Abstract
Aims/Introduction
Gastric inhibitory polypeptide (GIP) is an incretin secreted from the gastrointestinal tract after an ingestion of nutrients, and stimulates an insulin secretion from the pancreatic islets. Additionally, GIP has important roles in extrapancreatic tissues: fat accumulation in adipose tissue, neuroprotective effects in the central nervous system and an inhibition of bone resorption. In the current study, we investigated the effects of GIP signaling on the peripheral nervous system (PNS).
Materials and Methods
First, the presence of the GIP receptor (GIPR) in mouse dorsal root ganglion (DRG) was evaluated utilizing immunohistochemical analysis, western blotting and reverse transcription polymerase chain reaction. DRG neurons of male wild‐type mice (WT) were cultured with or without GIP, and their neurite lengths were quantified. Functions of the PNS were evaluated in GIPR‐deficient mice (gipr−/−) and WT by using current perception thresholds (CPTs), Thermal Plantar Test (TPT), and motor (MNCV) and sensory nerve conduction velocity (SNCV, respectively). Sciatic nerve blood flow (SNBF) and plantar skin blood flow (PSBF) were also evaluated.
Results
We confirmed the expression of GIPR in DRG neurons. The neurite outgrowths of DRG neurons were promoted by the GIP administrations. The gipr−/− showed impaired perception functions in the examination of CPTs and TPT. Both MNCV and SNCV were delayed in gipr−/− compared with these in WT. There was no difference in SNBF and PSBF between WT and gipr−/−.
Conclusions
Our findings show that the GIP signal could exert direct physiological roles in the PNS, which might be directly exerted on the PNS.
Keywords: Gastric inhibitory polypeptide, Incretins, Peripheral nervous system
Introduction
Gastric inhibitory polypeptide (GIP) is one of the gastrointestinal regulatory peptides synthesized by K cells of the duodenum and small intestine1. GIP potentiates meal‐induced insulin secretion and lower blood glucose level1. Recently, incretin‐based therapies have been used clinically as novel therapy for type 2 diabetes, using receptor agonists of glucagon‐like peptide‐1 (GLP‐1), another incretin, and inhibitors of the incretin‐degrading enzyme dipeptidyl peptidase‐4 (DPP‐4)2. Although both GLP‐1 receptor (GLP‐1R) agonists and DPP‐4 inhibitors (DPP‐4I) improve glycemic control in type 2 diabetes patients, there is no consensus regarding the antidiabetic effect for GIP receptor (GIPR) agonists5. In addition, the extrapancreatic physiological function of GIP, the increase of lipoprotein lipase activity and fat accumulation, might cause the delay of clinical application of GIPR agonists8. Furthermore, there are some reports of other extra‐islet functions of GIP: inhibition of bone resorption9, decrease of intestinal motility10, and neurotrophic effects in the central nervous system (CNS)11. Some of these functions could be beneficial for type 2 diabetes patients frequently complicated by osteoporosis14 and cognitive disorder15. Therefore, GIPR agonists should be considered as an independent therapeutic tool for type 2 diabetes treatment.
Recent studies have described the important roles of some intestinal peptides in nerve development, regeneration and neuronal survival16. Many reports have suggested that GLP‐1R agonists have neuroprotective properties in both the CNS18 and the peripheral nervous system (PNS)20. The expressions of GIP and GIPR have been reported in the large pyramidal neurons in the cortex and the hippocampus12. One of these reports also showed that the proliferation of neuronal progenitors was enhanced by exogenous GIP, and was decreased in the dentate gyrus of GIPR‐deficient mice (gipr−/−)12. In another study, it has been reported that protease‐resistant GIP facilitated hippocampal long‐term potential (LTP) and improved impaired LTP induced by beta‐amyloid11. In contrast to the CNS, there are few studies that evaluate the physiological function of GIP/GIPR signaling in the PNS23. Buhren et al.23 showed that axonal regenerations were impaired in the gipr−/− compared with wild‐type mice (WT) after crush injuries of sciatic nerves. With regard to DPP‐4I (vildagliptin), prevention of peripheral nerve degeneration in streptozotocin‐induced diabetic rats has recently been shown24. Although active GIP is certainly increased by DPP‐4I, many other bioactive peptides, such as neuropeptide Y (NPY), substance P (SP), GLP‐1, glucagon‐like peptide‐2 and stromal cell–derived factor‐1α (SDF‐1α), have also been reported as substrates of DPP‐425. Thus, the preventive effects of DPP‐4I on diabetic polyneuropathy (DPN) might be mediated through increased levels of GIP, but is attributed to these other peptides. Although we have already reported the beneficial effects of GLP‐1R agonist on DPN20, the effects of GIP on peripheral nerve functions have not yet been evaluated. Therefore, in the present study, we focused on the direct physiological roles of GIP/GIPR signaling in undamaged PNS, and assessed the neurological dysfunction of GIPR‐deficient mice (gipr−/−).
