Abstract
To achieve the desired therapeutic objective, the drug product must deliver the active drug at an optimal rate and amount. By proper biopharmaceutic design, the rate and extent of drug absorption (also called as bioavailability) or the systemic delivery of drugs to the body can be varied from rapid and complete absorption to slow and sustained absorption depending upon the desired therapeutic objective. Phytomedicine have served as the foundation for a larger fraction of the current pharmacopeia. But the delivery of phytomedicine is always problematic due to poor aqueous solubility, poor permeation, low systemic availability, instability and extensive first pass metabolism. Current review will discuss in detail about how nanotechnology can enhance the bioavilability and bioactivity of the phytomedicine.
Keywords: Bioavailability, Bioactivity, Nanotechnology, Phytomedicine
1. Introduction
The therapeutic effectiveness of any drug may obtained from plant, animal, sea or synthetic which depends upon the ability of the dosage form to deliver the medicament to its site of action at a rate and amount sufficient to elicit the desired pharmacological response. This attribute of the dosage form is referred to as physiologic availability, biological availability or simply bioavailability[1]. For most drugs, the pharmacological response can be related directly to the plasma levels. Thus, the term bioavailability is defined as the rate and extent (amount) of absorption of unchanged drug from its dosage form. Sometimes, a fast absorption is desired when a rapid onset of action is needed in the treatment of acute conditions such as asthma attack, pain etc. A slow rate of absorption is needed when the objective is to prolong the duration of action or to overcome the adverse effect and extent of absorption which is also significant in the treatment of chronic conditions such as hypertension, epilepsy, etc. These can be achieved by altering the physicochemical properties of the drug and characteristics of the dosage form[1].
Phytomedicine have been serving as a crucial source of drugs since ancient times. Today, about 50% of useful drugs is obtained from natural sources[2]. The usage of phytomedicine has been increased due to their better therapeutic activity and less side effects as compared to the allopathic medicines. Phytochemical and pharmacological investigation have been done extensively and well established. Phytomedicines shows impressive in-vitro activity but less in-vivo efficacy due to their poor water solubility, lipophilicity and inappropriate molecular size resulting in poor absorption and hence poor systemic availability. A better understanding of the biopharmaceutics and pharmacokinetics of phytomedicine can also help in designing rational dosage regimens[3].
Nanotechnology is on the threshold of providing a host of new materials and approaches in revolutionizing medical and pharmaceutical field. Several areas of medical care are already profiting from the advantage of nanotechnology[4].
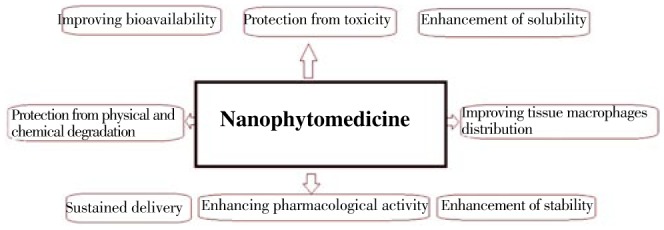
The use of nanotechnology for treatment, identification, monitoring, and managing biological systems have recently been referred to as nanomedicine. In the herbal formulation research, incorporating the nano-based formulation has a great number of advantages for phytomedicine, including improvement of solubility and bioavailability, safeguard from toxicity, enhancement of pharmacological activity, improvement of stability, increase in tissue macrophages distribution, sustained delivery, protection from physical and chemical degradation, etc.[5]. Thus nano phytomedicine have a prospective future for improving the activity and overcoming problems associated with herbal drugs (Figure 1).
Figure 1. Applications of nanotechnology based phytomedicine formulation.
2. Problems encountered in phytomedicine product development
Mostly, phytomedicines are called as secondary metabolites and these metabolites are being chemically isolated and identified. The production of active constituents of phytomedince represents a lot of challenges[6]. These secondary metabolites which are present in plant are very low and their active constituents vary depending on a number of factors, such as botanical species, used chemotypes, the anatomical part of the plant used (seed, flower, root, leaf, and so on) and also storage, sun, humidity, type of ground, time of harvest and geographic area[7]. Phytomedicine screening from the plant is another challenge, and even though the high throughput methods are normally employed in the screening of drugs in pharmaceutical field, it is not suitable for the phytomedicine as crude extracts contain numerous drug compounds. Moreover, some active constituents present in the plant gave false information when screening by high throughput techniques[2]. In addition to this, identification, isolation of active constituents and fractionation process also takes weeks or even months and active ingredient supply is also another challenge, which needs several hundreds of grams for preclinical development depending upon the utility.
