Abstract
Objective
To revise morphological identification keys to the anophelines in Sri Lanka.
Method
Samples were collected from selected entomological sites in different districts in the country. Stage III and IV larvae were identified under a light microscope with an objective (×10) using standard larval keys developed for Sri Lankan anophelines. Key larval characters were recorded for each species based on original observations and previous usage in literature.
Results
This manuscript describes an illustrated key for the identification of 22 of 23 mosquitoes which are currently recognized as local anopheline species in Sri Lanka, as a guide to workers engaged in malaria surveillance and control in the country.
Conclusions
Revised morphological keys to the larval of these species may be helpful in easy and accurate identification at the field level.
Keywords: Anopheline, Immature, Mosquitoes, Control, Keys
1. Introduction
The identification keys to the immature stages of Anopheles mosquitoes have long been a necessity for entomologists dealing with malaria vectors. Many of the illustrated keys to the Anopheles of Sri Lanka are of limited value[1],[2], as these were published more than 20 years ago and significant advances in our knowledge of the Anopheles mosquitoes have occurred in the intervening years. The number of anopheline species has not changed substantially from Carter's checklist; there have been many changes in the identity of the species actually listed, as evidenced by the checklist of Jayasekera and Chelliah[2]. Changes subsequently to this checklist include the invalidation of one species record and description of Anopheles jeyporiensis (An. jeyporiensis ). This paper presents an updated illustrated key for the identification of larval anophelines occurring in the country.
The purpose of the keys presented here is to assist field surveillance teams to identify the larval stages of Anopheles mosquitoes. The keys can be used initially to identify specimens to species group and then to species. Discriminating characteristics are highlighted in drawings wherever possible.
2. Materials and methods
2.1. Collecting samples
Samples were collected from 17 entomological sites in Ampara, Batticaloa, Mannar, Trincomalee and Killinochchi districts as part of the malaria elimination program of Tropical and Environmental Diseases and Health Associates (TEDHA) Pvt. Ltd. Further, wet zone specimens were obtained by the entomological teams attached to the Anti Malaria Campaign.
2.2. Identification of field samples
Stage III and IV larvae were placed individually in microscopic slides and identified under a light microscope with an objective (×10) using standard larval keys developed for Sri Lankan anophelines[2]. Key larval characters were recorded for each species. Further, larval species identification was reconfirmed through adult identification[3].
2.3. Revising larval identification key for Sri Lankan Anopheles
The morphological characters used here are based on original observations and previous use in the literature. The following were referred to during the construction of this key: Amarasinghe, 1992[2], Christophers, 1933[4], Colless, 1957[5], Reid, 1968[6], Harrison, 1980[7], Harrison and Scanlon, 1975[8], Linton et al., 2001[9], Junkum et al., 2005[10], Linton et al., 2005[11], Rattanarithikul et al., 2005[12], Sallum et al., 2005[13] and Rattanarithikul et al., 2006[14]. Taxonomic characteristics were cross checked in relation to Sri Lankan specimens by examining the reference materials archived at the Molecular Medicine Unit, Faculty of Medicine, University of Kelaniya, Sri Lanka.
Species nomenclature follows that proposed by Knight and Stone[15], and abbreviations used in the text follow that used by Reinert[16],[17]. Morphological terminology and chaetotaxy follow that used by Harbach and Knight[18]. Abdominal segments are numbered by roman numerals. Twenty two Sri Lankan anopheline species have been included into this key. The species considered include:
Subgenus Anopheles aitkenii James 1903 (An. aitkenii), Anopheles barbirostris Van der Wulp 1884 (An. barbirostris), Anopheles barbumbrosus Strickland and Choudhury 1927 (An. barbumbrosus), Anopheles gigas var. refutans Alcock 1913, Anopheles interruptus Puri 1929 (An. interruptus), Anopheles nigerrimus Giles 1900 (An. nigerrimus), Anopheles peditaeniatus (Leicester) 1908 (An. peditaeniatus), Anopheles peytoni Kulasekera (An. peytoni), Harrison and Amerasinghe 1988.
