Abstract
Objective
To justify the use of Artocarpus altilis (A. altilis), Ficus exasperata (F. exasperata) and Kigelia africana (K. africana) in ethnomedicine for the treatment of several ailments and to evaluate the in vitro antioxidant, radical scavenging and arginase inhibitory potentials of these herbs and compared with catechin (Standard).
Methods
Antioxidant activities were determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide, hydrogen peroxide (H2O2) and hydroxyl (OH) radicals scavenging methods. The flavonoids and phenolics content, inhibition of arginase activity, Fe2+/ascorbate-induced lipid peroxidation (LPO) and reducing power were also determined.
Results
The A. altilis, F. exasperata and K. africana showed dose-dependent and significant scavenging of DPPH, H2O2 and OH radicals in vitro relative to catechin. The A. altilis and F. exasperata effectively scavenged DPPH radical with IC50 of 593 and 635 µg/mL and, OH radical with IC50 of 487 and 514 µg/mL, respectively. The DPPH and OH radicals scavenging activities followed the order A. altilis>F. exasperata>K. africana. In addition, A. altilis and F. exasperata significantly (P<0.05) inhibited LPO in a dose-dependent manner. The A. altilis extract had the most potent inhibitory activity against LPO with 79% relative to catechin (28%) at 750 µg/mL. The reducing power followed the order: A. altilis>Catechin>F. exasperata>K. africana at 1 000 µg/mL. The A. altilis at 500 and 750 µg/mL significantly (P<0.05) inhibited arginase activity by 63% and 67%, respectively. The flavonoids contents were found to be highest in A. altilis.
Conclusions
Extracts of A. altilis and F. exasperata are potent antioxidative agents with strong radical scavenging activity and inhibition of lipid peroxidation.
Keywords: Antioxidant, lipid peroxidation, Arginase, Free radicals
1. Introduction
A free radical is a molecule with one or more unpaired electrons in the outer orbital[1]. Many of the free radicals, in the form of reactive oxygen species (ROS) and reactive nitrogen species are an integral part of normal physiology. These ROS are generated in the organelles such as mitochondria and microsomes under normal physiological conditions[2]. They can also be produced when a living system is exposed to radiation, toxic chemicals, cigarette smoking, alcohol intake and consumption of oxidized fats[3]–[5]. Overproduction of ROS can result in oxidative damage to various biomolecules including lipids, proteins, DNA and cell membranes[6],[7]. Formations of reactive species have been linked to the development of diseases such as coronary heart diseases, cancer, diabetes, hypertension and neurodegenerative disorders[8]–[10]. It is known that antioxidants or endogenous compounds capable of scavenging free radicals possess great potential in ameliorating ROS-induced diseases[11],[12]. The endogenous defense enzymes, viz.; catalase, superoxide dismutase, glutathione and associated enzymes may become depleted thereby limiting their functions during ROS overload[13]. Also, common synthetic antioxidants such as butylated hydoxyanisole, butylated hydoxytoluene, propylgallate used as supplements in foods are limited by shelves lifespan and adverse side effects[14]. Therefore, the search for natural antioxidants having little or no side effects to replace synthetic antioxidants for use in foods or as medicine is still an active area of research.
Breadfruit (Arocarpus altilis) (A. altilis) is a member of the Moraceae. The fruit is an excellent source of fiber, calcium, copper, iron, magnesium, potassium, thiamine, niacin, carbohydrates, vitamins and very low in fat[15]. Fruits can be eaten at all stages of growth as it can be baked, boiled, roasted, fried or steamed[16]. In Africa traditional herbal homes, the leaves of the plant are used for the treatment of liver disorders, hypertension and diabetes[17]. However, little information is available to give scientific support to the traditional use of breadfruit.
Ficus exasperata (F. exasperata), commonly known as “sand paper tree”, is a deciduous shrub. Phytochemical and toxicological analyses of the leaf and stem extracts of the plant revealed the presence of flavonoids, tannis, saponins, alkaloids and cyanogenic glycosides[18]. In ethnomedicine, the leaf extract from the plant has been used to treat patients with hypertension[19], heamostative opthalmia, coughs and haemorrhoid[20]. However, there is dearth of information on the scientific basis of its use in traditional medicine.
Kigelia africana (K. africana) is a member of Bignoniaceae family. In traditional herbal homes, the bark is used for the treatment of rheumatism, dysentery and veneral diseases. In addition, the bark, leaves and roots of the plant are used for treatment against ringworm, tapeworm, haemorrhagic conditions, malaria, diabetes, hypertension and pneumonia[21]. Furthermore, studies showed that various parts of the plant elicited antimicrobial, anti-malarial, anti-inflammatory, anticancer and hepatoprotective effects[22]–[25]. From the aforementioned, this study was designed to evaluate and compare the antioxidant and free radical scavenging activities of these selected medicinal plants using panels of in vitro assays.
2. Materials and methods
2.1. Collections and extraction of plant materials
The plant parts; stem bark of A. altilis, leaves of F. exasperata and fruits of K. africana were collected in Ibadan (Oyo State) and Iwo (Osun State) of Nigeria. The authentication was done in the Botanical Garden, University of Ibadan. The stem bark and leaves were air-dried and crushed into fine powder. The powdered samples were de-fatted with n-hexane and, extracted with methanol using soxhlet extractor. The methanolic extracts were concentrated in vacuum at 40 °C with rotary evaporator and water bath to dryness. The fruits of K. africana were sliced and soaked in water for 48 h. The water extract was concentrated in vacuum at 40 °C with rotary evaporator and water bath to dryness.
