medication therapy | ||||||
| Antihypertensive therapy | 22 (30.6) | 135 (39.1) | 94 (39.7) | 35 (30.7) | 60 (31.4) | 0.14 |
| Antidyslipidemic therapy | 11 (15.3) | 82 (23.8) | 59 (24.9) | 20 (17.5) | 44 (23.0) | 0.30 |
| Antidiabetic therapy | 13 (18.1) | 22 (6.4) | 50 (21.1) | 63 (55.3) | 158 (82.7) | <0.001 |
Values are presented as n (%) or as mean ± standard deviation (n = 959). †Values are presented as mean ± standard error.
Angiographic Data and Interventional Therapy
Patients with higher admission glucose levels tended to have greater incidence of multivessel disease, although this difference was only borderline significant (P = 0.05). They had significantly lower rates of initial success of primary PCI and poorer post‐PCI TIMI flow grades, were significantly more likely to require intra‐aortic balloon pump (IABP) implantation, and tended to have poorer left ventricular ejection fraction (Table 2).
Table 2. Coronary angiographic features of the admission glucose groups.
| Admission glucose level (mg/dL) | <100 (n = 72) | 100 – 139 (n = 345) | 140 – 189 (n = 237) | 190 – 249 (n = 114) | ≥250 (n = 191) | P‐value |
|---|---|---|---|---|---|---|
| No. diseased vessels | ||||||
| Single‐vessel disease | 24 (33.3) | 125 (36.2) | 74 (31.2) | 34 (29.8) | 45 (23.6) | 0.05 |
| Multiple‐vessel disease | 48 (66.7) | 220 (63.8) | 163 (68.8) | 80 (70.2) | 146 (76.4) | |
| Coronary artery intervention | ||||||
| Initial success of primary PCI | 65 (90.3) | 325 (94.2) | 219 (92.4) | 103 (91.2) | 155 (82.4) | <0.001 |
| Post‐PCI TIMI flow grade | 2.8 ± 0.7 | 2.9 ± 0.6 | 2.8 ± 0.7 | 2.7 ± 0.8 | 2.5 ± 1.0 | <0.001 |
| Intra‐aortic balloon pump | 10 (13.9) | 49 (14.2) | 42 (17.7) | 20 (17.7) | 60 (31.4) | <0.001 |
| Emergency CABG | 1 (1.4) | 3 (0.9) | 0 (0) | 0 (0) | 3 (1.6) | 0.29 |
| LVEF, by heart echo | 54.1 ± 12.9 | 57.8 ± 11.7 | 56.2 ± 13.1 | 52.6 ± 12.7 | 51.2 ± 13.6 | <0.001 |
Values are presented as n (%) or as mean ± standard deviation (n = 959). CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Outcomes
Higher admission glucose levels were associated with significantly higher in‐hospital morbidity, including those as a result of cardiogenic shock, ventricular arrhythmia requiring defibrillation, sepsis and acute renal failure requiring hemodialysis, but were not associated with differences in bleeding complications requiring blood transfusion and cardiac rupture/tamponade. Higher admission glucose levels were also associated with significantly increased risk of in‐hospital mortality and morbidity, and longer durations of ICU stay and length of hospitalization.
In terms of long‐term outcomes, there was no significant association between hyperglycemia on admission and the incidence of reinfarction or heart failure requiring hospital admission. However, the overall mortality rate at follow up and the incidence of reinfarction or heart failure requiring admission or leading to mortality at follow up were significantly higher among patients with higher admission hyperglycemia (Table 3).