Subgenus Cellia: Anopheles aconitus Donitz 1902 (An. aconitus), Anopheles annularis Vander Wulp 1884, Anopheles culicifacies Giles 1901 (An. culicifacies), Anopheles elegans (James) 1903 (An. elegans), Anopheles jamesii Theobald 1901, An. jeyporiensis (James) 1902, Anopheles karwari (James) 1902, Anopheles maculatus Theobald 1901, Anopheles pallidus Theobald 1901, Anopheles pseudojamesi Strickland and Chowdhury 1927, Anopheles ramsayi Covell 1927, Anopheles subpictus Grassi 1899 (An. subpictus ), Anopheles tessellatus Theobald 1901 (An. tessellatus), Anopheles vagus Donitz 1902 (An. vagus), Anopheles varuna Iyengar 1924 (Figure 1).
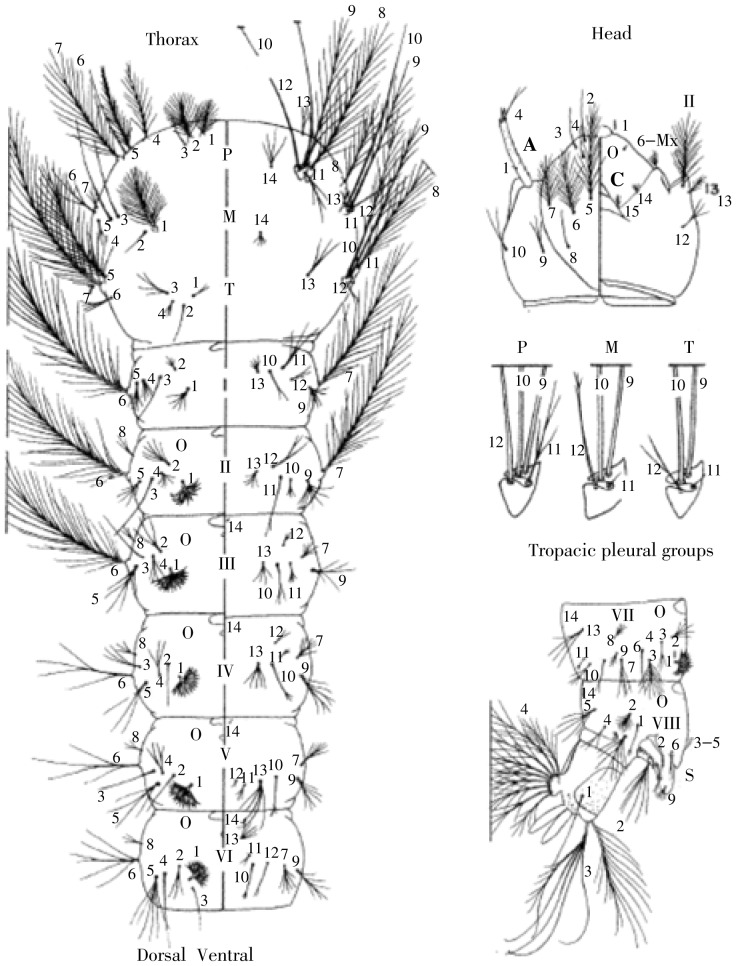
Figure 1. Larval morphology.
Head: A=Antenna, C=Cranium head capsule; Thorax: P=Pro-thorax, M=Meso-thorax, T=Meta-thorax, 1-14=setae on designated areas (seta 1M, setae 1-T); Abdomen: I-VII, X=Abdominal segments, S=Spiracular apparatus, 1-14= Setae on designated areas (seta 1-I, seta 5-IV).
3. Results
The revised larval morphological key is shown below. This key presents an illustrated key for the identification of larvae of 22 of 23 currently recognized local anopheline species in Sri Lanka. Morphological features noted in different regions of the body were recorded: head (antennal hairs, inner clypeal hairs, outer clypeal hairs, frontal hairs and sutural hairs), thorax (thoracic palmate hairs, shoulder hairs, pro, meso and meta thoracic hairs), abdomen (abdominal tergal plates, palmate hairs in abdominal segments, lateral hairs in the abdominal segments).
The inclusion of An. jeyporiensis is based on the reported collection during the TEDHA Malaria Elimination Program in 2011. This species was included in this key as local workers may encounter the species and may difficulty in its identification.