2.2. Chemicals
Ethylenediamine tetra-acetic acid (EDTA), 2,2-diphenyl-1-picryhydrazyl (DPPH), 2- deoxyribose, Folin-Ciocalteu reagent, catechin, 2-thiobarbituric acid (TBA), Trichloroacetic acid (TCA) and ascorbic acid were purchased from Sigma Chemical Co., Saint Louis, MO, USA. Ferrous ammonium sulphate, hydrochloric acid, naphthylenediamine dihydrochloride, phosphoric acid and sodium hydroxide were procured from British Drug House (BDH) Chemical Ltd., Poole, UK. Other chemicals were of analytical grade and purest quality available.
2.3.1. DPPH-radical scavenging activity
The radical scavenging activity of plant extracts were measured as described by Mensor et al[26]. The stable 2,2-diphenyl-1-picryldydrazyl (DPPH) radical was used for the determination of free radical scavenging activities of the extracts. A portion (1 mL) each of the different concentrations (10-1 000 ug/mL) of the extracts or standard (Catechin) was added to 1 mL of 1 mmol/L DPPH in methanol. The mixtures were vortexed and incubated in a dark chamber for 30 min after which the absorbance was measured at 517 nm against a DPPH control containing only 1 mL of methanol in place of the extract. All calculations were carried out in triplicates. The inhibition of DPPH was calculated as a percentage using the expression:
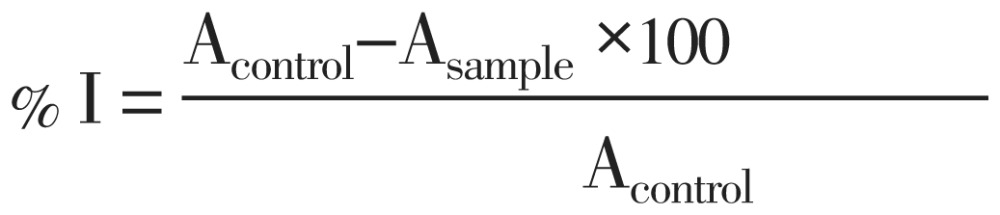 |
Where % I is the percentage inhibition of the DPPH radical; Acontrol is the absorbance of the control and Asample is the absorbance of the test compound.
2.3.2. Reducing power
This was determined according the method of Oyaizu[27]. The extract or standard (100 µg/mL) was mixed with phosphate buffer (pH 6.6) and potassium ferricyanide. The mixture was incubated at 50 °C for 20 min and trichloroacetic acid (10%, 2.5 mL) was added to the mixture. A portion of the resulting mixture was mixed with FeCl3 (0.1%, 0.5 mL) and the absorbance was measured at 700 nm in a spectrophotometer. Higher absorbance of the reaction mixture indicated reductive potential of the extract.
2.3.3. Determination of total phenolic content
The total phenolic content of the extract was determined using the method of Singleton et al.[28] with slight modifications. Folin-C reagent (1 mL) was added to 1mL of extract or standard. After 3 min, 1 mL of 15% Na2CO3 was added and the solution was made up to 5 mL with distilled water. The reaction mixture was kept in the dark for 90 min with intermittent shaking or placed in a water bath at 40 °C for 20 min. The absorbance was measured by a spectrophotometer at 760 nm. All experiments were done in triplicate. Catechin was used as standard.
2.3.4. Determination of total flavonoids
The total flavonoids content was determined by the method described by Meda et al[29] with slight modification. The extract (10-1 000 µg) in 1 mL of distilled water was added to 75 µL of 5% NaNO2. After 5 min, 150 µL of 10% AlCl3.6H2O was added, followed by 500 µL of 1 mol/L NaOH and 275 µL of distilled water. The solution was properly mixed and the colour intensity of the mixture read at 510 nm after 15 min. Catechin was used as the standard. All experiments were done in triplicate.
2.3.5. Scavenging of hydrogen peroxide
The ability of the extracts to scavenge hydrogen peroxide was determined according to methods described by Nabavi et al[30],[31]. A solution of hydrogen peroxide (40 mmol/L) was prepared in phosphate buffer (pH 7.4). The concentration of hydrogen peroxide was determined at 230 nm using a spectrophotometer. Extracts (10-1 000 µg/mL) in distilled water were added to hydrogen peroxide solution (0.6 mL, 40 mmol/L). The absorbance of hydrogen peroxide at 230 nm was determined after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide. The percentage of hydrogen peroxide scavenging by the extracts and catechin (standard) was calculated as follows:
 |
where Ao is the absorbance of the control and A1 is the absorbance in the presence of the extracts or standard.
2.3.6. Nitric oxide radical scavenging activity
The scavenging effect of extract on nitric oxide radical was measured according to the method of Ebrahimzadeh et al[32]. Sodium nitroprusside (5 mmol/L, 1 mL) in phosphate buffered saline was mixed with different concentration of extracts (10-1 000 µg/mL) and distilled water. This was incubated at room temperature for 150 min after which 0.5 mL of Griess reagent was added. The absorbance of the pink chromophore formed was read at 546 nm. Catechin was used as standard. All experiments were done in triplicate. The percentage inhibition was calculated as:
 |
2.3.7. Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity of extracts was carried out as described by Halliwell et al[33]. The assay was performed by adding 0.1 mL of EDTA, 0.01mL of FeCl3, 0.1 mL of H2O2, 0.36 mL of deoxyribose, 1.0 mL of plant extract (10-1 000 ug/mL), 0.33 mL of phosphate buffer (50 mmol/L, pH 7.4) and 0.1 mL of ascorbic acid in sequence. The mixture was then incubated at 37 °C for 1 h. About 1.0 mL of 10% TCA and 1.0 mL of 0.5% TBA were added to develop the pink chromogen and, was measured at 532 nm. The assay was conducted in triplicates and catechin served as standard.
 |
Where Acontrol is absorbance of control and Asample is absorbance of extract or catechin.