Materials and Methods
Primary Culture of Dorsal Root Ganglion Neurons
Dorsal root ganglion (DRG) neuron cultures were prepared from 5‐week‐old male C57BL/6 mice (Chubu Kagaku Shizai, Nagoya, Japan) and GIPR‐deficient mice as previously described26. The collected DRG were incubated in 0.12% collagenase (Wako Pure Chemical, Osaka, Japan) and dissociated using a flame‐narrowed glass pipette. DRGs were diluted in a medium consisting of F‐12 media supplemented with 30 nmol/L selenium and seeded on glass cover slips coated with poly‐L‐lysine.
Evaluation of Neurite Outgrowth
DRG neurons cultured for 24 h with or without human GIP (Peptide Institute, Osaka, Japan) were fixed with 4% paraformaldehyde (PFA) and incubated with rabbit polyclonal anti‐neurofilament heavy‐chain antibody (1:5000; Millipore, Billerica, MA, USA), followed by Alexa Fluor 594‐coupled goat anti‐rabbit immunoglobulin G (IgG) antibody (1:200; Invitrogen, Tokyo, Japan). Neurite outgrowths were analyzed in 10 neurons per cover slip.
Reverse Transcription Polymerase Chain Reaction
Ribonucleaic acids (RNAs) were extracted from frozen samples of DRGs and the pancreas using Isogen (Nippon Gene, Toyama, Japan). RNAs were reverse transcribed and real‐time polymerase chain reaction (PCR) was carried out utilizing the Mx3000P QPCR System (Stratagene Agilent Technologies, Santa Clara, CA, USA) using SYBR Green I (Applied Biosystem, Foster City, CA, USA). Primer sequences are as follows. GIP‐R, (f) GGATCTTGGAGAGACCACACTC, (r) TAAGATGAGTAGGGCTAGCAGCAG; β‐actin, (f) CATCCGTAAAGACCTCTATGCCAAC (r) ATGGAGCCACCGATCCACA. The PCR products were analyzed by agarose gel/ethidium bromide to confirm these predicted lengths.
Western Blotting
Samples were lysed in detergent lysis buffer (Cell Lysis Buffer; Cell Signaling Technology, Boston, MA, USA). The concentrations of proteins were quantitated with a bicinchoninic acid assay (Sigma Chemical, St Louis, MO, USA), and were transferred to polyvinylidene fluoride membranes (Millipore) after sodium dodecyl sulfate polyacrylamide gel electrophoresis. The membranes were incubated with goat polyclonal anti‐GIPR antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit polyclonal anti‐β‐actin antibody (1:10,000; Abcam, Cambridge, MA, USA). The antigen detection was carried out using ECL Plus Reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA) with horseradish peroxydase‐conjugated anti‐goat or rabbit IgG antibody (1:6,000; Cell Signaling Technology).
Animals
The generation and characterization of gipr−/− has been described previously8. The male gipr−/− and male wild‐type C57BL6/J mice (WT; Chubu Kagaku Shizai) were housed in an aseptic room with a 12‐h light cycle and fed ad libitum. Both WT and gipr−/− at 21‐weeks‐old were used for measurement of current perception thresholds, Thermal Plantar Test, motor and sensory nerve conduction velocity, sciatic nerve blood flow, plantar skin blood flow, and immunohistochemistry. The Nagoya University Institutional Animal Care and Use Committee approved the protocols of this experiment.
Measurement of Current Perception Threshold
To evaluate the plantar sensory perception, current perception thresholds (CPT) were measured in both WT and gipr−/− using a CPT/LAB Neurometer (Neurotron, Denver, CO, USA). Two electrodes for stimulation were attached to plantar surfaces of a mouse kept in a Ballman cage (Natsume Seisakusho, Tokyo, Japan). Transcutaneous electric stimuli with three different frequencies (2,000, 250 and 5 Hz) were applied to the plantar surfaces. The intensity of stimulation was gradually increased. The minimum intensity at which a mouse withdrew its paw was defined as the CPT. Six consecutive measurements were carried out at each frequency.
