Abstract
Objective
To evaluate pharmacologically the traditional use of Diospyros lotus as antipyretic and antinociceptive in various animal models.
Methods
In vivo experimental models were used in this study. Antipyretic activity of extract/fractions was evaluated in brewer's yeast induced hyperthermic mice while antinociceptive activity was studied in acetic acid induced writhing test at 50 and 100 mg/kg i.p.
Results
The crude extract strongly ameliorated the induced pyrexia during various assessment times. Upon fractionation, the antipyretic effects were strongly augmented by the chloroform and ethyl acetate fractions of the plant. However, hexane and butanol fractions were insignificant in their effect as antipyretic. The extract showed marked inhibition on the noxious simulation induced by post acetic acid injection. The effect was strongly supported by other fraction expect hexane.
Conclusions
In short, our study scientifically validated the traditional use of the plant as antipyretic.
Keywords: Diospyros lotus, Ebenaceae, Antipyretic, Antinociceptive activities
1. Introduction
Plants are richest source of bioactive secondary metabolites in a most effective way and with specific selectivity[1],[2]. From the start of human being development, men were using different medicinal plants as traditional medicines for their health care. Plants have the ability to produce several valuable classes of chemical constituents which showed interesting biological action[3],[4]. Diospyros is the most important genus which contains more than 500 species. The distinguishing features of the Diospyros species are trees, rarely shrubs, leaves alternate flowers green, white or yellow, few to many, axillary cymes or the pistillate solitary, corolla campanulate, urceolate or tubular, the lobes 3-7 (usually 4-5), stamens four to many mostly with 4-8 staminodia in pistillate flowers, ovary 4-16 celled; fruit is a large juicy 1-10 seeded berry and the sap wood is white and soft and heartwood is black and hard. The genus Diospyros is of more economic importance with many species yield edible fruits and valuable timbers. The best fruit yielding species is the kaki or Japanese persimmon (Diospyros kaki) has been cultivated in Japan for many centuries. The kaki fruits, a large orange-red berries, are very astringent until fully ripe, at which time the tannin content is completely transformed into insoluble crystals. The juice is then sweet and palatable. The other important fruit yielding species are Diospyros virginiana, Diospyros ebenaster, Diospyros lotus (D. lotus) L., Diospyros mespiliformis (D. mespiliformis) and Diospyros melanoxylon.
D. lotus is a deciduous tree, growing in China and Asia. D. Lotus has been cultivated for its edible fruits. The fruit of D. lotus is used as a sedative, astringent, nutritive, antiseptic, antidiabetic, antitumor, astringent, laxative, nutritive, antipyretic and for the treatment of constipation[3]. D. lotus fruits are used for the treatment of diarrhea, dry coughs and hypertension.
Phytochemical studies have been previously carried out on many Diospyros species and have revealed the widespread presence of naphthoquinones and naphthalene derivatives, dimeric naphthoquinones and lupine triterpenes[5]. Chemical investigation of the fruits led to the D. lotus identification of some fatty acids, sugars phenolic compounds and non-volatile acids[5],[6]. The aim of the current project deals with the antipyretic and antinociceptive activity of crude extract of D. lotus in experimental animals.
2. Materials and methods
2.1. Plant material
Roots of D. lotus were collected from Toormang Razagram, Dir, KPK, Pakistan, in May 2009. The sample was authenticated by Dr. Abdur Rashid, taxonomist, and Botany Department, University of Peshawar Pakistan. A voucher specimen (Bot/649) has been deposited at the herbarium, Department of Botany, University of Peshawar Pakistan.
2.2. Extraction and isolation
Shade-dried roots of D. lotus (14 kg) was powdered and then kept at room temperature in MeOH for 6 d with continuous stirring by simple percolation. After this period, the extracts were concentrated by evaporating solvents using rotary vacuum evaporator under reduced pressure at temperature 45 °C. This process was repeated four times until the extraction was completed and finally 202 g of dark red residue of roots and 155 g of barks was obtained. The MeOH extract of roots was suspended in water and successively partitioned with hexane, CHCl3, EtOAc and BuOH according to standard protocol[7].