Many phytomedicine and extracts of plant despite of their surprising potential in-vitro finding, exhibit least or no significant in-vivo activity due to their poor solubility, poor lipid solubility and improper size result in poor absorption and bioavailability. Another problem is their structural instability in biological milieu, premature drug loss through rapid clearance and biotransformation and some plant extracts are destroyed in gastric juice during gastric emptying when administered orally.
3. Various nanotechnologies approaches for enhancing the bioavailability and bioactivity of phytomedicine
Phytomedicines are attracting more popular in the current world for their application to cure a variety of diseases with less toxic effects and high therapeutic property. However some limitations of phytomedicine are discussed in the previous section. Nanotechnology can serve as an efficient tool in eradicating the limitations stated above. By reducing the size of the phytomedicine into nano phytomedicine and modifying surface properties of phytomedicine, the aqueous solubility and permeability through biological membrane. Various novel drug delivery systems such as liposomes, niosomes, nanospheres and phytosomes have been reported to have the ablility of delivering herbal drugs. Incorporation of herbal drugs in the delivery system also gives aid to increase in solubility, enhance stability, protect from toxicity, enhance pharmacological activity, improve tissue macrophage distribution, sustain delivery and protect from physical and chemical degradation[8]. Moreover, to use nanotechnology, it may be likely to accomplish (1) enhanced delivery of poorly water-soluble phytomedicine; (2) targeted delivery of phytomedicine in a cell- or tissue-specific way; (3) transcytosis of phytomedicine across tight epithelial and endothelial barriers; (4) delivery of large macromolecule phytomedicine to intracellular sites of action; (5) co-delivery of two or more phytomedicines or therapeutic modality for combination therapy; (6) observation of sites of drug delivery by incorporating phytomedicine with imaging modalities[9],[10].
3.1. Reducing the size of the phytomedicine into nanophytomedicine
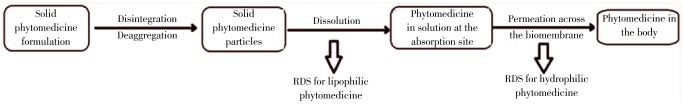
Most of the phytomedicine formulation is administered orally because of patient convenience and manufacturing advantages compared with other dosage forms. For the orally administered drug, there are two critical slower rate-determining steps (RDSs) in the absorption. First is the rate of dissolution and the rate of drug permeation through the membrane is an another step. Dissolution is the RDS for hydrophobic in poorly aqueous soluble drugs and absorption of such drugs is said to be dissolution rate limited. If the drug is hydrophilic with high aqueous solubility, then dissolution will be rapid and RDS in the absorption of such drugs will be the rate of permeation through the biomembrane. That is said to be permeation rate limited or transmembrane rate limited (Figure 2).
Figure 2. The two rate-determining steps in the absorption of orally administered phytomedicine formulation.
A well formulated nanophytomedicine prepared through various routes of synthesis, by virtue of their size, enhances the dissolution, absorption and bioavailability of drugs while reduces in the dose. Cuscuta chinensis (C. chinensis) is a Chinese drug containing flavonoids and lignins as active medicament, which is poor aqueous solubility and poor absorption upon oral administration. The nano sized C. chinensis were prepared by a nanosuspension method for hepatoprotective and antioxidant effects after oral administration. The 25 and 50 mg/kg oral doses showed similar activity as that of 125 and 250 mg/kg ethanolic extract of C. chinensis, five fold reduction in dose was observed with nano sized C. chinensis[11]. Su YL, et al., 2008[12] investigated Radix salvia nanoparticles prepared by spray drying method for coronary heart disease, angina pectoris and myocardial infarction, nanosized Radix salvia showing improved bioavailability. The reduction of the size of the phytomedicine improves aqueous solubility. Generally water soluble drugs whose size smaller than the diameter of the pore of the biomembrane can penetrate easily. The driving force is constituted by the hydrostatic pressure or the osmotic difference across the biomembrane due to which bulk flow of water along with small solid molecules occur through such aqueous channels.
3.2. Modification of surface properties
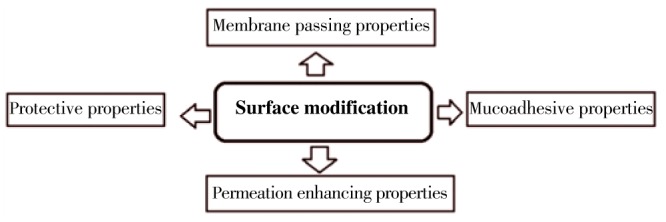
Surface modification can be achieved by surface coating with hydrophilic, stabilizing, mucoadhesive polymers/surfactants or by the production of biodegradable copolymers with hydrophilic segments[13]. These modifications change the zeta potential of nanoparticles, hydrophobicity, stability, mucoadhesive properties and protein adsorption on their surface. Surface properties of particles and size have a significant impact on particle uptake (Figure 3).