Thermal Plantar Test
Paw withdrawal response to thermal stimuli of radiant heat was measured using a device (Plantar Test, 7370; Ugo Basile, Comerio, Italy). The paw withdrawal latencies were measured five times per session, separated by a minimum interval of 10 min. Paw withdrawals as a result of locomotion or weight shifting were not counted.
Nerve Conduction Velocity
The anesthetized mice were placed on a heated pad to ensure a constant rectal temperature of 37°C. Motor nerve conduction velocity (MNCV) was determined between a sciatic notch and an ankle as previously described27. The sensory NCV (SNCV) was measured between a knee and an ankle with retrograde stimulation.
Sciatic Nerve Blood Flow and Plantar Skin Blood Flow
Sciatic nerve blood flow (SNBF) and plantar skin blood flow (PSBF) were measured by laser Doppler flowmetry (FLO‐N1; Omega Wave Inc, Tokyo, Japan) as previously described20. The sciatic nerves were exposed and the blood flows were measured by a probe placed 1 mm above the nerve. To determine PSBF, three different spots of plantar skin were selected to be measured. During this measurement, the mouse was placed on a heated pad.
Tissue Collection and Immunohistochemistry
Dissected pancreas and DRGs were fixed in 4% PFA, immersed in phosphate‐buffered saline containing 20% sucrose, embedded and cut into 5‐μm sections. Sections were blocked with 5% skim milk (Meiji Milk, Tokyo, Japan), and were applied with the goat polyclonal anti‐GIPR antibody (1:100; Santa Cruz Biotechnology), followed by the Alexa Fluor 594‐coupled donkey anti‐goat IgG antibody (1:200; Invitrogen). Nucleus staining was carried out using 4′,6‐diamidino‐2‐phenylindole (Merck).
Statistical Analysis
All the group values are expressed as means ± standard deviation. Statistical analyses were made by one‐way anova, with the Bonferroni correction for multiple comparisons. All analyses were carried out by personnel unaware of the animal identities.
Results
DRG Neurons Expressed GIPR
To confirm the quality of the GIPR antibody obtained from Santa Cruz Biotechnology, we compared the immunostaining of the islets of gipr−/− and WT. The antibody detected the GIPR protein in the islets of WT, but not in those of gipr−/− (Figure 1a). Using this antibody, GIPR proteins were detected in DRG neurons of WT, but not in those of gipr−/− (Figure 1b). The expressions of GIPR were observed in all sizes of neurons, and also in satellite glias. In addition to immunohistochemistry, GIPR proteins in the DRGs of WT were detected by western blot (WB) analysis, and those of gipr−/− were undetected by WB analysis (Figure 1c). GIPR messenger RNA in the pancreas and DRGs of WT were detected by reverse transcription (RT)–PCR (Figure 1d).
Figure 1.
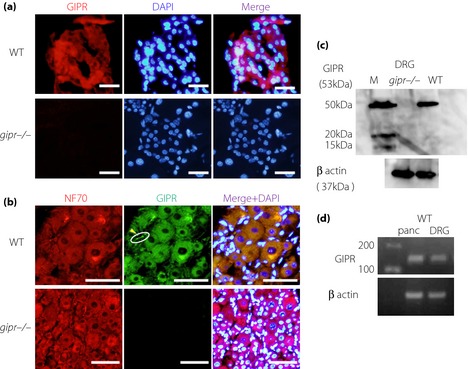
Expressions of gastric inhibitory polypeptide receptor (GIPR) in dorsal root ganglions (DRGs) and the pancreas. (a) GIPR proteins (red) were detected by the anti‐GIPR antibody in pancreatic islets of wild‐type mice (WT), but not in those of GIPR‐deficient mice (gipr−/−). Nuclei were stained with 4′,6‐Diamidino‐2‐phenylindole dihydrochloride (DAPI; blue). Scale bars, 50 μm. (b) GIPR proteins (green) were detected in DRG in WT. The expressions were detected in DRG neurons shown by NF70 antibody (red) and satellite glias (yellow arrowheads). Scale bars, 50 μm. (c) GIPR proteins in DRGs were detected with expected molecular weight in WT, but not in gipr−/− by western blot analysis. (d) The expressions of GIPR were confirmed in DRG neurons and pancreas by reverse transcription polymerase chain reaction. M, molecular markers; NF, neurofilament.
