Abstract
We report a case of a 6-week-old infant with diabetes mellitus based on a genetic defect in the sulfonylurea receptor 1 (SUR1), an ATP-sensitive potassium (KATP) channel protein. A spectacular improvement in glucose regulation was shown by real-time continuous glucose monitoring when switching her from insulin to oral glibenclamide. Children with neonatal onset of diabetes deserve genetic testing in order to replace insulin with oral medication.
Background
Diabetes mellitus (DM) in children is most likely caused by autodestruction of the pancreatic β cells. However, onset of DM in children before 6 months of age (estimated incidence of 1 : 400 0001) often has a distinct pathophysiology.2 The ATP-sensitive potassium (KATP) channel is an important component in the pathway of insulin production. Mutations in the genes coding for KATP channel proteins can result in DM as well as hyperinsulinism depending on whether they are inhibitory or activating.3 Glibenclamide inhibits the KATP channel, thereby stimulating insulin production. In patients with a proven KATP channel defect, insulin treatment can be switched to oral glibenclamide therapy, even many years after diagnosis.3 4 This case report shows that a therapy switch results in a better glucose management.
Case presentation
A 6-week-old female infant was brought to the emergency department with dyspnoea, vomiting and diarrhoea since 1 day. Urine production was present. It was the third child of non-consanguineous parents originating from the Sudan region. She was born term with a birth weight of 2800 g. At presentation the child was lethargic and pale, in hypovolemic shock and respiratory distress. According to advanced paediatric life support logarithms, airway, breathing and circulation were secured and stabilised. Blood results showed a glucose value of 37.5 mmol/L (676 mg/dL). Other laboratory results are shown in table 1. On the basis of these laboratory results, the diagnosis of diabetic ketoacidosis was performed and continuous intravenous insulin therapy was started (0.025 U/kg/h). Subcutaneous insulin therapy with an insulin pump was started after clinical and biochemical stabilisation. As in any young child stable glucose regulation was not easy to reach (figure 1A).
Table 1.
Laboratory data on admission
| Value measured | Reference values | |
|---|---|---|
| pH | 6.95 | 7.35–7.45 |
| pCO2 (kPa) | 3.4 | 4.4–6.3 |
| Base excess (mmol/L) | −27.2 | −2 to 2 |
| Na+ (mmol/L) | 149.2 | 136–147 |
| K+ (mmol/L) | 4.1 | 3.6–5.1 |
| Anion gap (mmol/L) | 15.1 | 4.3–11.1 |
| Effective osmolality* (mOsmol/kg) | 336.3 | 270–290 |
| Urine glucose | +++ | Negative |
| Blood ketones | 2.0 mmol/L | Negative |
*2[Na+]+ glucose.
Figure 1.
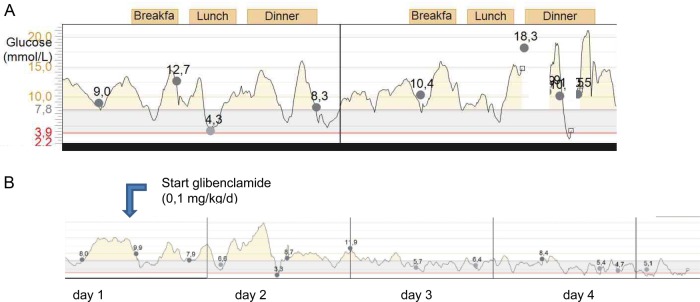
Real-time continuous glucose monitoring data: (A) on subcutaneous insulin pump; (B) transition to oral glibenclamide.
Investigations
Genetic testing (PCR of the coding genes (direct sequence), ABI 3730, applied Biosystems, Warrington UK) revealed a mutation in the ABCC8 gene (location 11p15.1), resulting in a mutation of the sulfonylurea receptor (SUR 1) protein, part of the KATP channel.
Treatment
Owing to positive results in the literature on glibenclamide therapy in patients with an ABCC8 mutation, insulin therapy was switched to oral glibenclamide therapy.3 This was performed under continuous glucose monitoring guidance, starting with 0.1 mg/kg/day in one dose and increased to 0.3 mg/kg/day in two dosages. Already after 1 day the insulin dose could be markedly reduced. Figure 1B shows the glucose values during the transition from insulin treatment to glibenclamide. After starting oral glibenclamide therapy glucose values were more in the physiological range as compared with the glucose values during insulin therapy. No side effects were observed and after discontinuation of insulin no hypoglycaemic episodes occurred.
Outcome and follow-up
From the age of 4 months glibenclamide dosage was reduced because of hypoglycaemia during the night. Owing to hyperglycaemia after the meals, repaglinide (short acting insulin secretagogue) was added to the treatment at a starting dose of 0.125 mg with good results. Glycated haemoglobin gradually decreased to 35 mmol/mol (5.4%). Clinically, there is no phenotype of developmental delay, epilepsy and neonatal diabetes (DEND) syndrome.