Table 3. Clinical outcomes of the admission glucose groups.
| Admission glucose level (mg/dL) | <100 (n = 72) | 100 – 139 (n = 345) | 140 – 189 (n = 237) | 190 – 249 (n = 114) | ≥250 (n = 191) | P‐value |
|---|---|---|---|---|---|---|
| Short‐term outcomes | ||||||
| In‐hospital morbidity | 19 (26.4) | 96 (27.8) | 71 (30.0) | 39 (34.2) | 93 (48.7) | <0.001 |
| Cardiogenic shock | 13 (18.1) | 48 (13.9) | 45 (19.0) | 30 (26.3) | 70 (36.6) | <0.001 |
| Ventricular arrhythmia required defibrillation | 5 (6.9) | 43 (12.5) | 17 (7.2) | 4 (3.5) | 23 (12.0) | 0.02 |
| Sepsis | 0 (0) | 11 (3.2) | 9 (3.8) | 8 (7.0) | 19 (9.9) | 0.001 |
| Bleeding complications requiring blood transfusion | 3 (4.2) | 20 (5.8) | 11 (4.6) | 6 (5.3) | 21 (11.0) | 0.06 |
| Cardiac rupture/tamponade | 0 (0) | 2 (0.6) | 1 (0.4) | 1 (0.9) | 2 (1.0) | 0.86 |
| Acute renal failure requiring hemodialysis | 0 (0) | 3 (0.9) | 3 (0.9) | 0 (0) | 7 (3.7) | 0.03 |
| In‐hospital mortality | 6 (8.3) | 10 (2.9) | 12 (5.1) | 11 (9.6) | 41 (21.5) | <0.001 |
| In‐hospital morbidity or mortality | 22 (30.6) | 98 (28.4) | 74 (31.2) | 42 (36.8) | 93 (48.7) | <0.001 |
| Length of intensive care unit stay (day) | 2.9 ± 1.8 | 3.6 ± 4.6 | 2.6 ± 3.1 | 4.9 ± 6.0 | 4.9 ± 7.9 | 0.004 |
| Length of total hospital stay (day) | 7.6 ± 6.3 | 7.7 ± 5.9 | 8.2 ± 6.4 | 10.0 ± 8.7 | 9.6 ± 9.8 | 0.005 |
| Long‐term outcomes | ||||||
| Reinfarction | 2 (2.8) | 10 (2.9) | 4 (1.7) | 8 (7.0) | 6 (3.1) | 0.12 |
| HF required hospital admission | 6 (8.3) | 15 (4.3) | 16 (6.8) | 10 (8.8) | 20 (10.5) | 0.09 |
| Mortality at follow up | 1 (1.4) | 12 (3.5) | 14 (5.9) | 10 (8.8) | 25 (13.1) | 0.002 |
| Reinfarction or HF or mortality | 7 (9.7) | 33 (9.6) | 34 (14.3) | 20 (17.5) | 42 (22.0) | 0.001 |
| Follow up (months)† | 66.2 ± 5.9 | 64.5 ± 2.7 | 65.9 ± 3.2 | 63.0 ± 4.8 | 53.3 ± 3.6 | 0.07 |
Values are presented as numbers (%) or mean ± standard deviation (n = 959). †Values are presented as mean ± standard error. HF, heart failure.
The Kaplan–Meier curves of event‐free survival showed that patients with higher admission glucose levels had higher rates of reinfarction, heart failure requiring admission and mortality at follow up (P < 0.001, log–rank test; Figure 1). After adjusting for sex, age, Killip class >1, current smoking, diabetes, hypertension, multivessel disease, chronic kidney disease and culprit artery TIMI flow after coronary intervention, admission glucose level ≥190 mg/dL was able to predict higher in‐hospital morbidity (OR 2.12, 95% CI 1.3–3.4, P = 0.001), in‐hospital mortality (OR 2.74, 95% CI 1.4–5.5, P = 0.004), in‐hospital morbidity or mortality (OR 1.97, 95% CI 1.3–3.1, P = 0.003), mortality at follow up (OR 2.52, 95% CI 1.2–5.1, P = 0.01), and risk of reinfarction, heart failure requiring admission and mortality at follow up (OR 1.70, 95% CI 1.03–2.8, P = 0.04; Table 4).
Figure 1.