GIP Promoted Neurite Outgrowth of DRG Neurons
It has been reported that axonal regrowth was impaired in gipr−/− after a sciatic nerve crush injury. Therefore, we used DRG culture system to evaluate the impact of the GIP on the PNS, especially sensory neurons. In our culture condition, only large neurons elongated their neurites, and the neurite outgrowths were promoted by the addition of GIP (Figure 2a). Joint numbers of the neurites were increased by GIP (control 25.2 ± 5.80/cell GIP 10 nmol/L; 82.2 ± 8.87, GIP 100 nmol/L; 91.8 ± 4.08, GIP 1,000 nmol/L; 113.8 ± 12.77 control vs GIP 10 nmol/L, P < 0.05; GIP 10 nmol/L vs GIP 100 nmol/L, P < 0.05; GIP 100 nmol/L vs GIP 1,000 nmol/L, P < 0.05; Figure 2b). In addition, total lengths of the neurites were significantly increased in all GIP‐loaded groups (control 430.0 ± 40.85 μm/cell, GIP 10 nmol/L; 901.0 ± 31.83, GIP 100 nmol/L; 1067.0 ± 85.12 GIP 1,000 nmol/L; 1667.4 ± 77.89 control vs GIP 10 nmol/L, P < 0.05; GIP 10 nmol/L vs GIP 100 nmol/L, P < 0.05; GIP 100 nmol/L vs GIP 1,000 nmol/L, P < 0.05; Figure 2b). Neurite outgrowths were not promoted in DRG neurons of GIPR‐deficient mice (joint number: control 25.1 ± 4.43/cell, GIP 1,000 nmol/L; 26.7 ± 2.49; P = 0.33, total length: control 462.1 ± 34.07 μm/cell, GIP 1,000 nmol/L; 452.5 ± 31.14; P = 0.51; Figure 2c).
Figure 2.

Neurite outgrowths of dorsal root ganglion (DRG) neurons. (a) Representative fluorescence micrographs of DRG neurons cultured in the absence or presence of gastric inhibitory polypeptide (GIP). Scale bars, 50 μm. (b) GIP significantly promoted total neurite length and increased joint number of neurites in a dose‐dependent manner. (c) GIP did not promote neurite outgrowth of DRG neurons in gipr−/−. Results are means ± standard deviation. Control vs GIP 10 nmol/L, *P < 0.05. GIP 10 nmol/L vs GIP 100 nmol/L, **P < 0.05. GIP 100 nmol/L vs GIP 1,000 nmol/L, ***P < 0.05. CNT; F‐12 control media.
Bodyweights and Blood Glucose Levels
Random blood glucose levels and bodyweight measured during the experimental period were not significantly changed between WT and gipr−/− (Table 1), consistent with the previous report8.
Table 1. Bodyweights and blood glucose levels.
| WT (n = 8) | gipr−/− (n = 8) | |
|---|---|---|
| Blood glucose (mmol/L) | 10.7 ± 0.3 | a11.4 ± 1.2 |
| Bodyweight (g) | 33.0 ± 1.1 | b29.4 ± 2.5 |
gipr−/−, gastric inhibitory polypeptide receptor deficient mice; WT, wild‐type mice.
Results are means ± standard deviation.
P = 0.35 vs wild‐type mice (WT).
P = 0.52 vs WT.
Sensory Perceptions Were Impaired and NCVs Were Decreased in the gipr−/−
We evaluated sensory functions using CPTs. In gipr−/−, all three CPTs were significantly increased compared with those in WT, representing hypoalgesia (Figure 3a–c). In the examination of CPTs, each electric pulse at 2,000, 250 and 5 Hz mainly stimulates large myelinated(Aβ‐), small myelinated (Aδ‐), and small unmyelinated (C‐) fibers, respectively29. However, these stimuli are not actual stimuli. Therefore, we reconfirmed the impaired sensory functions using the thermal plantar test (TPT). The delays of withdrawal response times were observed in gipr−/− compared with WT (Figure 3d), suggesting a significant reduction of thermal sensitivity or thermal nociception.
Figure 3.

