Abstract
Aims/Introduction
Type 2 diabetes is often complicated by diabetic foot syndrome (DFS). We analyzed the circulating stem cells, growth factor and anti‐oxidant gene expression profiles in type 2 diabetes patients without or with different forms of DFS.
Materials and Methods
Healthy volunteers (n = 13) and type 2 diabetes patients: (i) without DFS (n = 10); or with (ii) Charcot osteoneuropathy (n = 10); (iii) non‐infected (n = 17); (iv) infected (n = 11); and (v) healed ulceration were examined (n = 12). Peripheral blood endothelial progenitor cells (EPC), mesenchymal stem cells (MSC), hematopoietic stem cells (HSC) and very small embryonic‐like (VSEL) cells were phenotyped using flow cytometry. Plasma cytokine concentrations and gene expressions in blood cells were measured by Luminex and quantitative real‐time polymerase chain reaction assays, respectively.
Results
Patients with non‐complicated type 2 diabetes showed reduced HMOX1 expression, accompanied by HMOX2 upregulation, and had less circulating EPC, MSC or HSC than healthy subjects. In contrast, VSEL cells were elevated in the type 2 diabetes group. However, subjects with DFS, even with healed ulceration, had fewer VSEL cells, more CD45‐CD29+CD90+MSC, and upregulated HMOX1 when compared with the type 2 diabetes group. Patients with Charcot osteopathy had lowered plasma fibroblast growth factor‐2. Elevated plasma tumor necrosis factor‐α and decreased catalase expression was found in all diabetic patients.
Conclusions
Patients with type 2 diabetes and different forms of DFS have an altered number of circulating stem cells. Type 2 diabetes might also be associated with a changed plasma growth factor and anti‐oxidant gene expression profile. Altogether, these factors could contribute to the pathogenesis of different forms of DFS.
Keywords: Anti‐oxidant genes, Diabetic foot syndrome, Stem cells
Introduction
Diabetic foot syndrome (DFS), which includes ulcerations, infections and Charcot osteoneuropathy, is a frequent cause of amputations in diabetic patients. The pathogenesis of DFS, in addition to well established risk factors; that is, long diabetes duration and chronic hyperglycemia1, could be associated with alterations in stem or progenitor cell mobilization2, changes in growth factors3 and impairment in cellular anti‐oxidant capacity5; however, data supporting this hypothesis are scarce. Even less is known about how these factors might influence different forms of DFS, especially when the ulcer is further complicated with infection or when it is healed.
The number of smooth muscle or endothelial progenitors in peripheral blood (PB) is altered in diabetic patients, which might result in reduced vascular repair capacity7. Of note, type 2 diabetes also impairs endothelial progenitor cell proliferation, migration and the ability to incorporate to the pre‐existing blood vessels8. Furthermore, other populations, such as mesenchymal stem cells (MSC) or hematopoietic stem cells (HSC), which can contribute to revascularization and wound healing10, can be affected. Mesenchymal stem cells, which are precursors of connective tissue cells; that is, fibroblasts, osteoblasts and chondrocytes14, are of special interest in the Charcot foot that affects both bones and joints15. Additionally, the role of very small embryonic‐like (VSEL) cells, claimed to be pluripotent and mobilized in stroke, severe burns and acute myocardial infarction16, has not yet been studied in the complications of type 2 diabetes.
We compared the circulating stem cell subpopulations, growth factor, and anti‐oxidant gene expression profiles in type 2 diabetes patients with and without specific clinical forms of diabetic foot syndrome.
Methods
Patients
We examined healthy volunteers (H; n = 13) and five groups of type 2 diabetes patients: (i) without DFS (type 2 diabetes, n = 10); (ii) with Charcot peripheral osteoneuropathy (n = 10); (iii) with non‐infected ulceration (DFU; n = 17); (iv) with infected ulceration (DFU‐I; n = 11); and (v) with healed ulceration (DFU‐H; n = 12). The diagnosis of infection was based on clinical examination. We used the perfusion, extent or size, depth of tissue loss, infection, sensation or neuropathy (PEDIS) scale criteria17. The infected ulceration was diagnosed only in the case of sure and typical signs of infection, such as the presence of necrotic tissues, and/or purulent exudation and odor. All patients had at least moderate infection (grade 2 PEDIS). The non‐infected ulceration was diagnosed in patients without any clinical signs of infection (grade 1 PEDIS). The characteristics of the patients are shown in Table S1. The research complied with the Declaration of Helsinki and was approved by the local ethics committee. Patients provided written informed consent for the study.