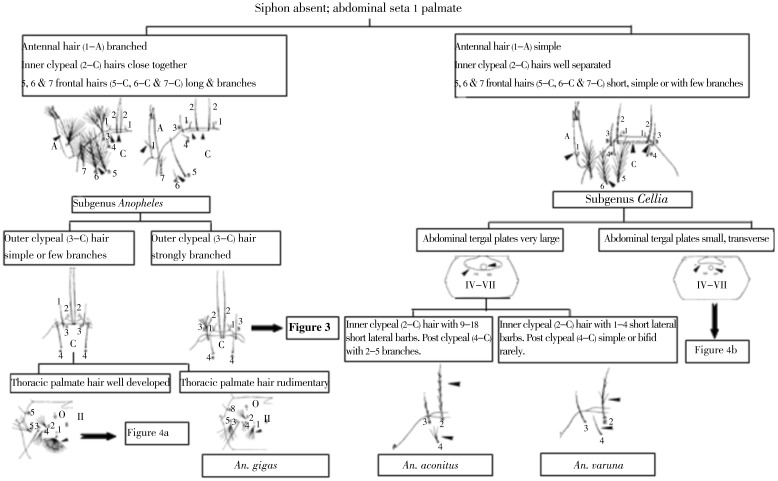
Generally, members of subgenus Anopheles are characterized by branched seta 1-A and closely situated setae 2-C. However, exceptions such as An. aitkenii sens. str., with widely spaced 2-C[2] and An. interruptus, with simple 1-A and closely spaced 2-C[2],[6],[8] have been reported in Sri Lanka. Figure 2 in the key has been worded to avoid initial misidentification of larvae.
Figure 2. Sri Lankan anopheline mosquito - key to the fourth instar larvae.
4. Discussion
The key to the Sri Lankan anopheline larvae has been revised according to the currently accepted classification of sections, series and groups in the Anophelinae.
Brief notes are provided at each step in the identification process along with illustrative diagrams. It is emphasized that taxonomic keys are only a rapid and convenient guide to identification based on the examination of a few important characters. In case of doubt, it is essential to consult published literature with detailed descriptions of species characters.
4.1. Subgenus Anopheles
4.1.1. Myzorhynchus series
The Myzorhynchus series can be separated from other Sri Lankan representatives of subgenus Anopheles by the outer clypeal hair (3-C) being strongly branched. Two species groups are present in Sri Lanka: the Hyrcanus and Barbirostris group (Figure 2).
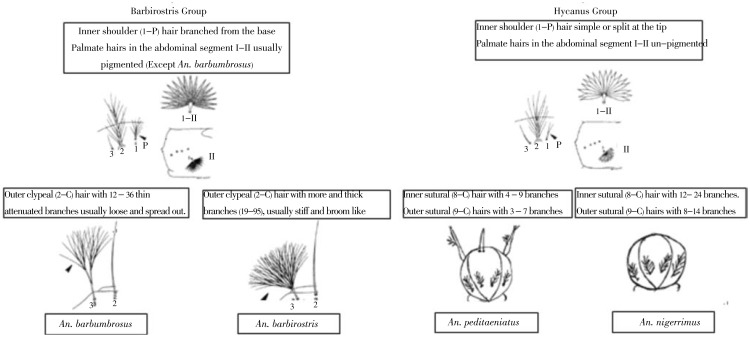
4.1.1.1. Hyrcanus group
The Hyrcanus group can be separated from the Barbirostris group by the morphological characters given in the key. This group bears a superficial resemblance to members of the Hyrcanus group, all of which have pale or un-pigmented I–II; the separation of An. peditaeniatus and An. nigerrimus needs care. The definitive identifying feature is the basally branched, sinuous seta 4-M of An. peditaeniatus which is small and often difficult to observe. This may be confused with 7-M resulting in misidentification as An. nigerrimus. In this instance, branching of setae 8-C and 9-C are useful in confirmation. The correct identification of An. nigerrimus is important as it is a suspected vector of malaria in Sri Lanka while An. peditaeniatus is considered a non-vector (Figure 3).
Figure 3. Outer clypeal hair strongly branched.
4.1.1.2. Barbirostris group
Within the Barbirostris group of subgenus Anopheles, the orientation of the inner shoulder hair (1-P) can be used as the primary character: 1P seta with 4 or more branches is characteristic of this group. This character should be checked carefully during identification. The pigmentation of seta I-II is an useful secondary character in separating barbirostris from barbumbrosus (pale or colorless seta). In addition, seta orientation of the outer clyepeal hair (3-C) can be used to separate these two species as the optional character in order to confirm the identification. It can be described as follows; 3-C of An. barbumbrosus has 12-36 thin attenuated branches usually loose and separated out, whereas 3-C of An. barbirostris has more branches which are thick (19-95), usually stiff and broom like (Figure 3). The larval morphology of An. reidi has still not been reported.