Functions of the peripheral nervous system. Measurements of current perception thresholds at (a) 5, (b) 250 and (c) 2,000 Hz by Neurometer were carried out. All current perception thresholds (CPTs) were significantly increased in the gastric inhibitory polypeptide receptor deficient mice (gipr−/−) compared with those of wild‐type mice (5 Hz: WT 50.7 ± 6.07 μA, gipr−/− 87.1 ± 12.53; 250 Hz: WT 48.5 ± 9.88, gipr−/− 87.8 ± 21.18; 2,000 Hz: WT 108.5 ± 8.99, gipr−/− 170.0 ± 19.14; 5 Hz: WT vs gipr−/−, *P < 0.0001; 250 Hz: WT vs gipr−/−, *P < 0.0001; 2,000 Hz: WT vs gipr−/−, *P < 0.0001). (d) The withdrawal response times using Thermal Plantar Test (TPT) were delayed in gipr−/− compared with those in WT. gipr−/−: GIP receptor deficient mice. The (e) motor nerve conduction velocities (MNCVs) and (f) sensory nerve conduction velocities (SNCV) of gipr−/− were significantly delayed compared with those of normal mice (MNCVs: WT 47.7 ± 1.49 m/s, gipr−/− 36.4 ± 9.17, *P < 0.0001; SNCVs: WT 46.2 ± 1.38, gipr−/− 29.0 ± 5.17, *P < 0.0001; n = 8 in each group).
The MNCVs and the SNCVs of gipr−/− were decreased significantly compared with those of WT (Figure 3e–f).
There was No Significant Aberration in the Peripheral Blood Flows of gipr−/−
As neurophysiological functions are influenced by a hemodynamic status, we examined the blood flows, SNBF and PSBF, using the laser Doppler measurement. The SNBF and PSBF in gipr−/− were comparable with those in WT (SNBF: WT 20.2 ± 1.73 mL/min/100 g; gipr−/−: 19.0 ± 2.49, P = 0.20; PSBF: WT 24.5 ± 1.98; gipr−/−: 22.7 ± 2.78, P = 0.09; Figure 4).
Figure 4.
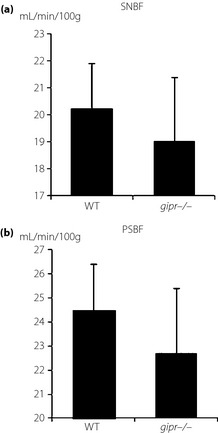
Sciatic nerve blood flow (SNBF) and plantar skin blood flow (PSBF). The SNBF and PSBF in gastric inhibitory polypeptide receptor deficient mice (gipr−/−) mice were comparable with those in wild‐type mice (WT). Results are means ± standard deviation (SNBF: WT 20.2 ± 1.73 mL/min/100 g, gipr−/− 19.0 ± 2.49, P = 0.20; PSBF: WT 24.5 ± 1.98, gipr−/− 22.7 ± 2.78, P = 0.09; n = 8 in each group).
Discussion
In the current study, we investigated whether the GIP/GIPR signal has some effects that maintain intact functions of the PNS in mice. First, we reconfirmed the expression of GIPR on DRG neurons using immunohistochemistry (IHC), WB and RT–PCR. Second, we showed that GIP promotes neurite outgrowths in the cultures of DRG neurons. Third, we showed that the sensory functions are reduced and NCVs are delayed in hindlimbs of gipr−/−. Finally, we confirmed that there is no difference in peripheral blood flow between WT and gipr−/−. These results show that GIP has direct beneficial effects on the PNS.
Although the expressions of GIP and GIPR in the CNS have been reported and proven12, proof of the expressions in the PNS are still insufficient23. In the present study, we confirmed expression of GIPR in DRG neurons using the GIPR antibody, the adequacy of which was assessed by comparison with positive and negative control staining. This result is consistent with the previous study in which GIPR was found to be expressed in DRG neurons and satellite glia23.