Figure 3. Use of surface modification in phytomedicine drug delivery.
The membrane passing and permeation can be increased by using the surface modification[14]–[16]. Intestinal transit results in reducing the phytomedicine residence time and limits the bioavailability of herbal drugs. One possible way to increase the bioavailability of phytomedicine which has low mucosal absorptive properties and to increase its residence time at mucosal or epithelial level is by incorporating phytomedicine in micro or nanoparticles. When a suspension of micro or nano phytomedicine is administered orally, they diffuse into the gastrointestinal medium and encounter the mucus at which they could adhere.
One of the big impediments of oral administration of phytomedicine is their lack of stability in the gastrointestinal tract. The surface-modified micro or nano phytomedicine can be used as an efficient strategy to circumvent this problem. The poly(lactic-co-glycolic) acid (PLGA) microspheres with chitosan[17], and PEGylated PLGA-based nanoparticles were used to modify the properties of formulations[18].
3.3. Attaching the polymers with phytomedicine
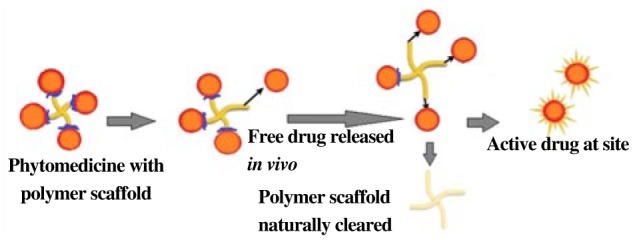
A variety of polymers are being utilized in these and other biomedical applications; for example, polyvinyl chloride used in the manufacture of cardiac catheters, surgical tapes, artificial hearts, blood pumps and artificial limbs[19]–[22]. By modifying polymer surfaces one may achieve a number of desirable properties ranging from blood clotting prevention to controllable drug release, and other applications, while maintaining useful bulk polymer properties. Each particle is a matrix of the drug dispersed in the polymer and drug is released as a first order process. The polymers used for the fabrication of the microspheres are biodegradable or non biodegradable. Various polymers have been used for the fabrication of these microparticulate carriers such as albumin, gelatin, modified starch, polypropylene, dextran, polylactic acid and poly lactide-co-glycolide etc. The drug release is controlled by the dissolution and degradation of the matrix (Figure 4).
Figure 4. Release of phytomedicine from the polymer scaffold.
R Garg, et al., 2010[23] encapsulated the phytomedicine, silymarin with the eudragit S 100 (ES100) and eudragit RL (ERL) polymers in order to increase the bioavailability. Silymarin is poorly soluble in water, therefore, an acidic medium is essential for its dissolution. R Garg, et al., 2010[23] developed gastroretentive floating microspheres of silymarin which following oral administration would exhibit prolonged gastric residence time, hence they would increase the bioavailability of the drug. Silymarin releases from the formulations followed zero order kinetics and the mechanism of drug release was diffusion-controlled. You J, et al., 2006[24] prepared the sustained-release formulation with self-emulsifying capability containing zedoary turmeric oil which were prepared by the quasi-emulsion-solvent-diffusion method. The plasma concentration-time profiles with improved sustained-release characteristics were achieved after oral administration of the formulation with a bioavailability of 135.6% with respect to the conventional self-emulsifying formulation (a good strategy for improving the bioavailability of an oily drug). In conclusion, the sustained-release formulation with self-emulsifying capability containing zedoary turmeric oil has an improved oral bioavailability. Chao P, et al., 2010[25] investigated the pulmonary targeting microparticulate camptothecin delivery system for anticancer evaluation in a rat orthotopic lung cancer model. The study shows that microparticulate is able to achieve effective local lung and low systemic drug concentrations at a dose which was 10 times lower than systemically administered drug resulting in a significant improvement in anticancer efficacy in an orthotopic rat model of lung cancer.
3.4. Nano carriers for phytomedicine
The main goals in designing nanoparticles as a delivery system is to manage particle size, surface properties and release of bioactive agents in order to accomplish the site-specific action of the drug at the therapeutically optimal rate and dose regimen. There are certain advantages of using nanoparticles in phytomedicine drug delivery system include, (1) particle size and surface characteristics of the nanoparticles can be modified easily for both passive and active target; (2) controlled release and degradation can be manipulated easily with matrix constituents, so drug can be incorporated into the system free from chemical reaction and with high loading of drug; (3) surface properties of the nanoparticles helps to achieve site specific delivery by attaching target ligands to the surface of the particles.