4.1.2. Lophoscelomyia series
An. interruptus is the only species in this series reported in the country. It can be distinguished by the simple inner clypeal hair (2-C), and branched outer clypeal hair (3-C), 4-A and 11-C. In the previous key for Sri Lankan anopheline larvae, the identification was confirmed through the presence of reduced setae 5, 6 and 7-C, some or all of which were short and a few-branched or simple; seta 4-C simple or branched only in distal half[2]. These characters are rather difficult in identification and controversial. Therefore, the new character which has been included in this key is useful to minimize misidentification of this species (Figure 4).
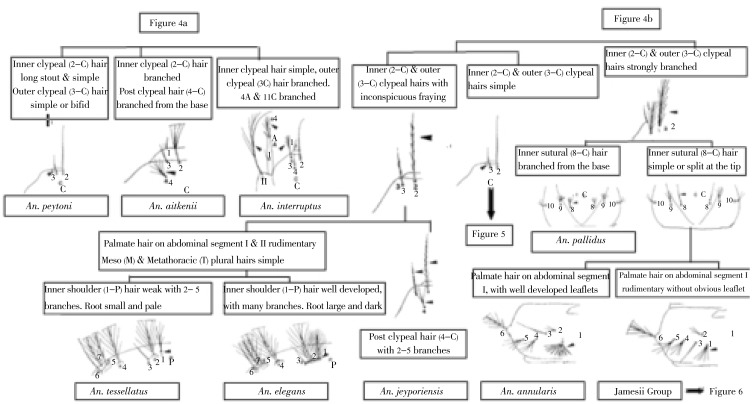
Figure 4. Thoracic palmate hair and abdominal tergal plates.
a: Thoracic palmate hair well developed; b: Abdominal tergal plates small, transverse.
4.1.3. Anopheles series
There are three species in the country, namely: Anopheles gigas, An. aitkenii and Anopheles peytoni (An. peytoni ). An. peytoni is an endemic species in Sri Lanka and had been confused with An. insulaeflorum; the larvae of An. peytoni has bases of setae 2-C nearly as wide apart as 2-C and 3-C on one side which is different from the larval morphology of An. insulaeflorum. In this key the following characters have been included to confirm identification of An. peytoni: 2-C long and simple, 3-C simple or bifid. These features facilitate the separation of An. aitkenii which has branched 2-C and 4-C (Figure 4).
4.2. Subgenus Cellia
4.2.1. Myzomyia series
Separating Anopheles varuna and An. aconitus in the Myzomyia series of subgenus Cellia is sometimes difficult when the barbed nature of setae 2-C, 3-C and branching of 4-C in An. aconitus are observed by drift or the larval mouth brushes. It is more difficult to distinguish An. jeyporiensis as it separates only by having a small tergal plate and 4-5 branches in 4-C (Figure 2).
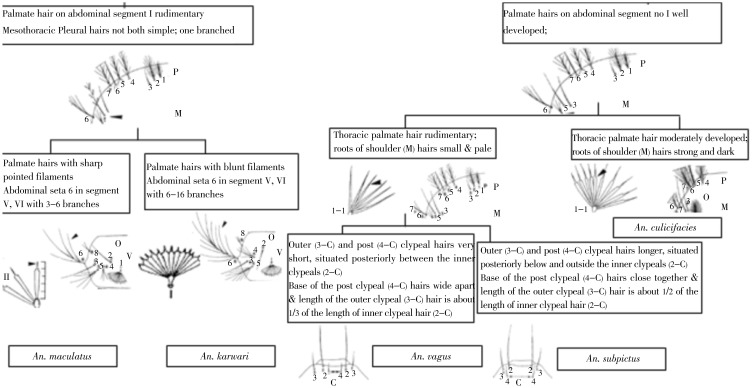
An. culicifacies, the main vector of malaria in Sri Lanka can be separated very easily among the members in Myzomyia series of subgenus Cellia by the presence of small tergal plates, unbranched 2-C and 3-C and moderately developed thoracic palmate hairs (Figure 5).
Figure 5. Palmate hairs on abdominal segments.
On abdominal segment II always well developed; hairs on segment I sometimes rudimentary; Meso and Metathoracic Pleural hairs not all simple; one or more branched.