BALB/c mice were used in various experiments. They were fed with standard laboratory food and water ad libitum. Animals were kept under standard condition of temperature and light. Before the start of experiment, animals were acclimatized with laboratory conditions. The rulings of the institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council were maintained during all the experiments performed.
2.3. Antipyretic test (yeast induced pyrexia)
The antipyretic activity was determined in BALB/c mice (25-30 g) of either sex[8]. The animals were divided in five groups (n=5). All groups were fasted overnight and allowed free accesses drinking water. Group one received saline as control group and second group received paracetamol as standard drug while the remaining groups received 50 and 100 mg/kg of extract/fractions. Normal temperature was recorded using digital thermometer and then pyrexia was induced in all minces by injecting 20% aqueous suspension of brewer's yeast (10 mL/kg s.c). After 24 h, rectal temperature was recorded and corresponding groups were injected with above doses. Rectal temperature was recorded periodically at 1.5, 3 and 5 h of drugs administration.
2.4. Acetic acid induced writhing test
BALB/c mice of either sex (n=6) weighing 25-30 g were used[9]. All animals were withdrawn from food 2 h before the start of experiment. All animals were divided in various groups. Group I was injected with normal saline intraperitoneally as control while group II was injected with standard drug diclofenac sodium (10 mg/kg body weight) and the remaining groups were injected with 50 and 100 mg/kg i.p. of methanolic extract and its various solvent fractions. After 30 min of saline, diclofenac sodium and various extract, the animals were treated i.p. with 1% acetic acid. The writhing was counted after 5 min of acetic acid injection. The number of abdominal constrictions (writhes) was counted for 10 min.
2.5. Statistical analysis
Results are expressed as mean±SEM. One-way ANOVA was used for comparison test of significant differences among groups followed by Dunnet's multiple comparison post test. A level of significance (P<0.05) was considered for each test.
3. Results
3.1. Effect of antipyretic activity
The results of yeast induced pyrexia test of extract/fractions of D. lotus in mice at 50 and 100 mg/kg are illustrated in Figure 1. The crude extract strongly ameliorated the induced pyrexia during various assessment times. The maximum antipyretic effect (60.33%) was observed after 5th of pretreatment of extract at 100 mg/kg i.p. (Figure 1A). Upon fractionation, activity differences were noted; hexane did not produce significant effect while chloroform fraction demonstrated outstanding action with maximum reversal of 69.28% after 5th h of drug administration at 100 mg/kg i.p. (Figure 1B). Of the extract/fractions, the ethyl acetate fraction was most active. It had maximum of 72.27% pyrexia control at 100 mg/kg i.p. after 5th hour of drug injection (Figure 1C). Butanol fraction was inactive like hexane. However, standard drug, paracetamol produced outstanding action during all assessment time at 100 mg/kg i.p. (Figure 1D).
Figure 1. Percent effect of yeast induced pyrexia in hyperthermic mice at 50 and 100 mg/kg i.p. during various assessment times.
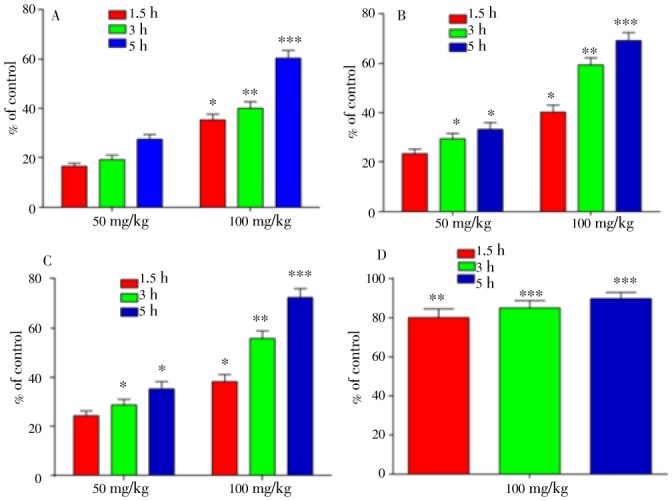
Crude extract (A), chloroform fraction (B), ethyl acetate (C) and standard drug, paracetamol (D). Data are reported as mean±SEM (n=6). The data were analyzed by ANOVA followed by Dunnett's test. *P<0.5, **P<0.01, ***P<0.001 was considered as statistically significant from control.