Blood Sample Collection
Blood samples were collected in three 5.0‐mL ethylenediaminetetraacetic acid tubes (Sarstedt, Numbrecht, Germany) at the Clinic of Metabolic Diseases at 08.00–09.00 h after overnight fasting, and processed within 1 h. Plasma levels of glucose, total cholesterol, blood urea nitrogen, total bilirubin and aspartate aminotransferase (GOT/AST) were measured with SpotChem Panel I Multi‐parameter Strips, using a SpotChem EZ SP‐4430 (Arkray, Amstelveen, the Netherlands) analyzer. Plasma was transferred to the 1.5‐mL tubes and frozen after the centrifugation for 10 min at 670 g. Total nucleated cells (TNCs) were obtained from the blood samples after the double ammonium chloride red blood cell lysis (0.15 mol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L ethylenediaminetetraacetic acid) and resuspended in autoMACS Running Buffer (Miltenyi Biotec, Auburn, CA, USA) supplemented with 2% of foetal bovine serum (Lonza, Basel, Switzerland).
Analysis of Cell Subpopulations
Progenitor and stem cells in peripheral blood were evaluated using flow cytometry. The following subpopulations were analyzed based on their surface markers: (i) EPC: CD45dimCD31+CD133+ and CD45dimCD31+CD34+KDR+; (ii) MSC: CD45−CD105+STRO1+ and CD45−CD29+CD90+; and (iii) HSC: Lin−CD45+CD133+ and Lin−CD45+CD34+. Additionally, we evaluated the Lin−CD45−CD133+ and Lin−CD45−CD34+ cells, described as VSEL. Peripheral blood TNC were stained using fluorescently conjugated antibodies: CD45dimCD31+CD133+ cells were stained with anti‐CD45‐FITC (clone HI30; BD Biosciences, San Diego, CA, USA), anti‐CD31‐PE (clone WM59; Biolegend, San Diego, CA, USA), anti‐CD133/AC133‐APC (clone AC133; Miltenyi Biotec), CD45dimCD31+CD34+KDR+ with anti‐CD45‐FITC (clone HI30; BD Biosciences), anti‐CD31‐PE (clone WM59; Biolegend), anti‐CD34‐PE‐Cy5 (clone 581; BD Pharmingen, San Diego, CA, USA), anti‐KDR(CD309)‐APC (clone Avas12; Biolegend), Lin−CD45+CD133+ and Lin−CD45−CD133+ anti‐CD45‐PE (clone HI30; BD Pharmingen), anti‐CD133/AC133‐APC (clone AC133; Miltenyi Biotec), Lin−CD45+CD34+ and Lin−CD45−CD34+ with anti‐CD45‐PE (clone HI30; BD Pharmingen), anti‐CD34‐APC (clone 581; BD Pharmingen) and a cocktail of antibodies for hematopoietic lineage markers (Lin) anti‐CD2‐FITC (clone RPA2.10), anti‐CD3‐FITC (clone UCHT1), anti‐CD14‐FITC (clone M5E2), anti‐CD16‐FITC (clone 3G8), anti‐CD19‐FITC (clone HIB19), anti‐CD24‐FITC (clone ML5), anti‐CD56‐FITC (clone NCAM16.2), anti‐CD66b‐FITC (clone G10F5), anti‐CD235a‐FITC (clone GA‐R2[HIR2]), all from BD Pharmingen; CD45−CD105+STRO1+ cells were stained with anti‐CD45‐FITC (clone HI30; BD Biosciences), anti‐CD105‐PE (clone 43A3; Biolegend), anti‐STRO1‐APC (clone STRO‐1; Biolegend), CD45−CD29+CD90+ with anti‐CD45‐FITC (clone HI30; BD Biosciences), anti‐CD29‐PE‐Cy5 (clone MAR4; BD Pharmingen) and anti‐CD90‐PE (clone 5E10; Biolegend). Cells were stained for 30 min at 4°C, then washed with phosphate‐buffered saline, collected on BD LSR II flow cytometer (Becton Dickinson, San Diego, CA, USA) and analyzed with BD FACS Diva software (Becton Dickinson). Number of cells per 1 mL of peripheral blood (PB) was calculated based on the total leukocyte count (WBC, 103 cells per 1 mL of PB) and the percentage of each population within the collected events. Gating strategies for flow cytometry are shown on Figures S1–S3.
Analysis of Gene Expression
Total ribonucleic acid was isolated from the peripheral blood nucleated cells with phenol–chloroform extraction, and reverse transcribed with the oligo(dT) primers and RevertAid reverse transcriptase (Fermentas; Thermo Fisher Scientific, Waltham, MA, USA). The expression of heme oxygenase‐1 (HMOX1), heme oxygenase‐2 (HMOX2), Cu/Zn superoxide dismutase (SOD1), catalase (CAT), nicotinamide adenine dinucleotide phosphate quinone oxidoreductase 1 (NQO1) in total nucleated cells (TNCs) was assessed with quantitative real‐time polymerase chain reaction, which was carried out in the StepOnePlus system (Applied Biosystems, Foster City, CA, USA) with the specific primers (Table S2), 50 ng of complementary deoxyribonucleic acid and SYBR Green Quantitative RT‐PCR kit (Sigma‐Aldrich, St. Louis, MO, USA), under conditions summarized in the Table S3.