4.2.2. Pyretophorus series
An. subpictus and An. vagus are the only two recorded species in this series in Sri Lanka. These two species can be easily separated from An. culicifacies by the moderately developed thoracic palmate hair and strong and dark roots of shoulder hairs. An. vagus has very short 3-C and 4-C, 4-C is situated between 2-C with the base of 4-C being wide apart, and the length of 3-C being about 1/3 of the length of 2-C; An. subpictus has longer 3-C and 4-C, and 4-C seta is situated below and outside 2-C; the base of the 4-C seta and length of the 3-C is about ½ of the length of 2-C (Figure 5).
4.2.3. Neocellia series
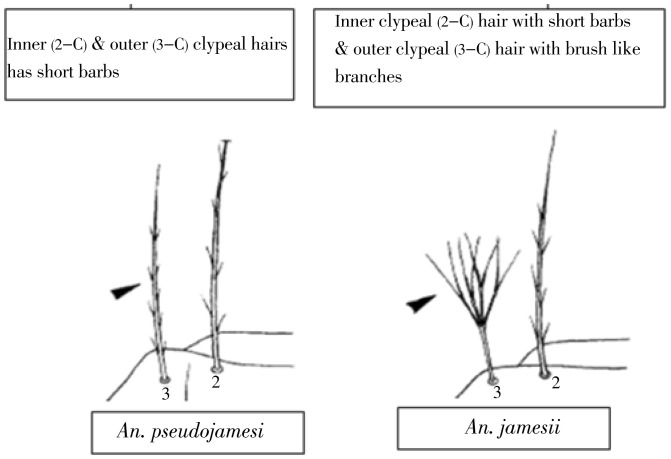
Six species of Sri Lankan Neocellia can be divided into those with bushy setae 3-C (Anopheles jamesii, Anopheles annularis, Anopheles pallidus) and those with simple or barbed 3-C (Anopheles maculatus, Anopheles kawari, Anopheles pseudojamesi). A further character that separates the two groups is the length of the filaments in abdominal palmate setae IV –VII, these being equal to or greater than ½ the length of the blades in the former and 1/3rd or less in the latter (Figure 6).
Figure 6. Palmate hair on abdominal segment I rudimentary without obvious leaflet.
4.2.4. Neomyzomyia series
Two species, An. tessellatus and An. elegans (the latter belonging to the leucosphyrus group) have been reported in Sri Lanka. An. elegans is an endemic species in the country. Identification of An. tessellatus is important in malaria vector surveillance programmes in the Sri Lanka as it has been incriminated as a secondary vector of malaria transmission in the country. Identification of this species can be confirmed by the presence of 2-5 branches in the inner shoulder hair (1-P) with small and pale roots. Individuals with opposite morphological features can be regarded as An. elegans (Figure 4).
Sri Lankan anopheline larvae can be differentiated easily by observing basic characters. However, this may become a difficult task when processing whole larvae in routine malaria entomological surveys.
Acknowledgments
I would like to thank Dr. Panduka Wijeyarathne, the Program Director of the Malaria Elimination Program of Tropical and Environmental Diseases and Health Associates (TEDHA) Pvt. Ltd for his encouraging support and financial assistance from the Global Fund for Aids, Tuberculosis and Malaria (GFATM) (Round 8) through TEDHA (Grant No. SRL809G11M).
Comments
Background
Morphological taxonomic keys are indispensable tools in malaria vectors surveillance and control. They help field operators to easily and accurately identify anopheline species in the field. In Sri Lanka, the published keys are outdated and need to be revised as knowledge on anophelines have advanced.
Research frontiers
This paper is carried out to revise a larval morphological identification key of the anopheline species of Sri Lanka.
Related reports
This key has reported one more species than the guide to the identification of anopheline mosquitoes of Sri Lanka published by Amarasinghe in 1992.
Innovations and breakthroughs
This paper contains the first illustrated key that allows the identification of the immature of 22 anopheline species currently known in Sri Lanka.
Applications
This key will assist field workers involved in malaria surveillance and control in Sri Lanka to firmly identify the anopheline species currently known in this island.
Peer review
This study is very important because the identification of mosquito species is always the first step in all surveillance and control of malaria strategies. It describes an updated and easy way to use illustrated key that will be useful for Sri Lankan malaria control personnel and researchers interested in local anopheline fauna.