The different method can be used for nano phytomedicine formulation such as high pressure homogenization method[5],[26], complex coacervation method[5], co-precipitation[5], salting out method[5], nanoprecipitation or solvent displacement method[5],[27], solvent emulsification-diffusion method[5], supercritical fluid method[5],[28] and self assembly method[5] (Figure 5). Different types of nanophytopharmaceuticals such as polymeric nanoparticles, solid lipid nanoparticles, magnetic nanoparticles, metal and inorganic nanoparticles, quantum dots, polymeric micelles, phospholipid micelles, colloidal nano-liposomes, dendrimers can be prepared with the help of the methods stated above (Figure 6).
Figure 5. Common techniques used in nanophytomedicine formulation.

Figure 6. Various types of nanophytopharmaceuticals.

A number of studies have been carried out to elucidate different nano carriers for phytomedicine delivery. Recently, Deepa V, et al., 2012[29] investigated nano emulsified ethanolic extract of Phyllanthus amarus Schum & Thonn ameliorated which was poorly water soluble. Their study illustrated that the nanophytomedcine formulation showed better hepatoprotective activity than Phyllanthus amarus Schum (100 mg/kg body weight) and also repeated dose oral toxicity proved to be safe.
Zhang J, et al., 2012[30] developed novel Panax notoginsenoside-loaded core-shell hybrid liposomal vesicles (PNS-HLV) were developed to resolve the restricted bioavailability of PNS and to enhance its protective effects in-vivo on oral administration. They compared the effect of PNS-HLV on global cerebral ischemia/reperfusion and acute myocardial ischemia injury with those of PNS solution, conventional PNS-loaded nanoparticles, and liposomes. They concluded that HLV had promising prospects for improving free drug bioactivity on oral administration.
Li Z, et al., 2012[31] evaluated the effect of celastrol nanoparticles on retinoblastoma and the potential mechanisms involved. Celastrol is a Chinese herbal medicine which is poorly soluble in water and inhibits the growth of retinoblastoma in a mouse xenograft model by inducing apoptosis in SO-Rb 50 cells, which may be related to the increased Bax/Bcl-2 ratio and the inhibition of NF-κB. Celastrol nanoparticles may represent a potential alternative treatment for retinoblastoma.
Shi F, et al., 2012[32] prepared solid lipid nanoparticles (SLNs) for the oral delivery of frankincense and myrrh essential oils by a high-pressure homogenization method using Compritol 888 ATO as the solid lipid and soybean lecithin and Tween 80 as the surfactants. They concluded that SLNs could be used as drug carriers for hydrophobic oil drugs extracted from traditional Chinese medicines.
Kundu P, et al., 2012[33] investigated antiglioma activity of curcumin-loaded lipid nanoparticles and its enhanced bioavailability in brain tissue for effective glioblastoma therapy. The greatest challenge in the administration of curcumin stems from its low bioavailability and high rate of metabolism. They demonstrated that curcumin-loaded nanoparticles inhibited cellular proliferation, migration and invasion along with a higher percentage of cell cycle arrest and telomerase inhibition, thus leading to a greater percentage of apoptotic cell death in glioma cells compared with native curcumin. An in-vivo study demonstrated an enhanced bioavailability of curcumin in blood serum and brain tissue when being delivered by curcumin-loaded glyceryl monooleate nanoparticles compared with native curcumin in a rat model. Thus, curcumin-loaded nanoparticles can be used as an effective delivery system to overcome the challenges of drug delivery to the brain, providing a new approach to glioblastoma therapy.
Han L, et al., 2012[34] prepared an injectable nanoparticulate system based on a monomethoxy (polyethylene glycol)-poly (lactide-co-glycolide)-monomethoxy (polyethyleneglycol)(PELGE) for co-encapsulation and sustained release of four active components (ginkgolides A, B, C and bilobalide) in Ginkgo biloba extract. Sustained and synchronized release of the four components from PELGE nanoparticles was observed both in vitro and in vivo, which was mainly contributed to the long circulation of PEGylated nanoparticles and the slow degradation of PLGA. The half-life time of the four terpenoid compounds was also significantly improved by incorporating into PELGE nanoparticles. The results indicate that a PELGE nanoparticle is a promising carrier system for sustained and synchronized release of herbal medicines containing multiple components.