2.3.8. Determination of Fe2+/ascorbate-induced lipid peroxidation
Lipid peroxidation was carried out by the method of Ohkawa et al[34]. The reaction mixtures contained 0.2 mL of rat liver homogenate in varying concentration of 30 mmol/L tris buffer, 0.38 mL of 0.16 mmol/L ferrous ammonium sulphate, 0.06 mL ascorbic acid and different concentration of the extracts (10-1 000 µg) and, were incubated for 1 h at 37 °C. The resulting thiobarbituric acid reacting substance formed was measured as followed; briefly, an aliquot (0.4 mL) of the reaction mixture was mixed with 1.6 mL of 0.15 mol/L Tris-KCl buffer and 0.5 mL of 30% TCA (to stop the reaction), and placed in a water bath for 45 min at 80 °C. After which it was cooled in ice and centrifuged at room temperature for 15 min at 3 000 r/min to remove precipitates. The absorbance of the clear pink coloured supernatant was measured against blank at 532 nm. Catechin was used as standard and experiment done in triplicate.
2.3.9. Determination of cardiac arginase activity
Arginase (l-Arginine amido hydrolase, EC. 3.5.3.1) assay was based on the method of Campbell[35]. Assay reaction mixture contained in 1 mL: 50 µmol NaHCO3 buffer (pH 9.5), 20 µmol arginine, 0.5 µmol MnCl2, 0.2 mL crude extracts (10-1 000 ug) and 0.79 mL of 10% rat heart homogenate. The reaction mixture was incubated at 37 °C for 1 h. The reaction was stopped with 1 mL of 0.5 mol/L HClO4 and centrifuged to obtain clear supernatant and the urea formed was determined using urea kits.
2.4. Statistical analysis
Experimental results were expressed as mean±standard deviation (SD). All measurements were replicated three times. The results were analyzed using One-way analysis of variance (ANOVA). The level of significance used was P<0.05.
3. Results
3.1. Total phenolic and flavonoids content of the extracts
The methanolic extract of A. altilis and F. exasperata showed considerable higher phenol contents than catechin (standard) while the phenol contents of aqueous extract of K. africana is lesser than catechin at the concentration of 10 µg/mL, the absorbance of extracts of A. altilis, F. exasperata, K. africana and catechin were 0.041, 0.059, 0.048 and 0.034 respectively; while at 1 000 µg/mL, the absorbance of the extracts of A. altilis, F. exasperata, K. africana and catechin were 0.797, 0.847, 0.523 and 0.709. The total phenol contents of the extracts and catechin showed dose-dependent increase (Figure 1). The flavonoid contents of the extracts showed a dose dependent (Figure 2). At 1 000 µg/mL, the flavonoid contents followed the order A. altilis> F. exasperata> Catechin> K. africana.
Figure 1. The total phenolic contents in the methanolic extracts of A. altilis) and F. exasperate as well as aqueous extract of K. africana and the standard (Catechin).

Figure 2. The flavonoid contents in the methanolic extracts of A. altilis and F. exasperate as well as aqueous extract of K. africana and the standard (Catechin).

3.2. Reducing power of extracts
The extracts of A. altilis, F. exasperata and K. africana showed a dose-dependent increase in the ferric ion reducing potential (Figure 3). At 10 µg/mL, the absorbance of A. altilis, F. exasperata, K. africana and catechin were 0.039, 0.049, 0.066 and 0.023, respectively while at 1 000 µg/mL the absorbance were 0.889, 0.236, 0.145 and 0.610, respectively.
Figure 3. The reducing property of methanolic extracts of A. altilis and F. exasperate as well as aqueous extract of K. africana and the standard (Catechin).

3.3. DPPH radical and nitric oxide scavenging activity of extracts
There were significant (P<0.05) and dose-dependent increases in scavenging activity of extracts on DPPH radicals (Table 1). At 100 µg/mL the percentage DPPH radical scavenging activity of the extracts were 2.3%, 10.5%, 22.7% and 42.2% while at 750 µg/mL the scavenging activity were 28.1%, 58.7%, 62.5% and 67.8% for K. africana, F. exasperata, A. altilis and catechin, respectively respectively. The aqueous extract of K. africana was found to have the highest scavenging activity on NO radical (Table 2). At 500 µg/mL, the scavenging activity of A. altilis, F. exasperata, K. africana and catechin on NO radical were 22.2%, 16.3%, 43.3% and 20.9% respectively.