Footnotes
Foundation Project: Supported by Global Fund for Aids, Tuberculosis and Malaria (GFATM) (Round 8) through TEDHA (Grant No. SRL809G11M).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Carter HF. Ceylon mosquitoes list species and names of mosquitoes recorded from Ceylon. Ceylon J Sci Sect B Zool. 1950;24:85–115. [Google Scholar]
- 2.Amarasinghe FP. A guide to the identification of anopheline mosquitoes (Diptera; Culicidae) of Sri Lanka. II. Larvae. Ceylon J Sci. 1992;22:1–13. [Google Scholar]
- 3.Amarasinghe FP. A guide to the identification of anopheline mosquitoes (Diptera; Culicidae) of Sri Lanka. I. Adult females. Ceylon J Sci. 1990;21:1–16. [Google Scholar]
- 4.Christophers SR. The fauna of British India, including Ceylon and Burma. London: Taylor & Francis; 1933. p. 371. [Google Scholar]
- 5.Colless DH. Further notes on the systematic of the Anopheles leucosphyrus group (Diptera: Culicidae) Proc R Entomol Soc London. 1975;26:131–139. [Google Scholar]
- 6.Amarasinghe FP, Reid JA. Anopheles mosquitoes of Malaya and Borneo. England: Staples Printers Limited; 1968. p. 518. [Google Scholar]
- 7.Harrison BA. Medical entomology studies–XIII. The Myzomyia series of Anopheles (Cellia). In Thiland, with emphasis on intra-interspecific variations (Diptera: Culicidae) Contrib Am Entomol Inst. 1980;17(4):1–195. [Google Scholar]
- 8.Harrison BA, Scanlon JE. The subgenus Anopheles in Thailand (Diptera: Culicidae) Contrib Am Entomol Inst. 1975;12(1):1–307. [Google Scholar]
- 9.Linton YM, Harbach RE, Chang MS, Anthony TG, Matusop A. Morphological and molecular identity of Anopheles (Cellia) sundaicus (Diptera: Culicidae), the nominotypical member of a malaria vector species complex in Southeast Asia. Syst Entomol. 2001;26:357–366. [Google Scholar]
- 10.Junkum A, Komalamisra N, Jitpakdi A, Jariyapan N, Min GS, Park MH, et al. Evidence to support two conspecific cytological races of Anopheles aconitus in Thailand. J Vector Ecol. 2005;30:213–224. [PubMed] [Google Scholar]
- 11.Linton YM, Dusfour I, Howard TM, Ruiz LF, Duc Manh N, Ho Dinh T, et al. Anopheles (Cellia) epiroticus (Diptera: Culicidae), a new malaria vector species in the Southeast Asian Sundaicus Complex. Bull Entomol Res. 2005;95:329–339. doi: 10.1079/ber2005364. [DOI] [PubMed] [Google Scholar]
- 12.Rattanarithikul R, Harrison BA, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand I. Background; geographic distributions; list of genera, subgenera, and species; and a key to genera. Southeast Asian J Trop Med Public Health. 2005;36(Suppl 1):1–80. [PubMed] [Google Scholar]
- 13.Sallum MA, Peyton EL, Wilkerson RC. Six new species of the Anopheles leucosphyrus group, reinterpretation of Anopheles elegans and vector implications. Med Vet Entomol. 2005;19:158–199. doi: 10.1111/j.0269-283X.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 14.Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand IV Anopheles. Southeast Asian J of Trop Med & Pub Health. 2006;37(Suppl 2):1–128. [PubMed] [Google Scholar]
- 15.Knight KL, Stone A. A catalog of the mosquitoes of the world (Diptera: Culicidae) 2nd ed. Maryland: Entomology Society of America; 1977. pp. 1–611. [Google Scholar]
- 16.Reinert JF. Mosquito generic and subgeneric abbreviations (Diptera: Culicidae) Mosquito Systematics. 1975;7:105–110. [Google Scholar]
- 17.Reinert JF. Revised list of abrreviations for genera and subgenera of Culicidae (Diptera) and notes on generic and subgeneric changes. J Am Mosq Control Assoc. 2001;17:51–55. [PubMed] [Google Scholar]
- 18.Harbach RE, Knight KL. Taxonomists' glossary of mosquito anatomy. Marlton: Plexus Publishing; 1980. pp. 415–420. [Google Scholar]