3.2. Effect of acetic acid induced writhing test
The effects of acetic acid induced writhing test of extract/fractions of D. lotus in mice are shown in Figure 2. The crude extract significantly antagonized the painful sensation in form of abdominal constriction produced by acetic acid injection up to 61.09% at 100 mg/kg i.p. (Figure 2A). The hexane fraction of the plant was inactive (Figure 2B) while chloroform fraction exhibited marked anti-nociceptive effect (68.87%) at 100 mg/kg i.p. (Figure 2C). The ethyl acetate fraction most dominantly inhibited (75.89%) noxious stimulation at 100 mg/kg i.p. (Figure 2D). In case of butanol fraction, pain inhibition was 55.23% at 100 mg/kg i.p. (Figure 2E). However, diclofenac was outstanding in the reversal of pain with 84.44% protection at 10 mg/kg i.p. (Figure 2F).
Figure 2. Percent protection against noxious stimulation induced by acetic acid at 50 and 100 mg/kg i.p. during various assessment times.
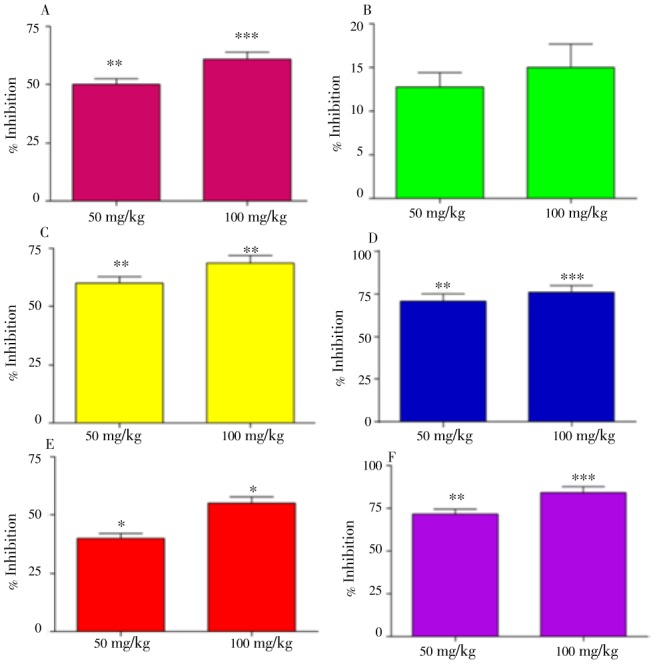
Crude extract (A), chloroform fraction (B), ethyl acetate (C) and standard drug, paracetamol (D). Data are reported as mean±SEM (n=6). The data were analyzed by ANOVA followed by Dunnett's test. *P<0.5, **P<0.01, ***P<0.001 was considered as statistically significant from control.
4. Discussion
The traditional therapeutic agent, D. lotus being used from time immemorial as antipyretic, rationalized here in the form of its crude methanolic extract and various fractions while using well established animal model accompanied by its antinociceptive potential.