Table 1. The scavenging activity of A. altilis, F. exasperata and K. africana extracts on 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) in vitro.
| Conccentration µg/mL | % Scavenging activity |
|||
| Catechin | A. altilis | F. exasperata | K. africana | |
| Control | 0.000 | 0.000 | 0.000 | 0.000 |
| 100 | 42.2±4.40* | 22.7±5.88* | 10.5±1.09* | 2.3±4.16* |
| 300 | 47.6±1.55* | 50.4±3.13* | 16.2±3.17* | 11.1±1.61* |
| 500 | 63.1±5.13* | 51.1±2.27* | 50.9±4.63* | 23.8±5.18* |
| 750 | 67.8±3.92* | 62.5±8.53* | 58.7±3.78* | 28.1±5.43* |
Data are expressed as mean±SD (n=3); * Dose-dependent increase from 100-750 µg/mL.
Table 2. The scavenging activity of A. altilis, F. exasperata and K. africana extracts on nitric oxide radical in vitro.
| Conccentration µg/mL | % Inhibition |
|||
| Catechin | A. altilis | F. exasperata | K. africana | |
| Control | 0.000 | 0.000 | 0.000 | 0.000 |
| 100 | 22.7±3.69 | 32.9±2.59 | 27.7±5.41 | 37.5±3.37 |
| 300 | 22.4±2.56 | 25.1±2.98 | 24.8±5.86 | 48.2±1.60 |
| 500 | 20.9±4.24 | 22.2±7.49 | 16.3±4.62 | 43.3±1.04 |
| 750 | 19.9±3.13 | 22.2±6.39 | 22.0±5.74 | 36.5±2.70 |
Data are expressed as mean±SD (n=3).
3.4. Inhibition of Fe2+/ascorbate-induced lipid peroxidation by the extracts
The lipid peroxidation (LPO) inhibition potential of extracts from A. altilis, F. exasperata and K. africana was compared with catechin. Extracts of A. altilis, F. exasperata and catechin exhibited dose-dependent and significant (P<0.05) inhibition of Fe2+/ascorbate-induced lipid peroxidation in vitro. The order of inhibition of LPO was A. altilis> F. exasperata> catechin> K. africana at 750 µg/mL (Table 3).
Table 3. Inhibition of Fe2+/ascorbate-induced lipid peroxidation by extracts of A. altilis, F. exasperata and K. africana in vitro.
| Conccentration µg/mL | % Inhibition |
|||
| Catechin | A. altilis | F. exasperata | K. africana | |
| Control | 0.000 | 0.000 | 0.000 | 0.000 |
| 100 | 21.6±8.17* | 60.6±1.15* | 60.5±0.31* | -75.4±7.09 |
| 300 | 22.5±4.03* | 74.5±5.67* | 62.8±8.03* | -70.8±5.79 |
| 500 | 26.8±5.65* | 78.2±2.06* | 63.7±5.49* | -01.8±7.17 |
| 750 | 28.4±4.42* | 79.0±1.11* | 66.8±3.70* | -65.1±3.43 |
Data are expressed as mean±SD (n=3); *Dose-dependent increase from 100-750 µg/mL.
3.5. Scavenging of hydrogen peroxide and hydroxyl radical by the extracts
The extracts (F. exasperata and K. africana) showed low scavenging activity of hydrogen peroxide when compared with catechin. The highest activity was found in A. altilis (32%) when compared with catechin (29%) at 1 000 µg/mL. At 750 µg/mL, the scavenging activity of A. altilis, F. exasperata, K. africana and catechin were 31%, 19%, 10% and 29%, respectively (Table 4). The hydroxyl radical scavenging activities of the three extracts were compared with catechin in Table 5. The hydroxyl radical scavenging activities of K. africana and F. exasperata showed dose-dependent and significant increase from 100-750 µg/mL. At 100 µg/mL, both catechin and A. altilis showed highest hydroxyl radical scavenging activities which were 90% and 81%, respectively.
Table 4. The hydrogen peroxide scavenging activity of extracts from A. altilis, F. exasperata and K. africana in vitro.
| Conccentration µg/mL | Scavenging activity (%) |
|||
| Catechin | A. altilis | F. exasperata | K. africana | |
| Control | 0.000 | 0.000 | 0.000 | 0.000 |
| 100 | 19.5±1.56* | 9.6±1.05* | 4.8±1.23* | 22.4±1.50 |
| 300 | 26.8±0.98* | 28.6±1.04* | 5.4±1.19* | 23.1±6.23 |
| 500 | 28.2±1.15* | 30.8±1.12* | 8.3±0.52* | 17.2±2.82 |
| 750 | 28.8±1.21* | 31.4±1.21* | 18.9±1.06* | 10.1±0.84 |
| 1000 | 29.0±1.12* | 31.6±1.15* | 25.1±2.15* | 6.4±1.34 |
Data are expressed as mean±SD (n=3); *Dose-dependent increase from 100-1 000 µg/mL.
Table 5. The hydroxyl radical scavenging activity of A. altilis, F. exasperata and K. africana extracts in vitro.
| Conccentration µg/mL | % Scavenging activity |
|||
| Catechin | A. altilis | F. exasperata | K. africana | |
| Control | 0.000 | 0.000 | 0.000 | 0.000 |
| 100 | 89.9±3.62 | 80.9±1.99 | 64.6±4.25* | 39.5±7.04* |
| 300 | 73.9±8.73 | 80.2±3.42 | 68.4±3.92* | 40.6±8.19* |
| 500 | 82.5±8.38 | 73.8±5.38 | 70.9±2.12* | 56.7±6.77* |
| 750 | 69.9±8.19 | 76.9±4.91 | 72.5±7.06* | 58.9±7.14* |
Data are expressed as mean±SD (n=3); * Dose-dependent increase from 100-750 µg/mL.