Zhang J, et al., 2011[35] investigated the potential of oral and pulmonary nanocrystal to enhance the bioavailability of baicalein, a bioactive flavonoid isolated from the root of Scutellaria baicalensis Georgi. The baicalein nanocrystal was prepared by anti-solvent recrystallization followed by high pressure homogenization. They concluded that the mean relative bioavailability of oral baicalein nanocrystal was 1.67-fold that of oral baicalein crystal. The pulmonary baicalein nanocrystal had rapid and extensive absorption and had almost identical pharmacokinetic parameters to intravenous baicalein injection.
Chang LC, et al., 2011[36] described an optimal method for preparing tanshinone IIA nanoemulsions (TA-NEs) to solve the problems of tanshinone IIA poor solubility in water and insufficient dissolution rate. Optimization of the method for preparing nanoemulsions of lipid soluble TA by emulsification/high pressure homogenization was carried out with a 2 (3) factorial design, using Tween 80, lecithin and water content as independent variables. TA-NE-F4 particles were spherical and intact, with a mean particle size of 95.6 nm and a high entrapment efficiency of 99.3%. TA-NE-F4 showed a fast dissolution rate of 80% in 10 min and 100% in 20 min. The cytotoxicity of TA-NE-F4 against T24 human bladder cancer cells was 103.4-fold greater than TA alone in both a time- and a dose-dependent manner. Overall, they described a feasible method for preparing TA-NEs that exhibited potent cytotoxicity.
Zhang H, et al., 2011[37] prepared and studied the in vitro release characteristics of curcumin in nano suspensions by the solvent evaporation method. The method described to prepare curcumin albumin nano suspensions is simple. It might be a novel vehicle potentially for a nanoparticle drug delivery system of curcumin.
Hu L, et al., 2010[38] investigated SLNs prepared by an ultrasonic and high-pressure homogenization method to improve the oral bioavailability of the poorly water-soluble drug cryptotanshinone. The particle size and distribution, drug loading capacity, drug entrapment efficiency, zeta potential and long-term physical stability of the SLNs were characterized in detail. These results indicate that cryptotanshinone absorption is enhanced significantly by employing SLN formulations, and SLNs represent a powerful approach for improving the oral absorption of poorly soluble drugs.
Zhao XL, et al., 2010[39] studied the preparation and characteristics of zedoary turmeric oil, a traditional Chinese oily medicine, loaded with nanostructured lipid carriers. The authors suggested that the presented nanostructured lipid carrier system might be a promising intravenous dosage form of water-insoluble oily drugs.
Sutthanut K, et al., 2009[40] formulated extracts of Kaempferia parviflora (K. parviflora) in solid lipid nanoparticles (SLNs) in order to enhance their transdermal permeability. The K. parviflora extracts were entrapped within SLNs by adding them to a melted mixture of oils, surfactants and PEGylated agents and subsequently forming an oil-in-water microemulsion at an elevated temperature. Cooling of this microemulsion resulted in the formation of SLNs. The formulation with the optimum properties was composed of stearyl alcohol as the nanoparticle matrix and tocopheryl polyethylene glycol succinate as the surfactant. Particle sizes of 82-108 nm were obtained with entrapment efficiencies as high as 87%. The amount of total K. parviflora flavonoids in the SLNs and gel that had permeated through the skin after 25 h [(95.57±9.08) and (81.04±5.82) g, respectively] were found to be significantly different (P<0.05).
Zhao J et al., 2009[41] prepared and evaluated brucine-loaded polylacticacid nanoparticles (Bru-PLA-NPs). The Bru-PLA-NPs were prepared by solvent diffusion method. The physical, chemical properties and in vitro release behavior of the prepared Bru-PLA-NPs were evaluated, respectively. The Bru-PLA-NPs prepared by solvent diffusion method exhibited small particle size, high Bru-loading efficiency, and obvious sustained release in vitro. The nanoparticle will increase the bioavailability and bioactivity of phytomedicine by reducing the size of the particles[42], surface modification[43], attaching or entrapping the phytomedicine with different polymers of micro or nano materials (Figure 7)[44],[45]. The collection of recent advances of nanophytomedicne is listed in Table 1.
Figure 7. Nanophytomedicine enhances the bioavailability and bioactivity of phytomedicine.