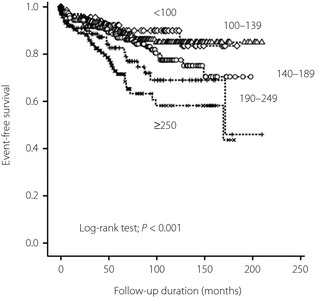
Event‐free survival curves in different admission glucose groups.
Table 4. Odds ratios and 95% confidence intervals for hyperglycemia (admission glucose ≥190 mg/dL).
| Short‐term outcomes | Long‐term outcomes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In‐hospital morbidity | In‐hospital mortality | In‐hospital morbidity or mortality | Mortality at follow up | ReMI/HF/mortality at follow up | |||||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Glucose ≥190 mg/dL | 2.12 | 1.3 – 3.4 | 0.001 | 2.74 | 1.4 – 5.5 | 0.004 | 1.97 | 1.3 – 3.1 | 0.003 | 2.52 | 1.2 – 5.1 | 0.01 | 1.70 | 1.03 – 2.8 | 0.04 |
Adjusted for sex, age, Killip class >1, current smoking, diabetes, hypertension, chronic kidney disease, multivessel disease and culprit artery thrombolysis in myocardial infarction flow >1 before coronary intervention. CI, confidence interval; HF, heart failure; OR, odds ratio; ReMI, reinfarction.
In the present study, 68.1% of patients had no previously documented diabetes. Compared with those with previously known diabetes, those without previously documented diabetes had worse in‐hospital morbidity rates in the group with admission glucose levels ≥250 mg/dL (44% vs 73%, P = 0.03; Figure 2a) although there was no difference in the in‐hospital mortality rate (Figure 2b). The in‐hospital morbidity or mortality rate was also higher in patients without previously known diabetes if admission glucose levels were ≥250 mg/dL (44% vs 70%, P = 0.03), compared with known diabetics with similar admission glucose levels (Figure 2c).
Figure 2.
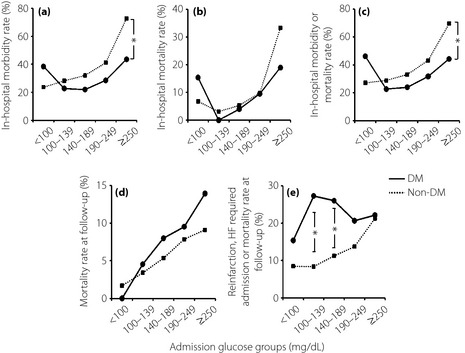
Relationships between admission glucose level and (a) in‐hospital mortality rate, (b) in‐hospital morbidity or mortality rates, (c) mortality rate at follow up, (d) reinfarction or heart failure (HF) requiring admission, or mortality rate at follow up, and (e) patients with and without previously recognized diabetes (DM). *P < 0.05 for comparison between DM and non‐DM.
In regard to long‐term outcomes, the overall mortality rates at follow up for patients with and without previously known diabetes did not differ significantly (Figure 2d). However, known diabetic patients had greater rates of reinfarction, heart failure requiring admission, or mortality at follow‐up when the admission glucose levels were 100–139 mg/dL (8% vs 27%, P = 0.04) and 140–189 mg/dL (11% vs 26%, P = 0.02; Figure 2e).
Discussion
The present study focuses on STEMI patients undergoing primary PCI, and shows that admission hyperglycemia can predict unfavorable short‐ and long‐term outcomes. Patients with higher admission glucose levels tend to have a higher prevalence of coexisting risk factors, such as old age, previously recognized diabetes, atypical presentation, poor Killip class and unfavorable coronary angiographic features, all of which contribute to poor clinical outcomes. Admission glucose levels (≥190 mg/dL) can therefore be a simple and useful prognosticating tool for such patients.