Fever is the primary feature of diseases right from the very beginning of human civilization. The febrile response is synchronized by the central nervous system through endocrine, neurological, immunological and behavioural mechanisms[10]. The initiation, manifestations and regulation of the febrile response are dependent on the pyrogenic and anti-pyrogenic properties of various exogenous and endogenous substances. Medical experts believe that fever is based on consistent rise in body temperature above normal daily fluctuations originating in combination with an elevated thermoregulatory set point[9],[11]. These neurons are sensitive not only to changes in blood temperature but also to cold and warm receptors located in skin and muscle, thus maintaining an appropriate balance between the heat production and loss[1]. The extract/fractions of D. lotus showed profound antipyretic activity against yeast evoked hyperthermic mice when administered intraperitoneally during various assessment times. Pyrexia was recovered in a dose dependent manner and remained significant up to 5th h of drug administration. The nonsteroidal antiinflammatory drugs are the drug of choice for the control of febrile conditions in routine practice. Over a period of time after thorough investigation, it believed that nonsteroidal antiinflammatory drugs inhibit prostaglandins synthesis via cyclooxygenase pathway[12]. The extract/fractions of D. lotus might contain active principle(s) that exhibited inhibitory action on cyclooxygenase. As a result, they produced antipyretic activity by preventing the formation of prostaglandins or by increasing the concentration of body's own antipyretic components[13]. Our finding of the analgesic property of this plant is in accordance with the effect of other species of this genus. The crude n-hexane fraction of Dudleya variegata demonstrated significant antipyretic effect in brewer's yeast model[14]. The methanol extract of D. mespiliformis has been reported as significant antipyretic effect in tested animals[15].
Acetic acid induced writhing test has been primarily used by various research groups for the assessment of antinociceptive of natural compounds worldwide[16],[17]. Acetic acid caused the release of different endogenous noxious mediators such as bradykinin, serotonin, histamine, substance P[17],[18]. The resulting pain is symbolized by contraction of the abdominal muscle accompanied by an extension of the forelimbs and body elongation. Peripheral nociceptive fibers are sensitive to both narcotics analgesic and non-steroid anti-inflammatory drugs[19],[20]. The crude n-hexane fraction of Dudleya variegata demonstrated significant antinociceptive effect in acetic acid and tail immersion models[21]. The methanol extract of D. mespiliformis was evaluated for its claimed folkloric usage in the relief of pain in acetic acid-induced and formalin test induced pain model. A significant (P<0.05) analgesic effect was observed in all tested animals[20]. One of the active constituent of D. mespiliformis (taraxeren-3-one) has been published as good analgesic when tested against acetic acid induced writhing[22]. The methanolic extract of leaves of Diospyros cordifolia has been reported as analgesic after testing in tail flaking animal model[23]. The methanolic extract D. lotus has been proved as central and peripheral analgesic[24].
The extract/fractions of D. lotus showed marked reduction in the abdominal constriction provoked by the acetic acid in a dose dependent manner. Consequently, one possible mechanism of antinociceptive activity of the extract/fractions of D. lotus could be due to the blockade of the effect or the release of endogenous substances (arachidonic acid metabolites) that excite pain nerve endings.
In conclusion, our study illustrated strong antipyretic and antinociceptive effect of the extract/fractions of D. lotus in in vivo studies and thus provided scientific rationale to the traditional uses of the plant as antipyretic.
Acknowledgments
The authors are grateful for the financial supported by Higher Education Commission of Pakistan and PNRL lab Institute of Chemical Sciences, University of Peshawar, Pakistan. The work was supported by HEC, Pakistan with grant number 112-26510-2PS1-258.
Comments
Background
Natural products considered safe is a very common perception among the public as they have less side effects. Management of pain through plants based therapy is popular in the developing as well as in third world countries. Pain may be chronic or acute in both cases. The use of synthetic painkiller causes peptic ulcer and natural products are devoid of such side effects. Therefore, the search for safe, effective and potent natural products is a beg challenge to the scientist of the present modern era.
Research frontiers
D. lotus is used as antipyretic and analgesic in the traditional system of medicines. In the present research work, the crude extract of D. lotus is evaluated for antipyretic and analgesic effects using brewer's yeast and acetic acid writing tests.