Discussion
Insulin is produced in the endocrine β cell of the pancreas in response to a rise in serum glucose that is ‘measured’ by glucokinase, after an influx of glucose through the glucose transporter (GLUT2 transporter). ATP concentration rises, and blocks the KATP channel. The following cell membrane depolarisation results in the opening of the voltage-dependent calcium channels. The rise in intracellular calcium concentration is the trigger for excretion of insulin.5
SUR1 proteins are part of the KATP channel (figure 2). Neonatal DM is caused by an inhibiting mutation of the KATP channel gene causing an open KATP channel and no insulin secretion.
Figure 2.
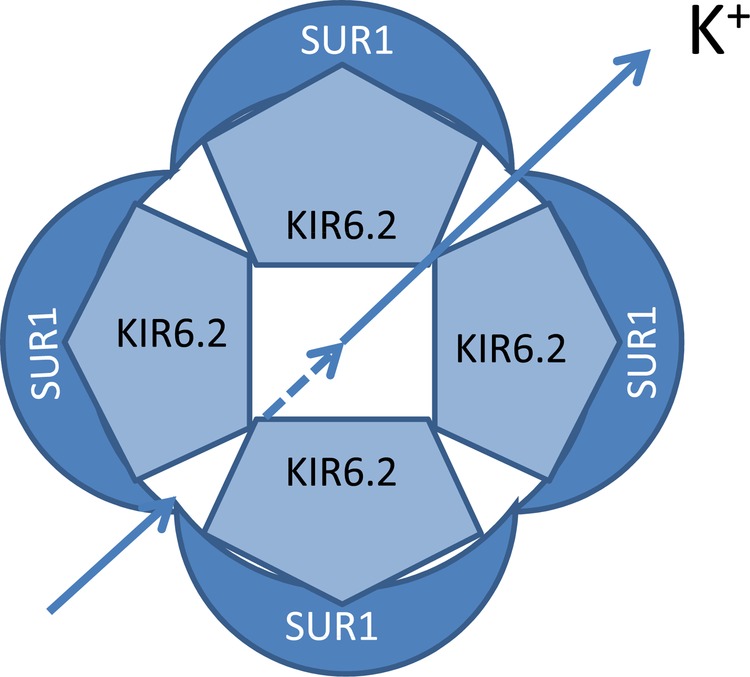
Schematic representation of ATP-sensitive potassium channel (KATP).
In 21% of children with neonatal onset DM, a genetic cause can be detected. In 40–60% the diabetes is transient; medication can be discontinued before the age of 18 months. There is often a relapse in puberty. The cause of this relapse remains unclear. Around 40–50% of patients need lifelong medication.2
Glibenclamide is commonly used in is type 2 DM. It is also indicated in neonatal DM due to the KATP channel-protein binding action, resulting in insulin excretion.2
Glibenclamide therapy can be initiated after proven mutation in the genes coding for KATP channel proteins. Glibenclamide therapy is highly effective, in 90% of patients insulin therapy can be discontinued and glucose values are better regulated.3 Glibenclamide therapy is also effective in older patients with neonatal onset DM who were treated with insulin therapy for long time.6
Learning points.
Neonatal onset diabetes mellitus (DM) requires genetic analysis of genes coding for the ATP-sensitive potassium (KATP) channel of the β cell.
Insulin treatment can be switched off and replaced by glibenclamide treatment in proven KATP channel defects.
Real-time continuous glucose monitoring is a valuable tool for the guidance of transition from insulin to oral medication, especially in very young children.
Stable glucose regulation can be achieved by glibenclamide treatment in children diagnosed with neonatal onset DM.
Footnotes
Contributors: SV wrote the initial case report and its improved versions, supervised by DM, who was the treating paediatric endocrinologist.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Shield J, Gardner R, Wadsworth EJ, et al. Aetiopathology and genetic basis of neonatal diabetes. Arch Dis Child Fetal Neonatal Ed 1997;76:F39–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanagan SE, Patch AM, Mackay DJ, et al. Mutations in ATP-Sensitive K channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 2007;56:1930–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson ER, Flechtner I, Njølstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 2006;355:467–77 [DOI] [PubMed] [Google Scholar]
- 4.Karges B, Meissner T, Icks A, et al. Management of diabetes mellitus in infants. Nat Rev Endocrinol 2012;8:201–11 [DOI] [PubMed] [Google Scholar]
- 5.Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell 2012;148:1160–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanciullo L, Iovane B, Gkliati D, et al. Sulfonylurea-responsive neonatal diabetes mellitus diagnosed through molecular genetics in two children and in one adult after a long period of insulin treatment. Acta Biomed 2012;83:56–61 [PubMed] [Google Scholar]