3.6. Inhibition of cardiac arginase activity in vitro by the extracts
The ability of the extracts to inhibit cardiac arginase activity expressed as the amount of urea liberated per minute per milligram protein is given in Table 6. The extracts; F. exasperata and K. africana did not produced significant (P>0.05) effect on the activity of cardiac arginase in vitro from 50-750 µg/mL. However at 500 and 750 µg/mL, A. altilis and catechin significantly (P<0.05) inhibited cardiac arginase activities by 63%, 67% and 42%, 52%, respectively when compared with the control (Table 6).
Table 6. The inhibitory effects of A. altilis, F. exasperata and K. africana on cardiac arginase activity in vitro.
| Conccentration µg/mL | Activities of arginase (mg Urea/ min/ mg protein) |
|||
| Catechin | A. altilis | F. exasperata | K. africana | |
| Control | 1.13±0.05 | 1.29±0.15 | 1.19±0.09 | 1.19±0.15 |
| 50 | 1.01±0.17 | 1.21±0.12 | 1.04±0.13 | 1.12±0.13 |
| 100 | 1.03±0.11 | 1.26±0.21 | 1.07±0.08 | 1.08±0.07 |
| 250 | 0.97±0.06 | 1.01±0.06 | 1.01±0.10 | 1.08±0.10 |
| 500 | 0.66±0.08* | 0.48±0.07* | 1.07±0.11 | 1.13±0.10 |
| 750 | 0.54±0.05* | 0.42±0.06* | 1.03±0.14 | 1.06±0.12 |
Data are expressed as mean±SD (n=3); *Significantly different from control (P<0.05) at 500 and 750 µg/mL.
4. Discussion
Several techniques have been used to determine the antioxidant activity in vitro in order to allow rapid screening of substances. Free radicals are known to play a definite role in a wide variety of pathological manifestations. Antioxidants combat free radicals and protect from various degenerative diseases. They exert their action either by scavenging the ROS or protecting the antioxidant defense mechanisms[36]. The electron donation ability of natural products can be measured by DPPH purple-coloured solution bleaching[37]. The method is based on scavenging of DPPH through the addition of a radical species or antioxidant that decolourizes the DPPH solution. The degree of colour change is proportional to the concentration and potency of the antioxidants. A large decrease in the absorbance of the reaction mixture indicates significant free radical scavenging activity of the compound under test[38]. In the present study, the three extracts (A. altilis, F. exasperata and K. africana) showed dose-dependent scavenging of DPPH radical. However, A. altilis and F. exasperata at 500 µg/mL significantly scavenged DPPH radical by over 50% which is directly related to the high phenolic contents of A. altilis and F. exasperata. The results indicate A. altilis and F. exasperata contain phytochemical constituents that are capable of donating hydrogen to a free radical to scavenge the potential damage.
One of the mechanisms of action of antioxidants is to chelate and deactivate transition metals thereby preventing such metals from participating in the initiation of LPO and oxidative stress through metal catalysed reaction[39]. Overproduction of ROS may have a direct attack on the polyunsatureated fatty acids of the cell membrane to induce peroxidation reactions[39]. The deleterious effect caused by iron is done by reacting with hydrogen peroxide to produce hydroxyl radical (OH.) through Fenton reaction. Superoxide can also react with Fe3+ to regenerate Fe2+ which can participate in the Fenton reaction[40]. In this study, LPO of rat liver homogenates was induced by ferric ion plus ascorbic acid. The LPO inhibitory activity of A. altilis and F. exasperata was found to be very high, significant and dose-dependent. The inhibition of LPO by A. altilis was the highest relative to others (F. exasperata and K. africana) at concentration of 750 µg/mL. These results indicated that A. altilis and F. exasperata have potential to be studied for use in treating liver disease. Hydroxyl radical is one of the potent ROS in the biological system. It reacts with polyunsatureated fatty acids moieties of cell membrane phospholipids and causes damage to cell[41]. The hydroxyl radical is regarded as a detrimental species in pathophysiological processes and capable of damaging almost every molecule of biological system and contributes to carcinogenesis, mutagenesis and cytotoxicity[42]. It is known that the mutagenic capacity of free radicals is due to the direct interactions of hydroxyl radicals with DNA and therefore playing an important role in cancer formation[43]. The results demonstrated that the methanolic extract of A. altilis and F. exasperata had appreciable OH radical scavenging effects when compared to catechin. The results indicate that both A. altilis and Fe may serve as anticancer agents by inhibiting the interaction of hydroxyl radical with DNA. The ability of these extracts to quench hydroxyl radicals might directly relate to the prevention of lipid peroxidation.