Related reports
Brewer's yeast and acetic acid induced writhing tests are used for antipyretic and analgesic effects. In the present research work, authors reported the antipyretic and acetic acid potential of crude extract of D. lotus as for the first time. Ample of medicinal plants have been reported for their antipyretic and analgesic effects.
Innovations and breakthroughs
The current research work strongly supports the ethno-medicinal use of this valuable plant for its antipyretic and analgesic effect. The results clearly demonstrate the significant fever reducing and pain reliving property of the plant. To the best of my information, this is a novel study in accordance to the topic of the MS.
Applications
The applications of this MS are that the plan is tested in animal models for their pharmacological activities (antipyretic and analgesic). The results revealed that the plant is recommended for the use in traditional medicine for fever and pain.
Peer review
This is a valuable research work for investigation of safe, effective and potent antipyretic and analgesic phytomedicines. In the present research work, the authors reported the antipyretic and analgesic effect of the said plant. The antipyretic effect has been tested using brewer's yeast induced fever and acetic acid induced writhing for pain. Both of these animal paradigms are recommended and well established.
Footnotes
Foundation Project: Supported by HEC, Pakistan with grant number 112-26510-2PS1-258.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Uddin G, Rauf A, Rehman TU, Qaisar M. Phytochemical screening of Pistacia chinensis var. integerrima. Middle-East J Sci Res. 2011;7(5):707–711. [Google Scholar]
- 2.Uddin G, Rauf A, Arfan M, Ali M, Qaisar M, Saadiq M, et al. Preliminary phytochemical screening and antioxidant activity of Bergenia caliata. Middle-East J Sci Res. 2012;11(8):1140–1142. [Google Scholar]
- 3.Uddin G, Rauf A, Siddiqui BS, Shah SQ. Preliminary comparative phytochemical screening of Diospyros lotus Stewart. Middle-East J Sci Res. 2011;10(1):78–81. [Google Scholar]
- 4.Rauf A, Muhammad N, Khan A, Uddin N, Atif M, Barkatullah Antibacterial and phytotoxic profile of selected Pakistani medicinal plants. World Appl Sci J. 2012;20(4):540–544. [Google Scholar]
- 5.Loizzo MR, Said A, Tundis R, Hawas UW, Rashed K, Menichini F, et al. Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods Hum Nutr. 2009;64:264–270. doi: 10.1007/s11130-009-0133-0. [DOI] [PubMed] [Google Scholar]
- 6.Rashed K, Zhang X, Luo M, Zheng Y. Anti-HIV-1 activity of phenolic compounds isolated from Diospyros lotus fruits. Phytopharmacology. 2012;3:199–207. [Google Scholar]
- 7.Uddin G, Rauf A, Arfan M, Waliullah, Khan I, Ali M, et al. Pistagremic acid a new leishmanicidal triterpene isolated from Pistaci integerrima Stewart. J Enzyme Inhib Med Chem. 2012;27(5):646–648. doi: 10.3109/14756366.2011.604853. [DOI] [PubMed] [Google Scholar]
- 8.Khan H, Saeed M, Gilani AH, Muhammad N, Haq IU, Ashraf N, et al. Antipyretic and anticonvulsant activity of Polygonatum verticillatum: comparison of rhizomes and aerial parts. Phytother Res. 2013;27(3):468–471. doi: 10.1002/ptr.4721. [DOI] [PubMed] [Google Scholar]