Table 1. Compilation of recent advances in nanophytomedicine.
| Formulations | Methods | Active ingredients | Purpose |
| Panax notoginsenoside-loaded core-shell hybrid liposomal vesicles | Water-in-oil-in-water double emulsion solvent evaporation method | Panax notoginsenoside | To increase the bioavailability and to enhance its protective effects |
| Celastrol nanoparticles | Poly (ethylene glycol)-block-poly (ε-caprolactone) nanopolymeric micelles | Celastrol | To improve the hydrophilicity |
| Solid lipid nanoparticles | High-pressure homogenization method | Frankincense and myrrh essential oils | To improve the hydrophilicity & bioavailability |
| Celastrol poly (ethylene glycol)-block-poly (ε-caprolactone) nanopolymeric micelles | Ultrasonic agitation using a sonic dismembrator followed by solvent evaporation | Celastrol | To improve the hydrophilicity |
| Glyceryl monooleate nanoparticles | High-pressure homogenization method | Curcumin | To enhance bioavailability in brain tissue |
| PEGylated nanoparticles | Co-encapsulation | Ginkgolides A, B, C and bilobalide | Sustained and synchronized release of the four components |
| Pulmonary nanocrystal | Anti-solvent recrystallization followed by high pressure homogenization | Baicalein | To enhance the bioavailability |
| Tanshinone IIA nanoemulsions | Emulsification/high pressure homogenization | Tanshinone IIA | To improve the hydrophilicity & bioavailability |
| Curcumin in nanosuspensions | Solvent evaporation method | Curcumin | To improve the hydrophilicity & bioavailability |
| Solid lipid nanoparticles | Ultrasonic and high-pressure homogenization method | Cryptotanshinone | To improve the bioavailability |
| Solid lipid nanoparticles | Oil-in-water microemulsion | Kaempferia parviflora | To enhance their transdermal permeability |
| Polylacticacid nanoparticles | Solvent diffusion method | Brucine | Sustained release |
| Artemisinin nanocapsules | Self assembly procedure | Artemisinin | To increase the biovailability |
| Taxel loaded nanoparticles | Emulsion solvent evaporation method | Taxel | To increase the biovailability |
4. Conclusion
Phytomedicine posses a significant therapeutic efficacy that should be explored with nanotechnology. Literatures reported that phytomedine posses excellent in-vitro bioactivity but poor aqueous solubility, larger molecular size, degradation during gastric emptying, extensive metabolism are the certain problems, which limits the utility of these plant extracts in-vivo. Application of nanotechnology leads to increase in bioavailability and bioactivity of phytomedicine by reducing the size of the particles, surface modification, attaching or entrapping the phytomedicine with different polymers of micro or nano materials. Nanomaterials aids the targeted delivery, sustained delivery and improves the pharmacokinetics profile, diffusion of drugs into various organs by crossing the barriers including the blood brain barrier. The current research should focus on designing and development of multifunctional nanomaterials and in-vivo studies of their formulations.
Acknowledgments
The authors are thankful to the Research, Knowledge and Technology Transfer, Ambo University, Ambo, Ethiopia for the financial support, Grant No. AU/PHAR 54/2004 EC. The authors thank for the support and assistance of the Mrs. Vanapalli V Satya Veni, Lecturer, Department of Pharmacy, College of Medicine and Health Sciences, Ambo University, Ambo, Ethiopia.
Comments
Background
This is a review article summarising the purpose and methods used for the formulation of phytomedicines into nano-sized drug delivery systems. The review describes the general advantages of nanoformulation before outlining specific examples from the literature using plant-derived actives.
Research frontiers
This review article is a useful summary of some recent studies in nanoformulation of phytomedicines.
Related reports
It does cite general nanoformulation papers and specific phytoformulations highlighting the absence of significant literature attempting a review of nanoformulation of phytomedicines.
Innovations and breakthroughs
The innovation is in bringing together a range of formulation techniques which can be applied to phytomedicines. It has a general introduction before highlighting some particular studies using a variety of actives and formulation strategies and summarising the results.
Applications
This paper serves as a useful introduction to researchers without a background in pharmaceutical formulation of the wide range of different formulation strategies that can be applied to phytomedicines. Strategies to improve solubility and absorption are highlighted.
Peer review
This is a useful summary of a range of different formulation approaches that can be used to improve bioavailability. The authors have focused on a range of actives and this paper gives a welcome insight into how pharmaceutical formulation has been applied to overcome a number of challenges.