Hydrogen peroxide occurs naturally at low levels in the air, water, human body, plants, microorganisms and food[44]. H2O2 is rapidly decomposed into oxygen and water, and may produce hydroxyl radicals that can initiate LPO and cause DNA damage[45]. The extracts; A. altilis, F. exasperata and K. africana exhibited low scavenging effect on hydrogen peroxide in vitro at 100-300 µg/mL. However, at higher concentrations (500-1 000 µg/mL), the hydrogen peroxide scavenging effect of A. altilis is statistically similar to catechin (about 30%). Hence, A. altilis showed appreciable scavenging of hydrogen peroxide and may be attributed to the presence of phenolic groups that could donate electrons to hydrogen peroxide, thereby neutralizing it into water. In reducing power assay, the yellow colour of the test solution changes to green depending on the reducing power of the extracts. The presence of the reductants in the solution causes the reduction of the Fe3+/ ferricyanide complex to the ferrous form. Therefore, Fe2+ can be monitored by absorbance measurement at 700 nm. It is known that the reducing properties of an extract could serve as a measure of its antioxidant action by donating hydrogen atom to break the free radical chain[46]. Increasing absorbance at 700 nm indicates an increase in reducing ability. The antioxidants present in the fractions of A. altilis, F. exasperata and K. africana caused the reduction of Fe3+/ ferricyanide complex to the ferrous form, and thus proved their reducing power. The reducing power of the plant extracts followed the order; A. altilis>F. exasperata>K. africana at 1 000 µg/mL. The release of cyanide and/or nitric oxide in sodium nitroprusside can cause cytotoxicity[47]. NO is a free radical with a short half-life (<30 s) and its independent action may cause neuronal damage, especially in conjunction other ROS such as superoxide radical to form peroxynitrite radical[47]. However, the result revealed that these extracts had higher NO scavenging potentials than standard at 750 µg/mL. Hence, these extracts may elicit inhibitory action against NO-induced cellular damage.
Arginine metabolism is important to vascular function in health and disease. The key enzymes required in arginine metabolism in the vascular systems are the nitric oxide synthases and the arginases, both of which use arginine as a substrate[48], and dysregulated activity of these enzymes has been linked to multiple types of endothelial dysfunction and cardiovascular disease[49]. Cardiac arginase is hemodynamically sensitive to blood pressure fluctuations and arginase inhibitors such as hydrazalazine and nor- hydroxyl arginine have been demonstrated to hold promise as future antihypertensive agents courtesy of their abilities to cause reduction in arginase activity by up to 30% and arterial blood pressure by 30-35 mmHg, modulate arterial resistance and promote blood flow[50]. It is important to note that cardiovascular diseases such as hypertension, arrythmias, angina pectoris, myocardial infarction, stroke, and left ventricular hypertrophy have become a major cause of morbidity and mortality in the world with increasing prevalence in developing countries[51]. In the present study, the plant extract (A. altilis) caused significant reduction in the activity of cardiac arginase in vitro at concentrations of 500 and 750 µg/mL. Therefore, the observed reduction in arginase activity is a pointer to the possible antihypertensive action of A. altilis.
In conclusion, the replacement of synthetic with natural antioxidants because of implications for human health may be advantageous. In the present study, methanolic extracts of A. altilis and F. exasperata had stronger hydroxyl and DPPH radical scavenging activities and inhibitory activity against lipid peroxidation than K. africana. The reducing capacity of A. altilis and F. exasperata on ferrous ion was higher than that of other extract (K. africana). In addition, the potent antioxidative activities of A. altilis and F. exasperata might result from their high contents of phenolic compounds. Hence, the methanolic extracts from A. altilis and F. exasperata could be used as a health-care food supplement or in the pharmaceutical industry as natural antioxidants. Further studies may be necessary to identify, isolate and characterize the active components in A. altilis and F. exasperata that is responsible for the observed effects.
Acknowledgments
This research was partly supported by Senate Research Grants from University of Ibadan, Nigeria given to Dr. O.A. Adaramoye (SRG/COM/2010/7A).
Comments
Background
Free radicals are essential in human physiology, but may be responsible of various diseases when overproduced. The search for active molecules from natural sources has become attractive since synthetic medicines are costly and present some severe side effects.
Research frontiers
This study is carried out to investigate the free radical scavenging (DPPH, NO, H2O2, OH) activity by extracts from Nigerian plants that are extensively used in traditional medicine). Total phenols and flavonoid contents, inhibition of arginase activity and Fe2+-ascorbate-induced lipid peroxidation were also assessed.
Related reports
The results indicate that Artocarpus altilis and F. exasperata extracts showed a high and dose-dependent DPPH scavenger activity. The above extracts also inhibited lipid peroxidation efficiently. Artocarpus artilis extracts inhibited arginase activity by 63% and 67% at concentrations of 500 and 750 µg/mL, respectively.
Innovations and breakthroughs
Authors have demonstrated new biological activities that have never been reported for the plants involved in this study. This opens new perspectives of research using these plants.
Applications
The preliminary results reported in this study can be considered as the premise for supporting the use of this plant in traditional medicine.
Peer review
This paper that describes the antioxidant, anti-lipid peroxidation and anti-arginase activities of selected Nigerian plants is indeed interesting. The antioxidant activity and the inhibitory potential of arginase activity exerted by A. altilis and F. exasperata extracts, although at relatively high doses, are promising.