Among patients without prior diabetes, hyperglycemia might reflect previously undiagnosed diabetes, stress hyperglycemia or a combination of both. In the present study, patients without previously known diabetes, but with admission glucose levels >250 mg/dL, had a worse short‐term outcome than those previously diagnosed with diabetes. This is consistent with findings of previous studies3. Previously undiagnosed and untreated diabetes leads to a greater risk of vascular damage. Hyperglycemia might not cause obvious symptoms or signs for years, thus leading to delays in treatment. However, cardiovascular risk is known to increase even in the early stages of impaired glucose tolerance23, and might develop years before a confirmed diagnosis of diabetes24.
In contrast, stress hyperglycemia at the time of hospital admission can also play an important role in the clinical outcomes of MI patients25. Some patients without previously known diabetes, but presenting with admission hyperglycemia, show normal glucose levels after the acute phase of MI. Acute hyperglycemia in MI has been independently associated with impaired left ventricular function, inducing arrhythmias, increased platelet activation, amplified inflammatory immune reactions and poor cardiac functional outcomes26. Thus, stress hyperglycemia might be one explanation for the worse short‐term outcomes of patients with higher glucose levels, but without previously recognized diabetes.
Patients with previously diagnosed diabetes also have worse long‐term outcomes compared with those without previously diagnosed diabetes. The diagnosis of diabetes itself is associated with adverse outcomes in acute coronary syndrome28, suggesting that overall vascular damage in patients with previous diabetes is worse than that in patients without a history of diabetes because of the longer duration of hyperglycemia in the former. These results echo findings by Ishihara et al.30, who showed that admission hyperglycemia predicts worse short‐term mortality, whereas a previous diagnosis of diabetes is associated with increased long‐term mortality in MI patients undergoing PCI. Overall, these findings further emphasize the importance of early diagnosis and aggressive management of diabetes, as good glycemic control can greatly affect long‐term outcomes in STEMI patients undergoing PCI.
Anti‐diabetic therapy might be a factor influencing admission glucose level. In the present study, the proportion of patients receiving antidiabetic therapy was greater in the group with admission glucose levels ≥100 mg/dL, showing that patients with higher admission glucose levels tend to have previously known diabetes. The proportion of patients receiving anti‐diabetic therapy is also slightly higher in the group of admission glucose levels <100 mg/dL compared with the 100–139 mg/dL group. Patients with admission glucose levels <100 mg/dL tended to have poorer short‐term outcomes, particularly patients receiving antidiabetic therapy. Hypoglycemia seems to cause worse short‐term outcomes, for which antidiabetic drugs might be an underlying reason. Previous studies have also shown this J‐curve phenomenon. In addition to the influence of antidiabetic drugs, stress‐induced hepatic gluconeogenesis dysfunction or relative adrenal insufficiency might also have contributed to hypoglycemia in such patients7.
The present study had certain limitations. The diagnostic criteria of previous diabetes was based on the use of antidiabetic medication, which could be a factor influencing admission glucose level, but we did not clarify it clearly. Although patients with and without a previous history of diabetes were compared, glycated hemoglobin levels and the duration of diabetes were not evaluated. In addition, although admission hyperglycemia was associated with poor outcomes, the effect of treating hyperglycemia after admission was not assessed. Further studies are required for more in‐depth examination of these factors. Despite these limitations, these findings remain valid and might be corroborated by further investigations.
In conclusion, admission glucose level above 190 mg/dL is an indicator of poor short‐ and long‐term prognosis in STEMI patients undergoing primary PCI. Patients with admission hyperglycemia ≥250 mg/dL and undiagnosed diabetes have poorer short‐term outcomes, whereas patients with admission hyperglycemia and previously confirmed diabetes have worse long‐term outcomes. Early diagnosis and intensive treatment of diabetes should be emphasized in order to decrease cardiovascular complications, and improve clinical outcomes in such patients.
Acknowledgement
There was no financial support or relationship that could pose a conflict of interest for any of the authors.