Analysis of Growth Factor Concentrations
Concentrations of stem cell factor (SCF), leukemia inhibitory factor (LIF), thrombopoietin (TPO), epidermal growth factor (EGF), fibroblast growth fator‐2 (FGF‐2), fms‐like tyrosine kinase‐3 ligand (Flt‐3‐L), granulocyte colony‐stimulating factor (G‐CSF), tumor necrosis factor‐α (TNF‐α), and stromal cell‐derived factor‐1α and β (SDF‐1α+β) in plasma were evaluated using Milliplex FlexMap 3D (Millipore, Billerica, MA, USA) according to the vendor's protocol.
Analysis of Plasma Total Anti‐oxidant Capacity
Plasma total anti‐oxidant capacity was measured with the 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid; ABTS) assay according to the protocol by Erel18. Briefly, 5 μL of plasma was mixed with 200 μL of 0.4 mol/L acetate buffer, pH 5.8, 20 μL of 10 mmol/L ABTS+ in 30 mmol/L acetate buffer pH 3.6 and incubated for 5 min at room temperature. Absorbance at λ = 660 nm was read using the plate reader. Trolox was used for the standard curve.
Statistical Analysis
Normality of data was checked with the D'Agostino–Pearson omnibus test. Statistical significance was assessed with the Mann–Whitney U‐test and accepted at P < 0.05 level. All data obtained was correlated using Spearman's rank correlation.
Results
Circulating Stem and Progenitor Cells
The number of EPC defined as CD45dimCD31+CD133+ (Figure 1a) or CD45dimCD31+CD34+KDR+ (Figure 1b) was decreased in type 2 diabetes patients in comparison with healthy controls, and in general was not further modified by diabetic foot syndrome forms. The exemption was infected diabetic foot ulcer, which led to an increase in the CD45dimCD31+CD34+KDR+ subpopulation (P = 0.01 for DFU‐I vs DFU). Both EPC subpopulations correlated negatively with glycated hemoglobin (HbA1c) (CD45dimCD31+CD133+ Spearman's r = −0.29 P = 0.014, CD45dimCD31+CD34+KDR+ Spearman's r = −0.47, P < 0.0001), although only in the DFU‐H group was HbA1c lower than in the type 2 diabetes and DFU groups (P = 0.0393 and P = 0.0375, respectively). CD45−CD29+CD90+ MSC were less numerous in type 2 diabetes and Charcot osteoneuropathy patients than in healthy volunteers (Figure 1c), whereas in groups with infected or healed ulcers their numbers increased to the control values. However, a similar pattern without an increase in the DFU‐I group was observed for MSC defined as CD45−CD105+STRO1+ (Figure 1d). Patients with type 2 diabetes either with or without DFS had also less Lin−CD45+CD133+ HSC (Figure 1e). Differences for Lin−CD45+CD34+ HSC were similar, but less pronounced (Figure 1f).
Figure 1.
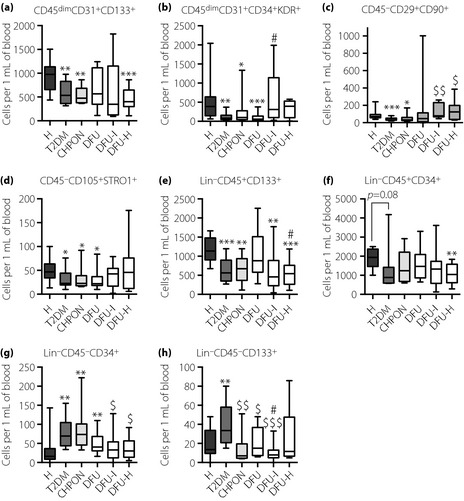
Numbers of circulating stem and progenitor cells in the peripheral blood of patients. (a) CD45dimCD31+CD133+ endothelial progenitor cells (EPC); (b) CD45dimCD31+CD34+KDR+ EPC; (c) CD45−CD29+CD90+ mesenchymal stem cells (MSC); (d) CD45−CD105+STRO‐1 MSC; (e) Lin−CD45+CD133+ hematopoietic stem cells (HSC); (f) Lin−CD45+CD34+ HSC; (g) Lin−CD45−CD34+ very small embryonic‐like (VSEL) cells; (h) Lin−CD45−CD133+ VSEL cells. Flow cytometry phenotyping. *P < 0.05, **P < 0.01, ***P < 0.001 vs healthy volunteers (H); $P < 0.05, $$P < 0.01, $$$P < 0.001 vs type 2 diabetes group (T2DM); #P < 0.05 vs type 2 diabetes patients with non‐infected ulceration (DFU). Results are shown as box and whisker charts displaying minimum, maximum, median, upper and lower quartiles. CHPON, Charcot peripheral osteoneuropathy; DFU‐H, type 2 diabetes patients with healed ulceration; DFU‐I, type 2 diabetes patients with infected ulceration.