Footnotes
Foundation Project: Supported by Senate Research Grants from University of Ibadan, Nigeria, (SRG/COM/2010/7A).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Gryn'ova G, Marshall DL, Blanksby SJ, Coote ML. Switching radical stability by pH-induced orbital conversion. Nat Chem. 2013;5(6):474–481. doi: 10.1038/nchem.1625. [DOI] [PubMed] [Google Scholar]
- 2.Visavadiya NP, McEwen ML, Pandya JD, Sullivan PG, Gwag BJ, Springer JE. Antioxidant properties of Neu2000 on mitochondrial free radicals and oxidative damage. Toxicol In Vitro. 2013;27(2):788–797. doi: 10.1016/j.tiv.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham DL, Carail M, Caris-Veyrat C, Lowe GM. (13Z)- and (9Z)-lycopene isomers are major intermediates in the oxidative degradation of lycopene by cigarette smoke and Sin-1. Free Radic Res. 2012;46(7):891–902. doi: 10.3109/10715762.2012.686663. [DOI] [PubMed] [Google Scholar]
- 5.Liang JY, Yuann JM, Cheng CW, Jian HL, Lin CC, Chen LY. Blue light induced free radicals from riboflavin on E. coli DNA damage. J Photochem Photobiol B. 2013;119:60–64. doi: 10.1016/j.jphotobiol.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Betancor MB, Caballero MJ, Terova G, Corà S, Saleh R, Benítez-Santana T, et al. Vitamin C enhances vitamin E status and reduces oxidative stress indicators in sea bass larvae fed high DHA microdiets. Lipids. 2012;47(12):1193–1207. doi: 10.1007/s11745-012-3730-x. [DOI] [PubMed] [Google Scholar]
- 7.Braga PC, Ceci C, Marabini L, Nappi G. The antioxidant activity of sulphurous thermal water protects against oxidative DNA damage: a comet assay investigation. Drug Res (Stuttg) 2013;63(4):198–202. doi: 10.1055/s-0033-1334894. [DOI] [PubMed] [Google Scholar]
- 8.D'souza D, Subhas BG, Shetty SR, Balan P. Estimation of serum malondialdehyde in potentially malignant disorders and post-antioxidant treated patients: a biochemical study. Contemp Clin Dent. 2012;3(4):448–451. doi: 10.4103/0976-237X.107438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habib SL. Diabetes and renal tubular cell apoptosis. World J Diabetes. 2013;4(2):27–30. doi: 10.4239/wjd.v4.i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumitrascu R, Heitmann J, Seeger W, Weissmann N, Schulz R. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev. 2013;2013:234631. doi: 10.1155/2013/234631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim H, Jang JY, Lee SH, Lee JG. Correlation of the oxygen radical activity and antioxidants and severity in critically ill surgical patients-study protocol. World J Emerg Surg. 2013;8:18. doi: 10.1186/1749-7922-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha M, Apostolova N, Herance JR, Rovira-Llopis S, Hernandez-Mijares A, Victor VM. Perspectives and potential applications of mitochondria-targeted antioxidants in cardiometabolic diseases and type 2 diabetes. Med Res Rev. 2014;34(1):160–189. doi: 10.1002/med.21285. [DOI] [PubMed] [Google Scholar]
- 13.Stanley JA, Sivakumar KK, Nithy TK, Arosh JA, Hoyer PB, Burghardt RC, et al. Postnatal exposure to chromium through mother's milk accelerates follicular atresia in F1 offspring through increased oxidative stress and depletion of antioxidant enzymes. Free Radic Biol Med. 2013;61C:179–196. doi: 10.1016/j.freeradbiomed.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Toxicology Program Butylated hydroxyanisole (BHA) Rep Carcinog. 2002;10:40–42. [PubMed] [Google Scholar]
- 15.Rincón AM, Bou Rached L, Aragoza LE, Padilla F. [Effect of acetylation and oxidation on some properties of breadfruit (Artocarpus altilis) seed starch] Arch Latinoam Nutr. 2007;57(3):287–294. Spanish. [PubMed] [Google Scholar]
- 16.Ragone D. Breadfruit. In: Caballlero B, Trugo L, Finglas PM, editors. Encyclopaedia of food sciences and nutrition. San Diego, California: Academic press; 2003. pp. 655–661. [Google Scholar]
- 17.Zerega NYC, Ragone D, Motley TJ. Systematics and species limits of breadfruit (Artocarpus, Moraceae) Syst Bot. 2005;30:603–615. [Google Scholar]
- 18.Ijeh II, Iheanacho AI. Acute effect of administration of ethanol extracts of Ficus exasperata vahl on kidney function in albino rats. J Med Plant Res. 2007;1(2):27–29. [Google Scholar]
- 19.Ayinde BA1, Omogbai EK, Amaechina FC. Pharmacognosy and hypotensive evaluation of Ficus exasperata VVahl. (Moraceae) leaves. Acta Pol Pharm. 2007;64(6):543–546. [PubMed] [Google Scholar]
- 20.Odunbaku OA, Ilusanya OA, Akasoro KS. Antibacterial activity of ethanolic leaf extract of Ficus exasperata on Escherichia coli and Staphylococcus albus. Sci Res Essays. 2008;3:562–564. [Google Scholar]
- 21.Agyare C, Dwobeng AS, Agyepong N, Boakye YD, Mensah KB, Ayande PG, et al. Antimicrobial, antioxidant, and wound healing properties of Kigelia africana (Lam.) Beneth. and Strophanthus hispidus DC. Adv Pharmacol Sci. 2013;2013 doi: 10.1155/2013/692613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olaleye MT, Rocha BT. Acetaminophen-induced liver damage in mice: effects of some medicinal plants on the oxidative defense system. Exp Toxicol Pathol. 2008;59(5):319–327. doi: 10.1016/j.etp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Zofou D, Tene M, Tane P, Titanji VP. Antimalarial drug interactions of compounds isolated from Kigelia africana (Bignoniaceae) and their synergism with artemether, against the multidrug-resistant W2mef Plasmodium falciparum strain. Parasitol Res. 2012;110(2):539–544. doi: 10.1007/s00436-011-2519-9. [DOI] [PubMed] [Google Scholar]