Footnotes
Foundation Project: Supported by Research, Knowledge and Technology Transfer, Ambo University, Ambo, Ethiopia (Grant No. AU/PHAR 54/2004 EC).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Brahmankar DM, Jaiswal SB. Biopharmaceutics and pharmacokinetics-A treatise. 1st ed. Delhi: Vallabh Prakashan Publishers; 1995. pp. 296–297. [Google Scholar]
- 2.Kingston DGI. Modern natural products drug discovery and its relevance to biodiversity conservation. J Nat Prod. 2011;74:496–511. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia L, Zhao Y. Current evaluation of the millennium phytomedicine-ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem. 2009;16:2475–2484. doi: 10.2174/092986709788682146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunasekaran T, Nigusse T, Dhanaraju MD. Silver nanoparticles as real topical bullets for wound healing. J Am Coll Clin Wound Spec. 2012;3:82–96. doi: 10.1016/j.jcws.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahni JK, Baboota S, Ali J. Promising role of nanopharmaceuticals in drug delivery. Pharma Times. 2011;43:16–18. [Google Scholar]
- 6.Qureshi NA, Al-Bedah AM. Mood disorders and complementary and alternative medicine: a literature review. Neuropsychiatr Dis Treat. 2013;9:639–658. doi: 10.2147/NDT.S43419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egert S, Rimbach G. Which sources of flavonoids: complex diets or dietary supplements? Adv Nutr. 2011;2:8–14. doi: 10.3945/an.110.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain D, Raturi R, Jain V, Bansal P, Singh R. Recent technologies in pulsatile drug delivery systems. Biomatter. 2011;1:57–65. doi: 10.4161/biom.1.1.17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert WJ. Considerations in developing a target product profile for parenteral pharmaceutical products. AAPS PharmSciTech. 2010;11:1476–1481. doi: 10.1208/s12249-010-9521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen induced hepatotoxicity in rats. Food Chem Toxicol. 2008;46:1771–1777. doi: 10.1016/j.fct.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Su YL, Fu ZY, Zhang JY, Wang WM, Wang H, Wang YC, et al. Microencapsulation of Radix salvia miltiorrhiza nanoparticles by spray-drying. Powder Technol. 2008;184:114–121. [Google Scholar]
- 13.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: an updated review. Int J Pharm Investig. 2012;2:2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadiyala I, Loo Y, Roy K, Rice J, Leong KW. Transport of chitosan-DNA nanoparticles in human intestinal M-cell model versus normal intestinal enterocytes. Eur J Pharm Sci. 2010;39:103–109. doi: 10.1016/j.ejps.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mi S, Cai T, Hu Y, Chen Y, Hodges E, Ni F, et al. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell. 2008;133:116–127. doi: 10.1016/j.cell.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Wolfson SN, Gharib A, Sagasti A. LAR receptor tyrosine phosphatases and HSPGs guide peripheral sensory axons to the skin. Curr Biol. 2012;22:373–382. doi: 10.1016/j.cub.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer S, Foerg C, Merkle HP, Gander B. Chitosan coated PLGA-microsperes-a modular system for targeted drug delivery. Eur Cell Mater. 2004;7:11–12. [Google Scholar]
- 18.Garinot M, Fiévez V, Pourcelle V, Stoffelbach F, des Rieux A, Plapied L, et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release. 2007;120:195–204. doi: 10.1016/j.jconrel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Muskovich M, Bettinger CJ. Biomaterials-based electronics: polymers and interfaces for biology and medicine. Adv Healthc Mater. 2012;1:248–266. doi: 10.1002/adhm.201200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawar SK, Vavia PR. Rice germ oil as multifunctional excipient in preparation of self-microemulsifying drug delivery system (SMEDDS) of tacrolimus. AAPS PharmSciTech. 2012;13:254–261. doi: 10.1208/s12249-011-9748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaikh R, Singh TRR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3:89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fackler K, Schwanninger M. How spectroscopy and microspectroscopy of degraded wood contribute to understand fungal wood decay. Appl Microbiol Biotechnol. 2012;96:587–599. doi: 10.1007/s00253-012-4369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg R, Gupta GD. Gastroretentive floating microspheres of silymarin: preparation and in vitro evaluation. Trop J Pharm Res. 2010;9:59–66. [Google Scholar]