The number of Lin−CD45−CD34+ VSEL cells was increased in type 2 diabetes and Charcot osteoneuropathy patients (P < 0.01 and P < 0.05 vs the healthy group, respectively; Figure 1g) and correlated positively with HbA1c (Spearman's r = 0.32, P = 0.007). This rise was weaker in diabetic patients with foot ulceration, especially in the DFU‐I and DFU‐H groups. The Lin−CD45−CD133+ VSEL population was also less numerous in comparison with the non‐complicated type 2 diabetes group in all groups with DFS (Figure 1h). Of note, populations of endothelial progenitor cells, mesenchymal stem cells and hematopoietic stem cells/progenitors in general correlated positively with each other. Interestingly, numbers of Lin−CD45−CD133+ and Lin−CD45−CD34+ VSEL cells also correlated positively with each other, and the number of Lin−CD45−CD34+ cells correlated negatively with CD45dimCD31+CD34+KDR+ EPC and CD45−CD29+CD90+ MSC populations (Table S4).
Next, we checked whether the presence of stem cell subpopulations allowed for detection of the POU5F1 transcript, which encodes OCT4A – a key regulator of pluripotency. The results show that primers designed to avoid detection of known pseudogenes (Figure 2a) did not show any POU5F1 expression in PB TNCs (Figure 2b), although amplified in the positive control (Figure 2c). Additionally, no signal was detected in human Lin−CD45− cells sorted from the bone marrow (data not shown).
Figure 2.
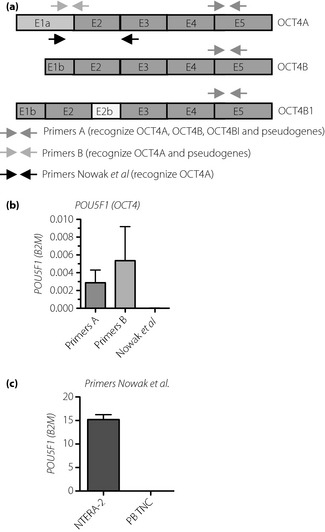
Schematic localization of recognized sequences in the gene encoding OCT4. (a) E1a‐E5 exons. (b) Analysis of POU5F1 expression in peripheral blood total nucleated cells (PB TNC) of patients (mean ± standard deviation, n = 8, randomly chosen patients). Primers A and B provide false‐positive signals. (c) Primers by Nowak et al. produce no signal. Comparison of signal from NTERA‐2 human embryonal carcinoma cell line (positive control) and PB TNC using primers by Nowak et al.
Expression of Anti‐oxidant Genes
Peripheral blood nucleated cells in all groups of diabetic patients showed a downregulation of catalase (Figure 3a), which correlated negatively with the Lin−CD45−CD34+ population (P = 0.024, Spearman's r = −0.30) and HbA1c (P = 0.023, Spearman's r = −0.33). A similar decrease in patients with non‐complicated type 2 diabetes was found for HMOX1 (Figure 3b), regulated in response to oxidative stress by Nrf2 transcription factor. Here, the inhibition of HMOX1 was possibly not associated with Nrf2, as NQO1 – another Nrf2 target – showed an opposite tendency (Figure S4A). The expression of HMOX1 was augmented in all groups with foot disorders (Figure 3b), and correlated negatively with the Lin−CD45−CD34+ VSEL population (P = 0.024, Spearman's r = −0.291). Furthermore, patients with healed foot ulcerations had higher expression of HMOX1 than those with non‐infected wounds (P < 0.05). Interestingly, the expression of HMOX2, which is considered a constitutive gene, was higher in type 2 diabetes, Charcot osteoneuropathy and diabetic foot ulcer groups than in healthy subjects (Figure 3c), what might be considered as a kind of compensatory mechanism. Expression of SOD1 was higher in patients with the non‐complicated type 2 diabetes than in healthy controls (Figure 3d), and correlated positively with both VSEL populations (Lin−CD45−CD133+ P = 0.057, Spearman's r = 0.24; Lin−CD45−CD34+ P = 0.006, Spearman's r = 0.34), NQO1 (P = 0.037, Spearman's r = 0.28), HMOX2 (P < 0.0001, Spearman's r = 0.72), HbA1c (P = 0.036, Spearman's r = 0.28), but negatively with CD45dimCD31+CD34+KDR+ EPCs (P = 0.024, Spearman's r = −0.26) and expression of HMOX1 (P = 0.002, Spearman's r = −0.40). Finally, using the ABTS assay we checked the plasma total anti‐oxidant capacity. Surprisingly, the anti‐oxidant capacity was higher in plasma of non‐complicated type 2 diabetes or DFU and DFU‐H groups than in healthy controls (P < 0.01, P < 0.05, P < 0.05, respectively; Figure S4B). Furthermore, plasma anti‐oxidant capacity correlated positively with the HMOX2 (Spearman's r = 0.017, P = 0.33) and SOD1 (Spearman's r = 0.28, P = 0.045) expression. The expression of anti‐oxidant genes, CAT, SOD1 or HMOX1, was not affected by the metformin therapy (data not shown).
Figure 3.