- 9.Muhammad N, Saeed M, Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med. 2012;12(1):59. doi: 10.1186/1472-6882-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu B, Descalzi G, Ye H, Zhuo M, Wang YM. Translational investigation and treatment of neuropathic pain. Mol Pain. 2012;8:15. doi: 10.1186/1744-8069-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhammad N, Barkatullah, Ibrar M, Khan H, Saeed M, Khan AZ, et al. In vivo screening of essential oils of Skimmia laureola leaves for antinociceptive and antipyretic activity. Asian Pac J Trop Biomed. 2013;3(3):202–206. doi: 10.1016/S2221-1691(13)60050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blandizzi C, Tuccori M, Colucci R, Fornai M, Antonioli L, Ghisu N, et al. Role of coxibs in the strategies for gastrointestinal protection in patients requiring chronic non-steroidal anti-inflammatory therapy. Pharmacol Res. 2009;59:90–100. doi: 10.1016/j.phrs.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Okokon JE, Nwafor PA. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak J Pharm Sci. 2010;23:385–392. [PubMed] [Google Scholar]
- 14.Muhammad N, Saeed M, Khan H, Raziq N, Halimi SM, Abass M. Antipyretic and anticonvulsant activity of n-hexane fraction of Viola betonicifolia. Asian Pac J Trop Biomed. 2013;3(4):280–283. doi: 10.1016/S2221-1691(13)60063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trongsakul S, Panthong A, Kanjanapothi D, Taesotikul T. The analgesic, antipyretic and anti-inflammatory activity of Diospyros variegate Kruz. J Ethnopharmacol. 2003;85(2–3):221–225. doi: 10.1016/s0378-8741(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 16.Khan H, Saeed M, Gilani AU, Khan MA, Dar A, Khan I. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J Ethnopharmacol. 2010;127(2):521–527. doi: 10.1016/j.jep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Ibrar M, Muhammad N, Barkatullah, Khan H, Jahan F, Ashraf N. Antinociceptive and anticonvulsant activities of essential oils of Zanthoxylum armatum. Phytopharmacology. 2012;3(1):191–198. [Google Scholar]
- 18.Mazid MA, Datta BK, Nahar L, Rashid MA, Bachar SC, Bashar SA, et al. Analgesic and diuretic properties of alpha-santalone from Polygonum flaccidum. Phytother Res. 2010;24:1084–1087. doi: 10.1002/ptr.3053. [DOI] [PubMed] [Google Scholar]
- 19.Chavan MJ, Kolhe DR, Wakte PS, Shinde DB. Analgesic and antiinflammatory activity of kaur-16-en-19-oic acid from Annona reticulata L. bark. Phytother Res. 2012;26(2):273–276. doi: 10.1002/ptr.3544. [DOI] [PubMed] [Google Scholar]
- 20.Muhammad N, Saeed M, Gilani SN, Haq IU, Khan H. Analgesic and anti-inflammatory profile of n-hexane fraction of Viola betonicifolia. Tropical J Pharm Res. 2012;11(6):963–969. [Google Scholar]
- 21.Adzu B, Amos S, Dzarma S, Muazzam I, Gamaniel KS. Pharmacological evidence favouring the folkloric use of Diospyros mespiliformis Hochst in the relief of pain and fever. J Ethnopharmacol. 2002;82(2–3):191–195. doi: 10.1016/s0378-8741(02)00179-4. [DOI] [PubMed] [Google Scholar]
- 22.Chang TN, Huang SS, Chang YS, Chang CI, Yang HL, Deng JS, et al. Analgesic effects and mechanisms of anti-inflammation of taraxeren-3-one from Diospyros maritima in mice. J Agric Food Chem. 2011;59(17):9112–9119. doi: 10.1021/jf201375u. [DOI] [PubMed] [Google Scholar]
- 23.Das S, Haldar PK, Pramanik G, Panda SP, Bera S. Evaluation of analgesic and anti-inflammatory activity of Diospyros cordifolia extract. Afr J Tradit Complement Altern Med. 2011;8(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- 24.Said A, Hawas UW, Nofal SM, Rashed K, Huefner A. Pharmaco-chemical studies on the aqueous methanolic extract of Diospyros lotus leaves. Res J Phytochem. 2009;3(1):1–12. [Google Scholar]