- 24.Chivandi E, Cave E, Davidson BC, Erlwanger KH, Moyo D, Madziva MT. Suppression of Caco-2 and HEK-293 cell proliferation by Kigelia africana, Mimusops zeyheri and Ximenia caffra seed oils. In Vivo. 2012;26(1):99–105. [PubMed] [Google Scholar]
- 25.Chenia HY. Anti-quorum sensing potential of crude Kigelia africana fruit extracts. Sensors (Basel) 2013;13(3):2802–2817. doi: 10.3390/s130302802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mensor LI, Menezes FS, Leitao GG, Reis AS, dos Santos T, Coube CS, et al. Screening of Brazilian plants extracts for antioxidants activity by the use of DPPH free radical method. Phytother Res. 2001;15(2):127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 27.Oyaizu M. [Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucoseamine] Jpn J Nutr Diet. 1986;44(6):307–315. Japanese. [Google Scholar]
- 28.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Cioalteau reagent. Meth Enzymol. 1999;299:152–178. [Google Scholar]
- 29.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Faso honey, as well as their radical scavenging activity. Food Chem. 2005;91(3):571–577. [Google Scholar]
- 30.Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Hamidinia A, Bekhradnia AR. Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. Pharmacologyonline. 2008;2:560–567. [Google Scholar]
- 31.Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Fazelian M, Eslami B. In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran. Pharmacogn Mag. 2009;4(18):122–127. [Google Scholar]
- 32.Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antioxidant activities of methanol extract of Sambucus ebulus L. flower. Pak J Biol Sci. 2009;12(5):447–450. doi: 10.3923/pjbs.2009.447.450. [DOI] [PubMed] [Google Scholar]
- 33.Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987;1(5):358–364. [PubMed] [Google Scholar]
- 34.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 35.Campbell JW. Studies on tissue arginase and ureogenesis in the elasmobranch Mustelus canis. Arch Biochem Biophys. 1961;93:448–455. doi: 10.1016/0003-9861(61)90292-2. [DOI] [PubMed] [Google Scholar]
- 36.Umamaheswari M, Chatterjee TK. In vitro antioxidant activities of the fractions of Coccinnia grandis L. leaf extract. Afr J Tradit Complement Altern Med. 2008;5(1):61–73. [PMC free article] [PubMed] [Google Scholar]
- 37.Nunes PX, Silva FS, da S.Almeida JRG, de Lima JT, de Araujo Ribeiro LA, Quintans LJ, Junior, et al. Biological oxidations and antioxidant activity of natural products. In: Rao V, editor. Phytochemicals as nutraceuticals-global approaches to their role in nutrition and health. Croatia: In Tech; 2012. [Google Scholar]
- 38.Krishnaiah D, Sarbatly R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–233. [Google Scholar]
- 39.Oboh G, Puntel RL, Rocha JBT. Hot pepper (Capsicum annuum, Tepin and Capsicum chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain in vitro. Food Chem. 2007;102:178–185. [Google Scholar]
- 40.Moreira da Silva F, Marques A, Chaveiro A. Reactive oxygen species: a double-edged sword in reproduction. Open Vet Sci J. 2010;4:127–133. [Google Scholar]
- 41.Khan RA, Khan MR, Sahreen S, Ahmed M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem Cent J. 2012;6(1):12. doi: 10.1186/1752-153X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner JR, Cadet J. Oxidation reactions of cytosine DNA components by hydroxyl radical and one-electron oxidants in aerated aqueous solutions. Acc Chem Res. 2010;43(4):564–571. doi: 10.1021/ar9002637. [DOI] [PubMed] [Google Scholar]
- 43.Dizdaroglu M, Kirkali G, Jaruga P. Formamidopyrimidines in DNA: mechanisms of formation, repair, and biological effects. Free Radic Biol Med. 2008;45(12):1610–1621. doi: 10.1016/j.freeradbiomed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Gulcin I, Berashvili D, Gepdiremen A. Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J Ethnopharmacol. 2005;101(1–3):287–293. doi: 10.1016/j.jep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Sahreen S, Khan MR, Khan RA. Phenolic compounds and antioxidant activities of Rumex hastatus D. Don. Leaves. J Med Plants Res. 2011;5(13):2755–2765. [Google Scholar]
- 46.Sarkar R, Hazra B, Mandal N. Reducing power and iron chelating property of Terminalia chebula (Retz.) alleviates iron induced liver toxicity in mice. BMC Complement Altern Med. 2012;12:144. doi: 10.1186/1472-6882-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oboh G, Rocha JB. Hot pepper (Capsicum spp.) protects brain from sodium nitroprusside- and quinolinic acid-induced oxidative stress in vitro. J Med Food. 2008;11(2):349–355. doi: 10.1089/jmf.2007.341. [DOI] [PubMed] [Google Scholar]
- 48.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32(8):1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 50.Bagnost T, Berthelot A, Bouhaddi M, Laurant P, André C, Guillaume Y, et al. Treatment with the arginase inhibitor N(omega)-hydroxy-nor-L-arginine improves vascular function and lowers blood pressure in adult spontaneously hypertensive rat. J Hypertens. 2008;26(6):1110–1118. doi: 10.1097/HJH.0b013e3282fcc357. [DOI] [PubMed] [Google Scholar]
- 51.Ogah OS, Madukwe OO, Chukwuonye II, Onyeonoro UU, Ukegbu AU, Akhimien MO, et al. Prevalence and determinants of hypertension in Abia State Nigeria: results from the Abia State non-communicable diseases and cardiovascular risk factors survey. Ethn Dis. 2013;23(2):161–167. [PubMed] [Google Scholar]