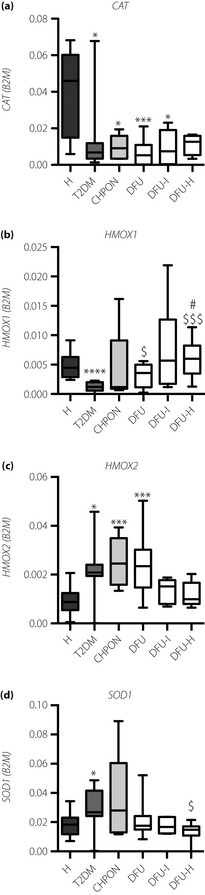
Expression of anti‐oxidant genes in circulating total nucleated cells and concentration of plasma cytokines in peripheral blood of patients. (a) Catalase. (b) HMOX1. (c) HMOX2. (d) SOD1. Expression of messenger ribonucleic acid was determined by quantitative real‐time polymerase chain reaction. B2M served as a constitutive control. *P < 0.05, **P < 0.01, ***P < 0.001 vs healthy volunteers (H); $P < 0.05 vs type 2 diabetes group (T2DM); #P < 0.05 vs type 2 diabetes patients with non‐infected ulceration (DFU). Results are shown as box and whisker charts displaying minimum, maximum, median, upper and lower quartiles. CHPON, Charcot peripheral osteoneuropathy; DFU‐H, type 2 diabetes patients with healed ulceration; DFU‐I, type 2 diabetes patients with infected ulceration.
Concentration of cytokines
TNF‐α was elevated in all diabetic patients, regardless of the presence of DFS (Figure 4a). Type 2 diabetes patients also had a trend towards the lowered concentration of EGF (Figure 4b), the effect was less pronounced in groups with DFS. Of note, the level of plasma EGF correlated positively with the number of circulating CD45−CD29+CD90+ MSC (Spearman's r = 0.34, P = 0.003). Interestingly, patients with Charcot osteoneuropathy were the only participants with a reduced level of FGF‐2 (Figure 4c). Concentrations of G‐CSF, Flt‐3‐L, SDF1α, SDF1β, LIF, TPO and SCF (Figure S4C–H) were similar in all groups, and did not correlate with the number of stem or progenitor cells.
Figure 4.
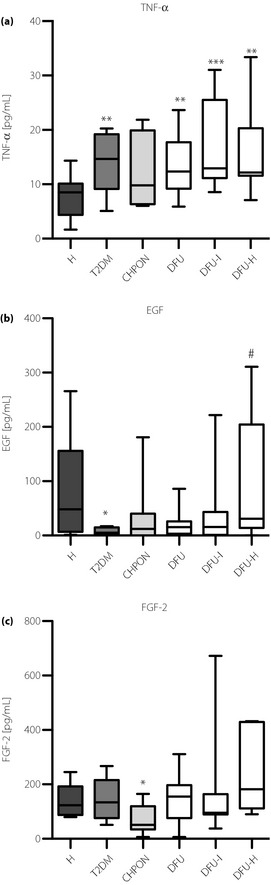
Plasma levels of (a) tumor necrosis factor‐α (TNF‐α); (b) epidermal growth factor (EGF); and (c) fibroblast growth fator‐2 (FGF‐2). The concentration of cytokines in plasma was measured using the Milliplex FlexMap 3D method. *P < 0.05, **P < 0.01, ***P < 0.001, vs healthy volunteers (H); $P < 0.05 vs type 2 diabetes group (T2DM); #P < 0.05 vs type 2 diabetes patients with non‐infected ulceration (DFU). Results are shown as box and whisker charts displaying minimum, maximum, median, upper and lower quartiles. CHPON, Charcot peripheral osteoneuropathy; DFU‐H, type 2 diabetes patients with healed ulceration; DFU‐I, type 2 diabetes patients with infected ulceration.
Discussion
The most consistent finding of the current study shows that all groups of diabetic patients, with and without DFS, had an elevated concentration of plasma TNF‐α and decreased expression of catalase in peripheral blood nucleated cells. This could indicate a chronic inflammatory reaction and reduced cellular anti‐oxidative capabilities. A low level of catalase in the blood cells of diabetic patients, accompanied by enhanced oxidative stress, has been already reported6. Of note, catalase expression is regulated by peroxisome proliferator‐activated receptor‐γ, the target for antidiabetic thiazolidinediones19. Furthermore, the inherited catalase deficiency has been reported to be a risk factor for the development of type 2 diabetes20.
Type 2 diabetes patients without DFS had also less circulating EPC, MSC and HSC, when compared with healthy subjects. The decrease found in EPC confirms the previously published data2, whereas HSC and MSC in diabetes have not been this thoroughly studied hitherto. Interestingly, both subpopulations of EPC correlated negatively with HbA1c. We can suggest that the simultaneous decrease in circulating EPC, MSC and HSC might reflect the impaired stem cell niche function in type 2 diabetes21.