J Diabetes Invest 2014; 5: 80–86
References
- 1.Svensson AM, McGuire DK, Abrahamsson P, et al Association between hyper‐ and hypoglycaemia and 2 year all‐cause mortality risk in diabetic patients with acute coronary events. Eur Heart J 2005; 26: 1255–1261 [DOI] [PubMed] [Google Scholar]
- 2.Norhammar AM, Ryden L, Malmberg K. Admission plasma glucose. Independent risk factor for long‐term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care 1999; 22: 1827–1831 [DOI] [PubMed] [Google Scholar]
- 3.Wahab NN, Cowden EA, Pearce NJ, et al Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol 2002; 40: 1748–1754 [DOI] [PubMed] [Google Scholar]
- 4.Stranders I, Diamant M, van Gelder RE, et al Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med 2004; 164: 982–988 [DOI] [PubMed] [Google Scholar]
- 5.de Mulder M, Cornel JH, van der Ploeg T, et al Elevated admission glucose is associated with increased long‐term mortality in myocardial infarction patients, irrespective of the initially applied reperfusion strategy. Am Heart J 2010; 160: 412–419 [DOI] [PubMed] [Google Scholar]
- 6.Squire IB, Nelson CP, Ng LL, et al Prognostic value of admission blood glucose concentration and diabetes diagnosis on survival after acute myocardial infarction; Results from 4702 index cases in routine practice. Clin Sci 2010; 118: 527–5357 [DOI] [PubMed] [Google Scholar]
- 7.Goyal A, Mehta SR, Diaz R, et al Differential clinical outcomes associated with hypoglycemia and hyperglycemia in acute myocardial infarction. Circulation 2009; 120: 2429–2437 [DOI] [PubMed] [Google Scholar]
- 8.Kosiborod M, Rathore SS, Inzucchi SE, et al Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation 2005; 111: 3078–3086 [DOI] [PubMed] [Google Scholar]
- 9.Pinto DS, Kirtane AJ, Pride YB, et al Association of blood glucose with angiographic and clinical outcomes among patients with ST‐segment elevation myocardial infarction (from the CLARITY‐TIMI‐28 study). Am J Cardiol 2008; 101: 303–307 [DOI] [PubMed] [Google Scholar]
- 10.Ishihara M, Kojima S, Sakamoto T, et al Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J 2005; 150: 814–820 [DOI] [PubMed] [Google Scholar]
- 11.Kosuge M, Kimura K, Kojima S, et al Effects of glucose abnormalities on in‐hospital outcome after coronary intervention for acute myocardial infarction. Circ J 2005; 69: 375–379 [DOI] [PubMed] [Google Scholar]
- 12.Busk M, Maeng M, Rasmussen K, et al The Danish multicentre randomized study of fibrinolytic therapy vs. primary angioplasty in acute myocardial infarction (the DANAMI‐2 trial): outcome after 3 years follow‐up. Eur Heart J 2008; 29: 1259–1266 [DOI] [PubMed] [Google Scholar]
- 13.Widimsky P, Bilkova D, Penicka M, et al Long‐term outcomes of patients with acute myocardial infarction presenting to hospitals without catheterization laboratory and randomized to immediate thrombolysis or interhospital transport for primary percutaneous coronary intervention. Five years' follow‐up of the PRAGUE‐2 Trial. Eur Heart J 2007; 28: 679–684 [DOI] [PubMed] [Google Scholar]
- 14.Le May MR, Labinaz M, Davies RF, et al Stenting versus thrombolysis in acute myocardial infarction trial (STAT). J Am Coll Cardiol 2001; 37: 985–991 [DOI] [PubMed] [Google Scholar]
- 15.Lazzeri C, Valente S, Chiostri M, et al Predictors of the early outcome in elderly patients with ST elevation myocardial infarction treated with primary angioplasty: a single center experience. Intern Emerg Med 2011; 6: 41–46 [DOI] [PubMed] [Google Scholar]