Promotion of axonal regrowth by GIP has been described in nerve‐injured model animals23, it was still unclear whether GIP had a direct impact on the PNS or whether GIP exerted its potential through systemic effects. Therefore, we tried to evaluate the beneficial effect of GIP on axonal growth using in vitro DRG cultures. The neurite outgrowth was promoted dose‐dependently by GIP. This result shows that GIP might have direct effects on the PNS. However, there were some limitations in our DRG culture system. First, many different types of cells were contained in the culture: neurons, satellite glia, fibroblasts and hematocytes. As a result, we could not conclude whether the effects of GIP were produced on neurons directly or indirectly through other types of cells. Second, only large sized neurons elongated their neurites in our culture. To minimize the influences of other biologically active substances, we refrained from the use of commercially available supplements or media for neuron cultures in our medium. Furthermore, the medium was tested many times to ascertain that neurons could survive in the F‐12 medium supplemented only by selenite. Unfortunately, although the neurons survived, only large neurons formed neurites in this medium. Therefore, our obtained data must be considered as inconclusive evidence and the medium needs to be additionally modified in the future studies.
To investigate the physiological role of the GIP/GIPR signal on the PNS, we used the gipr−/−. We evaluated sensory nerve functions through the use of CPTs. The CPT measurement is clinically used to examine peripheral nerve functions in various neuropathies30. In the present study, reduced responsiveness against each electrical stimulation was observed in the gipr−/−. These results represented multiple perception impairments. Additionally, we reconfirmed a part of the dysfunction using another test, the TPT. We evaluated the NCV of lower limbs, which is the most established method ascertaining dysfunction of the PNS. Both the MNCVs and SNCVs were delayed in the gipr−/−. The decrease of MNCV was consistent with previous data that found the GIP and GIPR proteins in spinal motor neurons23. However, the outcome of reduced NCVs should be interpreted carefully. Because we have limited data to explain the phenomenon, the functional impairment needs to be examined through both pathological and intercellular molecular biological aspects in the future. Additionally, as evaluation of structural changes on the PNS in the gipr−/− has not yet been carried out, the question remains whether these deficits in the gipr−/− could be comparable with those in other diabetic animal models or humans. We also consider the probability that maturational retardation might influence the development of the neuropathic phenotype in this model, although no maturational retardation in the gipr−/− has been shown up to the present. To resolve these questions, further experiments including sequential morphological examinations of the PNS should be carried out in the future.
Decreased nerve blood flow has been recognized as an important factor in the development of DPN. Although there is no report about the influence of GIP on hemodynamics, we examined the nerve and skin blood flow to exclude influence on functions of the PNS. As expected, the amounts of these blood flows in gipr−/− were equivalent to those in WT.
In conclusion, although these data might suggest important physiological roles of GIP/GIPR signals on the PNS, further intervention studies are required to ascertain the effect of incretin‐based drugs on DPN.
Acknowledgements
This research was supported in part by a Grant‐in‐Aid for Scientific Research (23591303) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. There is no conflict of interest for all listed authors. The authors thank Ms Michiko Yamada and Ms Mayumi Katagiri for technical assistance.
J Diabetes Invest 2014; 5: 31–47
References
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology 2007; 132: 2131–2157 [DOI] [PubMed] [Google Scholar]
- 2.Inagaki N, Atsumi Y, Oura T, et al Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26‐week, randomized, open‐label, parallel‐group, multicenter. noninferiority study. Clin Ther 2012; 34: 1892–1908 [DOI] [PubMed] [Google Scholar]
- 3.Kubota A, Maeda H, Kanamori A, et al Efficacy and safety of sitagliptin monotherapy and combination therapy in Japanese type2 diabetes patients. J Diabetes Invest 2012; 3: 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karagiannis T, Paschos P, Paletas K, et al Dipeptidyl peptidase‐4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta‐analysis. BMJ 2012; 344: e1369. [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi‐phenomenon of impaired beta‐cell function? Diabetes 2010; 59: 1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widenmaier SB, Kim SJ, Yang GK, et al A GIP receptor agonist exhibits beta‐cell anti‐apoptotic actions in rat models of diabetes resulting in improved beta‐cell function and glycemic control. PLoS ONE 2010; 5: e9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yabe D, Seino Y. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Invest 2010; 1: 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyawaki K, Yamada Y, Ban N, et al Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002; 8: 738–742 [DOI] [PubMed] [Google Scholar]