In contrast, Lin−CD45−CD34+ and, to a lesser extent, Lin−CD45−CD133+ populations, both described as VSEL pluripotent cells16, were elevated in type 2 diabetes patients. The latter ones were less numerous in groups with DFS than in patients without these complications. One could speculate that VSEL cell mobilization is a defensive mechanism, less effective in patients with diabetic complications. In contrast, a recent study showed that such populations (Lin−CD45−CXCR4+CD34+ among them) do not express pluripotency markers, including POU5F1, and contain many aneuploid cells and products of defective cell divisions23. Accordingly, although using meticulously designed primers, we were unable to detect any POU5F1 transcript in peripheral blood nucleated cells. Furthermore, in our recent study on the murine bone marrow‐derived FSClowLin−CD45−Sca‐1+ events, which fulfil the VSEL criteria, we showed that such cells can be contaminated with the nuclei expelled during the erythropoiesis. Murine VSELs are also highly enriched in terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labelling events, with deoxyribonucleic acid fragmentation. Importantly, we were not able to show Oct‐4A expression in either sorted VSEL populations or at the single‐cell level24.
The number of Lin−CD45−CD34+ cells correlated positively with HbA1c, and negatively with the expression of cytoprotective HMOX1. This might suggest that the elevation of these very rare populations results from hyperglycemia‐induced tissue injury. Increases in the number of circulating Lin−CD45−CD133+ and Lin−CD45−CD34+ events have been previously shown in myocardial infarction25, skin burns28, stroke29 and inflammatory bowel disease30. However, the reported numbers of such events are highly variable, ranging from 0.03530 to 0.33 per 1 μL of blood28, even in healthy controls. Finally, there is so far no proof for the active participation of human VSELs in the tissue regeneration or definitive evidence for their stemness. Altogether, we speculate that VSELs might be a heterogenous population of events that are rather the markers of tissue injury.
Apart from changes in TNF‐α and catalase levels, type 2 diabetes patients showed a trend towards decreased concentration of EGF, and reduced expression of HMOX1, compensated by an upregulation of HMOX2. In mice, a decrease in EGF or HMOX1 enhances the risk of diabetic complications and impairs wound healing31. In contrast, HMOX1 was higher in DFU patients, which could indicate an increased oxidative stress or inflammatory reaction. Rapid upregulation of HMOX1 in response to tissue injury is well‐known33. However, in the db/db diabetic mice, we showed that such induction was weaker and delayed as compared with that in healthy counterparts, and was not sufficient to maintain the proper vascularization and healing of the wounded tissue33. Additional overexpression of HMOX1 using adenoviral vector‐mediated gene transfer33 or injection of mesenchymal stem cells pretreated with EGF34 significantly improves neovascularization and healing of wounded skin33, or facilitates angiogenesis in ischemic limbs34 in diabetic mice.
The parameters, which were different in patients with DFS, even with ulceration that had already healed, than in those without this complication included a lower number of VSEL cells, higher CD45−CD29+CD90+ MSC, and augmented expression of HMOX1. Interestingly, intramuscular injection and topical administration of autologous MSC accelerated ulcer healing in the clinical study35. Of note, the number of circulating EPC and HSPC was no different between the DFU group and healthy controls. Interestingly, in db/db diabetic mice, both EPC and HSPC are normally released from the bone marrow, but poorly to the site of injury36.
Infected ulcers were additionally associated with increased CD45dimCD31+CD34+KDR+ endothelial progenitors and CD45−CD29+CD90+ mesenchymal stem cells, whereas numbers of hematopoietic stem and progenitor cells, as well as VSELs, remained low. Interestingly, the characteristic feature of patients with peripheral Charcot osteoneuropathy was a lowered level of plasma FGF‐2, which might be related to the decreased number of endothelial progenitors preventing neuropathy in mice37. FGF‐2 has been found to be neuroprotective in the rat model of diabetic neuropathy38, also when secreted by intramuscularly injected mesenchymal stem cells39.
In summary, although the present study included a limited number of participants, the results show that patients with type 2 diabetes and different forms of DFS have an altered number of circulating stem cells. Type 2 diabetes might also be associated with a changed serum growth factor and anti‐oxidant gene expression profile. Altogether, these factors can contribute to the pathogenesis of different DFS forms.
Supplementary Material
Table S1 | Characteristics of patients.
Table S2 | Sequence of primers used for the quantitative real‐time polymerase chain reaction.
Table S3 | Conditions for the quantitative real‐time polymerase chain reaction reactions.
Table S4 | Spearman's correlation of tested circulating cell populations.
Figure S1 | Gating strategy for CD45dimCD31+CD133+ and CD45dimCD31+CD34+KDR+ endothelial progenitor cells.
Figure S2 | Gating strategy for CD45−CD105+STRO‐1+ and CD45−CD29+CD90+ mesenchymal stem/progenitor cells.
Figure S3 | Gating strategy for Lin−CD45+CD133+ and Lin−CD45+CD34+ hematopoietic stem and progenitor cells, and Lin−CD45−CD133+ and Lin−CD45−CD34+ enriched with very small embryonic‐like cells [VSEL].