- 24.You J, Cui FD, Han X, Wang YS, Yang L, Yu YW, et al. Study of the preparation of sustained release microspheres containing zedoary turmeric oil by the emulsion solvent diffusion method and evaluation of the self emulsification and bioavailability of the oil. Colloids Surf B Biointerfaces. 2006;48:35–41. doi: 10.1016/j.colsurfb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Chao P, Deshmukh M, Kutscher HL, Gao D, Rajan SS, Hu P, et al. Pulmonary targeting microparticulate campothecin delivery system: anticancer evaluation in a rat orthotopic lung cancer model. Anticancer Drugs. 2010;21:65–76. doi: 10.1097/CAD.0b013e328332a322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diab R, Jaafar-Maalej C, Fessi H, Maincent P. Engineered nanoparticulate drug delivery systems: the next frontier for oral administration? AAPS J. 2012;14:688–702. doi: 10.1208/s12248-012-9377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anil M, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalani M, Yunus R. Application of supercritical antisolvent method in drug encapsulation: a review. Int J Nanomedicine. 2011;6:1429–1442. doi: 10.2147/IJN.S19021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deepa V, Sridhar R, Goparaju A, Reddy PN, Murthy PB. Nanoemulsified ethanolic extract of Phyllanthus amarus Schum & Thonn ameliorates CCl4 induced hepatotoxicity in Wistar rats. Indian J Exp Biol. 2012;50:785–794. [PubMed] [Google Scholar]
- 30.Zhang J, Han X, Li X, Luo Y, Zhao H, Yang M, et al. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomedicine. 2012;7:4299–4310. doi: 10.2147/IJN.S32385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Wu X, Li J, Yao L, Sun L, Shi Y, et al. Antitumor activity of celastrol nanoparticles in a xenograft retinoblastoma tumor model. Int J Nanomedicine. 2012;7:2389–2398. doi: 10.2147/IJN.S29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi F, Zhao JH, Liu Y, Wang Z, Zhang YT, Feng NP. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int J Nanomedicine. 2012;7:2033–2043. doi: 10.2147/IJN.S30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kundu P, Mohanty C, Sahoo SK. Antiglioma activity of curcumin-loaded lipid nanoparticles and its enhanced bioavailability in brain tissue for effective glioblastoma therapy. Acta Biomater. 2012;8:2670–2687. doi: 10.1016/j.actbio.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 34.Han L, Fu Y, Cole AJ, Liu J, Wang J. Co-encapsulation and sustained-release of four components in ginkgo terpenes from injectable PELGE nanoparticles. Fitoterapia. 2012;83:721–731. doi: 10.1016/j.fitote.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Lv H, Jiang K, Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;25:180–188. doi: 10.1016/j.ijpharm.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Chang LC, Wu CL, Liu CW, Chuo WH, Li PC, Tsai TR. Preparation, characterization and cytotoxicity evaluation of tanshinone IIA nanoemulsions. J Biomed Nanotechnol. 2011;7:558–567. doi: 10.1166/jbn.2011.1315. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Zhang L, Yuan P, Wang C. [Preparation and in vitro release characteristics of curcumin in nanosuspensions] Zhongguo Zhong Yao Za Zhi. 2011;36:132–135. Chinese. [PubMed] [Google Scholar]
- 38.Hu L, Xing Q, Meng J, Shang C. Preparation and enhanced oral bioavailability of cryptotanshinone-loaded solid lipid nanoparticles. AAPS PharmSciTech. 2010;11:582–587. doi: 10.1208/s12249-010-9410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao XL, Yang CR, Yang KL, Li KX, Hu HY, Chen DW. Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Dev Ind Pharm. 2010;36:773–780. doi: 10.3109/03639040903485716. [DOI] [PubMed] [Google Scholar]
- 40.Sutthanut K, Lu X, Jay M, Sripanidkulchai B. Solid lipid nanoparticles for topical administration of Kaempferia parviflora extracts. J Biomed Nanotechnol. 2009;5:224–232. doi: 10.1166/jbn.2009.1026. [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Liu Z, Xu J, Yu Y, Feng N. [Preparation and in vitro evaluation of brucine-loaded polylacticacid nanoparticles] Zhongguo Zhong Yao Za Zhi. 2009;34:2322–2324. Chinese. [PubMed] [Google Scholar]
- 42.Li Z, Yao L, Li J, Zhang W, Wu X, Liu Y, et al. Celastrol nanoparticles inhibit corneal neovascularization induced by suturing in rats. Int J Nanomedicine. 2012;7:1163–1173. doi: 10.2147/IJN.S27860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Lin X, Park H, Greever R. Study of artemisinin nanocapsules as anticancer drug delivery systems. Nanomedicine. 2009;5:316–322. doi: 10.1016/j.nano.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Li DC, Zhong XK, Zeng ZP, Jiang JG, Li L, Zhao MM, et al. Application of targeted drug delivery system in Chinese medicine. J Control Release. 2009;138:103–112. doi: 10.1016/j.jconrel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Yadav D, Suri S, Choudhary AA, Sikender M, Hemant , Beg MN, et al. A novel approach: herbal remedies and natural products in pharmaceutical science as nano drug delivery systems. Int J Pharm Technol. 2011;3:3092–3116. [Google Scholar]