- 9.Tsukiyama K, Yamada Y, Yamada C, et al Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol Endocrinol 2006; 20: 1644–1651 [DOI] [PubMed] [Google Scholar]
- 10.Ogawa E, Hosokawa M, Harada N, et al The effect of gastric inhibitory polypeptide on intestinal glucose absorption and intestinal motility in mice. Biochem Biophys Res Commun 2011; 404: 115–120 [DOI] [PubMed] [Google Scholar]
- 11.Gault VA, Holscher C. Protease‐resistant glucose‐dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta‐amyloid. J Neurophysiol 2008; 99: 1590–1595 [DOI] [PubMed] [Google Scholar]
- 12.Nyberg J, Anderson MF, Meister B, et al Glucose‐dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J Neurosci 2005; 25: 1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyberg J, Jacobsson C, Anderson MF, et al Immunohistochemical distribution of glucose‐dependent insulinotropic polypeptide in the adult rat brain. J Neurosci Res 2007; 85: 2099–2119 [DOI] [PubMed] [Google Scholar]
- 14.Hamann C, Kirschner S, Gunther KP, et al Bone, sweet bone–osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol 2012; 8: 297–305 [DOI] [PubMed] [Google Scholar]
- 15.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet 2012; 379: 2291–2299 [DOI] [PubMed] [Google Scholar]
- 16.Pavelock KA, Girard BM, Schutz KC, et al Bone morphogenetic protein down‐regulation of neuronal pituitary adenylate cyclase‐activating polypeptide and reciprocal effects on vasoactive intestinal peptide expression. J Neurochem 2007; 100: 603–616 [DOI] [PubMed] [Google Scholar]
- 17.Martin B. Lopez de Maturana R, Brenneman R, et al. Class II G protein‐coupled receptors and their ligands in neuronal function and protection. Neuromolecular Med 2005; 7: 3–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.During MJ, Cao L, Zuzga DS, et al Glucagon‐like peptide‐1 receptor is involved in learning and neuroprotection. Nat Med 2003; 9: 1173–1179 [DOI] [PubMed] [Google Scholar]
- 19.Bertilsson G, Patrone C, Zachrisson O, et al Peptide hormone exendin‐4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res 2008; 86: 326–338 [DOI] [PubMed] [Google Scholar]
- 20.Himeno T, Kamiya H, Naruse K, et al Beneficial effects of exendin‐4 on experimental polyneuropathy in diabetic mice. Diabetes 2011; 60: 2397–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolivalt CG, Fineman M, Deacon CF, et al GLP‐1 signals via ERK in peripheral nerve and prevents nerve dysfunction in diabetic mice. Diabetes Obes Metab 2011; 13: 990–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kan M, Guo G, Singh B, et al Glucagon‐like peptide 1, insulin, sensory neurons, and diabetic neuropathy. J Neuropathol Exp Neurol 2012; 71: 494–510 [DOI] [PubMed] [Google Scholar]
- 23.Buhren BA, Gasis M, Thorens B, et al Glucose‐dependent insulinotropic polypeptide (GIP) and its receptor (GIPR): cellular localization, lesion‐affected expression, and impaired regenerative axonal growth. J Neurosci Res 2009; 87: 1858–1870 [DOI] [PubMed] [Google Scholar]
- 24.Jin H, Liu W, Park J, et al Effect of dipeptidyl peptidase‐IV (DPP‐IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin‐induced diabetic rats. Arch Med Res 2009; 40: 536–544 [DOI] [PubMed] [Google Scholar]
- 25.De Meester I, Durinx C, Bal G, et al Natural substrates of dipeptidyl peptidase IV. Adv Exp Med Biol 2000; 477: 67–87 [DOI] [PubMed] [Google Scholar]
- 26.Tosaki T, Kamiya H, Yasuda Y, et al Reduced NGF secretion by Schwann cells under the high glucose condition decreases neurite outgrowth of DRG neurons. Exp Neurol 2008; 213: 381–387 [DOI] [PubMed] [Google Scholar]
- 27.Nakae M, Kamiya H, Naruse K, et al Effects of basic fibroblast growth factor on experimental diabetic neuropathy in rats. Diabetes 2006; 55: 1470–1477 [DOI] [PubMed] [Google Scholar]
- 28.Shibata T, Naruse K, Kamiya H, et al Transplantation of bone marrow‐derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes 2008; 57: 3099–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koga K, Furue H, Rashid M, et al Selective activation of primary afferent fibers evaluated by sine‐wave electrical stimulation. Mol Pain 2005; 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veves A, Young MJ, Manes C, et al Differences in peripheral and autonomic nerve function measurements in painful and painless neuropathy. A clinical study. Diabetes Care 1994; 17: 1200–1202 [DOI] [PubMed] [Google Scholar]