Figure S4 | Plasma total anti‐oxidant capacity, expression of anti‐oxidant genes in circulating total nucleated cells and concentration of plasma cytokines in peripheral blood of patients.
Acknowledgments
The authors have no relevant conflicts of interests to disclose. We thank Dr Ewa Zuba‐Surma (Jagiellonian University, Faculty of Biochemistry, Biophysics and Biotechnology, Department of Cell Biology, Krakow, Poland) for the initial technical assistance with immunolabelling protocols. The authors are grateful to Ms Aleksandra Malecka (Warsaw University, Warsaw, Poland) for her editorial and linguistic assistance. This work was supported by the European Union Innovative Economy Programme grants POIG 01.01.02‐00‐109/09, and 01.01.02.069/09 with the equipment purchased also in the frame of 02.01.00‐12‐064/08, 02.02.00‐00‐014/08 grants. MTM was supported by the Jagiellonian University Medical College grant K/ZDS/002808. JD and AJ participate in the HypoxiaNet COST Action TD0901 and European Network on Gasotransmitters BM1005.
J Diabetes Invest 2014; 5: 99–107
References
- 1.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–1625 [DOI] [PubMed] [Google Scholar]
- 2.Fadini GP, Miorin M, Facco M, et al Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 2005; 45: 1449–1457 [DOI] [PubMed] [Google Scholar]
- 3.Nandy D, Mukhopadhyay D, Basu A. Both vascular endothelial growth factor and soluble Flt‐1 are increased in type 2 diabetes but not in impaired fasting glucose. J Investig Med 2010; 58: 804–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeboah J, Sane DC, Crouse JR, et al Low plasma levels of FGF‐2 and PDGF‐BB are associated with cardiovascular events in type II diabetes mellitus (diabetes heart study). Dis Markers 2007; 23: 173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodovici M, Giovannelli L, Pitozzi V, et al Oxidative DNA damage and plasma antioxidant capacity in type 2 diabetic patients with good and poor glycaemic control. Mutat Res 2008; 638: 98–102 [DOI] [PubMed] [Google Scholar]
- 6.Pan H‐Z, Zhang L, Guo M‐Y, et al The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol 2010; 47(Suppl 1): 71–76 [DOI] [PubMed] [Google Scholar]
- 7.van Ark J, Moser J, Lexis CPH, et al Type 2 diabetes mellitus is associated with an imbalance in circulating endothelial and smooth muscle progenitor cell numbers. Diabetologia 2012; 55: 2501–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thum T, Fraccarollo D, Schultheiss M, et al Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 2007; 56: 666–674 [DOI] [PubMed] [Google Scholar]
- 9.Sorrentino SA, Bahlmann FH, Besler C, et al Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with Type 2 diabetes mellitus: restoration by the peroxisome proliferator‐activated receptor‐ agonist rosiglitazone. Circulation 2007; 116: 163–173 [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Chen L, Scott PG, et al Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007; 25: 2648–2659 [DOI] [PubMed] [Google Scholar]
- 11.Grant MB, May WS, Caballero S, et al Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 2002; 8: 607–612 [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Pompili VJ, Das H. Neovascularization and hematopoietic stem cells. Cell Biochem Biophys 2011; doi:10.1007/s12013‐011‐9298‐x (Epub ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falanga V, Iwamoto S, Chartier M, et al Autologous bone marrow–derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007; 13: 1299–1312 [DOI] [PubMed] [Google Scholar]
- 14.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997; 276: 71–74 [DOI] [PubMed] [Google Scholar]
- 15.Rogers LC, Frykberg RG, Armstrong DG, et al The charcot foot in diabetes. Diabetes Care 2011; 34: 2123–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratajczak MZ, Liu R, Marlicz W, et al Identification of very small embryonic/epiblast‐like stem cells (VSELs) circulating in peripheral blood during organ/tissue injuries. Methods Cell Biol 2011; 103: 31–54 [DOI] [PubMed] [Google Scholar]
- 17.Lipsky BA, Berendt AR, Cornia PB, et al Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54: 1679–1684 [DOI] [PubMed] [Google Scholar]
- 18.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 2004; 37: 277–285 [DOI] [PubMed] [Google Scholar]
- 19.Okuno Y, Matsuda M, Miyata Y, et al Human catalase gene is regulated by peroxisome proliferator activated receptor‐gamma through a response element distinct from that of mouse. Endocr J 2010; 57: 303–309 [DOI] [PubMed] [Google Scholar]
- 20.Góth L, Nagy T. Acatalasemia and diabetes mellitus. Arch Biochem Biophys 2012; 525: 195–200 [DOI] [PubMed] [Google Scholar]