- 16.Pres D, Gasior M, Strojek K, et al Blood glucose level on admission determines in‐hospital and long‐term mortality in patients with ST‐segment elevation myocardial infarction complicated by cardiogenic shock treated with percutaneous coronary intervention. Kardiol Pol 2010; 68: 743–751 [PubMed] [Google Scholar]
- 17.Cruz‐Gonzalez I, Chia S, Raffel OC, et al Hyperglycemia on admission predicts larger infarct size in patients undergoing percutaneous coronary intervention for acute ST‐segment elevation myocardial infarction. Diabetes Res Clin Pract 2010; 88: 97–102 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Ako J, Kadowaki T, et al Impact of acute hyperglycemia during primary stent implantation in patients with ST‐elevation myocardial infarction. J Cardiol 2009; 53: 272–277 [DOI] [PubMed] [Google Scholar]
- 19.Ryan TJ, Anderson JL, Antman EM, et al ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol 1996; 28: 1328–1428 [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K, Alpert JS, White HD, et al Universal definition of myocardial infarction. Circulation 2007; 116: 2634–2653 [DOI] [PubMed] [Google Scholar]
- 21.Chesebro JH, Knatterud G, Roberts R, et al Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987; 76: 142–154 [DOI] [PubMed] [Google Scholar]
- 22.Ergelen M, Uyarel H, Cicek G, et al Which is worst in patients undergoing primary angioplasty for acute myocardial infarction? Hyperglycaemia? Diabetes mellitus? Or both? Acta Cardiol 2010; 65: 415–423 [DOI] [PubMed] [Google Scholar]
- 23.Chien KL, Hsu HC, Su TC, et al Fasting and postchallenge hyperglycemia and risk of cardiovascular disease in Chinese: the Chin‐Shan Community Cardiovascular Cohort study. Am Heart J 2008; 156: 996–1002 [DOI] [PubMed] [Google Scholar]
- 24.Ramlo‐Halsted BA, Edelman SV. The natural history of type 2 diabetes. Implications for clinical practice. Prim Care 1999; 26: 771–789 [DOI] [PubMed] [Google Scholar]
- 25.Capes SE, Hunt D, Malmberg K, et al Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773–778 [DOI] [PubMed] [Google Scholar]
- 26.Ceriello A. Acute hyperglycaemia: a ‘new’ risk factor during myocardial infarction. Eur Heart J 2005; 26: 328–331 [DOI] [PubMed] [Google Scholar]
- 27.Ishihara M, Inoue I, Kawagoe T, et al Is admission hyperglycaemia in non‐diabetic patients with acute myocardial infarction a surrogate for previously undiagnosed abnormal glucose tolerance? Eur Heart J 2006; 27: 2413–2419 [DOI] [PubMed] [Google Scholar]
- 28.Malmberg K, Yusuf S, Gerstein HC, et al Impact of diabetes on long‐term prognosis in patients with unstable angina and non‐Q‐wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 2000; 102: 1014–1019 [DOI] [PubMed] [Google Scholar]
- 29.McGuire DK, Emanuelsson H, Granger CB, et al Influence of diabetes mellitus on clinical outcomes across the spectrum of acute coronary syndromes. Findings from the GUSTO‐IIb study. GUSTO IIb Investigators. Eur Heart J 2000; 21: 1750–1758 [DOI] [PubMed] [Google Scholar]
- 30.Ishihara M, Kagawa E, Inoue I, et al Impact of admission hyperglycemia and diabetes mellitus on short‐ and long‐term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol 2007; 99: 1674–1679 [DOI] [PubMed] [Google Scholar]
- 31.Pinto DS, Skolnick AH, Kirtane AJ, et al U‐shaped relationship of blood glucose with adverse outcomes among patients with ST‐segment elevation myocardial infarction. J Am Coll Cardiol 2005; 46: 178–180 [DOI] [PubMed] [Google Scholar]