- 21.Ferraro F, Lymperi S, Mendez‐Ferrer S, et al Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Science Transl Med 2011; 3: 104ra101 Available from: http://stm.sciencemag.org/cgi/doi/10.1126/scitranslmed.3002191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucia M, Halasa M, Wysoczynski M, et al Morphological and molecular characterization of novel population of CXCR4+ SSEA‐4+ Oct‐4+ very small embryonic‐like cells purified from human cord blood: preliminary report. Leukemia 2007; 21: 297–303 [DOI] [PubMed] [Google Scholar]
- 23.Danova‐Alt R, Heider A, Egger D, et al Very small embryonic‐like stem cells purified from umbilical cord blood lack stem cell characteristics. PLoS ONE 2012; 7: e34899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szade K, Bukowska‐Strakova K, Nowak WN, et al Murine bone marrow Lin−Sca‐1+CD45− very small embryonic‐like (VSEL) cells are heterogeneous population lacking Oct‐4A expression. PLoS ONE 2013; 8: e63329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel‐Latif A, Zuba‐Surma EK, Ziada KM, et al Evidence of mobilization of pluripotent stem cells into peripheral blood of patients with myocardial ischemia. Exp Hematol 2010; 38: 1131–1142.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karapetyan AV, Klyachkin YM, Selim S, et al Bioactive lipids and cationic antimicrobial peptides as new potential regulators for trafficking of bone marrow‐derived stem cells in patients with acute myocardial infarction. Stem Cells Dev 2013; 22: 1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wojakowski W, Ratajczak M, Tendera M. Mobilization of very small embryonic‐like stem cells in acute coronary syndromes and stroke. Herz 2010; 35: 467–473 [DOI] [PubMed] [Google Scholar]
- 28.Drukała J, Paczkowska E, Kucia M, et al Stem cells, including a population of very small embryonic‐like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Rev 2012; 8: 184–194 [DOI] [PubMed] [Google Scholar]
- 29.Paczkowska E, Kucia M, Koziarska D, et al Clinical evidence that very small embryonic‐like stem cells are mobilized into peripheral blood in patients after stroke. Stroke 2009; 40: 1237. [DOI] [PubMed] [Google Scholar]
- 30.Marlicz W, Zuba‐Surma E, Kucia M, et al Various types of stem cells, including a population of very small embryonic‐like stem cells, are mobilized into peripheral blood in patients with Crohn's disease. Inflamm Bowel Dis 2012; 18: 1711–1722 [DOI] [PubMed] [Google Scholar]
- 31.Grochot‐Przeczek A, Dulak J, Jozkowicz A. Heme oxygenase‐1 in neovascularisation: a diabetic perspective. Thromb Haemost 2010; 104: 424–431 [DOI] [PubMed] [Google Scholar]
- 32.Kasayama S, Ohba Y, Oka T. Epidermal growth factor deficiency associated with diabetes mellitus. Proc Natl Acad Sci USA 1989; 86: 7644–7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grochot‐Przeczek A, Lach R, Mis J, et al Heme oxygenase‐1 accelerates cutaneous wound healing in mice. PLoS ONE 2009; 4: e5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin AH, Elmageed ZYA, Nair D, et al Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind‐limb of type II diabetic mice. Lab Invest 2010; 90: 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dash NR, Dash SN, Routray P, et al Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Res 2009; 12: 359–366 [DOI] [PubMed] [Google Scholar]
- 36.Fiorina P, Pietramaggiori G, Scherer SS, et al The mobilization and effect of endogenous bone marrow progenitor cells in diabetic wound healing. cell Transplant 2010; 19: 1369–1381 [DOI] [PubMed] [Google Scholar]
- 37.Jeong JO, Kim MO, Kim H, et al Dual angiogenic and neurotrophic effects of bone marrow‐derived endothelial progenitor cells on diabetic neuropathy. Circulation 2009; 119: 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakae M, Kamiya H, Naruse K, et al Effects of basic fibroblast growth factor on experimental diabetic neuropathy in rats. Diabetes 2006; 55: 1470–1477 [DOI] [PubMed] [Google Scholar]
- 39.Shibata T, Naruse K, Kamiya H, et al Transplantation of bone marrow‐derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes 2008; 57: 3099–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Characteristics of patients.
Table S2 | Sequence of primers used for the quantitative real‐time polymerase chain reaction.
Table S3 | Conditions for the quantitative real‐time polymerase chain reaction reactions.
Table S4 | Spearman's correlation of tested circulating cell populations.
Figure S1 | Gating strategy for CD45dimCD31+CD133+ and CD45dimCD31+CD34+KDR+ endothelial progenitor cells.
Figure S2 | Gating strategy for CD45−CD105+STRO‐1+ and CD45−CD29+CD90+ mesenchymal stem/progenitor cells.
Figure S3 | Gating strategy for Lin−CD45+CD133+ and Lin−CD45+CD34+ hematopoietic stem and progenitor cells, and Lin−CD45−CD133+ and Lin−CD45−CD34+ enriched with very small embryonic‐like cells [VSEL].
Figure S4 | Plasma total anti‐oxidant capacity, expression of anti‐oxidant genes in circulating total nucleated cells and concentration of plasma cytokines in peripheral blood of patients.


