Abstract
Asian palm civets (Paradoxurus hermaphroditus), or toddy cats, belong to the family Viverridae. Little is known about the pathology of these animals and few articles have been published, mainly concerning their important role as wild reservoir hosts for severe infectious diseases of domestic animals and human beings. A 4-year-old, female Asian palm civet was found dead by the owner. At necropsy, large amount of adipose tissue was found in the subcutis and in the peritoneal cavity. Most of the pancreas appeared red, translucent. Hepatomegaly, discoloration of the liver were evident, with multifocal areas of degeneration, characterized by white nodular lesions. Histologically, the pancreas showed severe interstitial and perilobular necrosis and extensive haemorrhages, with separation of the interstitium, mild reactive inflammation at the periphery of the pancreatic lobules. Liver showed multifocal foci of vacuolar degeneration, lipidic accumulation, sometimes associated to hepatocyte necrosis. A diagnosis of acute severe hemorrhagic-necrotizing pancreatitis (or acute pancreatic necrosis) associated with pancreatic and hepatic lipidosis was made. To the best of our knowledge, this represents the first case report of acute lethal pancreatitis in an Asian palm civet. Although the exact cause of the disease remains undetermined, a hypothesis of the cause and pathogenesis is discussed, pointing out dietary indiscretion and consequent overweight as possible important risk factors.
Keywords: Necrotizing pancreatitis, Civet, Viverridae, Diet
1. Introduction
Asian palm civets (Paradoxurus hermaphroditus), or toddy cats, belong to the family Viverridae, together with other civets, genets and lisangs, for a total of 71 different species. They are small mammals, nocturnal frugivores, originated from the South and Southeast Asia, but they can be found throughout South-Western Europe, Asia, the East Indies, Africa, and Madagascar[1].
Little is known about the pathology of these animals. Most of the published studies refer to the importance of this animal as wild reservoir of important infectious diseases, such as distemper[2],[3], rabies[4]–[6], severe acute respiratory syndrome (SARS coronavirus)[7],[8], avian influenza H5N1[9], parvovirosis[10]. Interestingly, in a recent paper, civets have been identified as an important new reservoir for Bartonella henselae playing a role as potential sources of human infection[11].
Asian palm civets are frugivorous carnivoras[12],[13], using fruits as a major food source, even if they also eat small mammals and insects[1]. In captivities, overweight and obesity are common problems because of lack of exercise and difficulty to find an appropriated diet[14]. Indeed, the food used for these animals are often adapted from commercial diet designed for other species, with the possibility to induce nutritional imbalances.
To the best of our knowledge, this is the first case report of acute lethal pancreatitis in an Asian palm civet. Even though, in the present case, the exact cause of the disease remains unknown, a hypothesis of the pathogenesis is discussed.
2. Case report
A 4-year-old, female Asian palm civet (Paradoxurus hermaphroditus) was found dead by the owner (Figure 1A), which referred the animal as apparently healthy in the morning of the same day. The animal lived in a closed enclosure with a male. The diet consisted mainly in dry cat food, with two times per week raw egg, and rarely chicks, mice and rats.
Figure 1. A) Pictures of the Asian palm civet found dead; B) Pancreas: most of the organ appeared red, translucent, with more evident lobulated; C) Histological features of pancreas. Haematoxylin and eosin: severe interstitial and perilobular necrosis and extensive haemorrhages and oedema, with separation of the interstitium. 4×. Inset: scattered single or small clusters of round cells, with large clear cytoplasm and central hyperchromatic nuclei (lipidic macrophages) found in the pancreatic parenchyma. 40×.
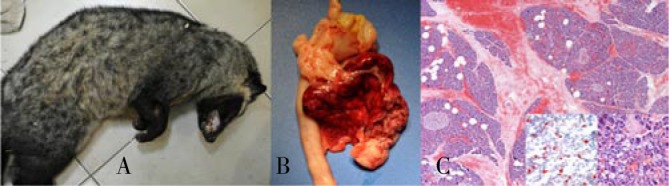
At necropsy, large amount of adipose tissue was found in the subcutis and in the peritoneal cavity. Most of the pancreas appeared red, translucent (Figure 1B). Hepatomegaly and discoloration of the liver were evident, with multifocal areas of degeneration, characterized by white nodular lesions. All the other organs were grossly normal.
Samples from pancreas, liver, spleen and kidney were taken and routinely fixed in 10% neutral buffered formalin, embedded in paraffin wax, and 5 µm-thick sections were examined using haematoxylin and eosin (H-E) staining and visualized by light microscope. In order to confirm the presence of lipid within pancreatic acinar cells, samples from the formalin fixed pancreas were also taken, washed in tape water for 2 h, and then fixed by immersion in OCT Compound (EMS 62550-01) for snap-freezing in a -80 °C freezer. The obtained frozen samples were then cryostat sectioned, and 7 µm-thick sections were stained with Oil red O (Fluka 75087, cf 25 g), specific for lipids.
Histologically, the pancreas showed severe interstitial and perilobular necrosis, oedema and extensive haemorrhages, with separation of the interstitium (Figure 1C) and mild reactive inflammation at the periphery of the pancreatic lobules, characterized by the presence of few neutrophils. Necrotic areas were characterized by pale eosinophilic, finely fibrillar or homogeneous material, admixed with free erythrocytes. Multifocally, small vessels showed fibrinoid necrosis of the vessel wall and were surrounded by numerous erythrocytes. Multifocal aspects of fat necrosis were also evident in the surrounding adipose tissue. At the periphery of the pancreatic lobules, beside areas of liquefaction, there were features of early modifications of the parenchyma, consisting of small foci of coagulative necrosis, where acinar cells appear shrunken and acidophilic. Duct system, endocrine pancreas, centrilobular parenchyma, as well as large vessels and nerves, were spared. In the spared portions of pancreatic parenchyma, and within the islets of langerhans, multifocal haemorrhages and scattered single or small clusters of round cells, with large clear cytoplasm and central nuclei (lipidic macrophages) were found (Figure 1, right inset). The presence of these cells and lipidic accumulation also within the acinar cells was confirmed by red oil staining on frozen sections (Figure 1, left inset). Multifocal post-mortem autolysis processes were also evident, due to the fact the animal was found death by the owner, and some time passed between death and necropsy.
Liver showed multifocal foci of vascular degeneration, lipidic accumulation, sometimes associated with hepatocyte necrosis. In the spleen, multifocal foci of extramedullary hematopoiesis were evident. The kidney showed multifocal interstitial infiltration of lymphocytes.
On the basis of the gross and histological features, a diagnosis of acute severe haemorrhagic-necrotizing pancreatitis (or acute pancreatic necrosis) associated with pancreatic and hepatic lipidosis was made.
3. Discussion
Unfortunately, the palm civet was found dead, and no haemato-biochemical diagnostic tests were performed, in order to define the metabolic profile of the animal and identify eventual metabolic dysfunctions (such as hyperadrenocorticism, hypothyroidism, hypercalcemia, uraemia) known to increase risk of acute pancreatitis in domestic animals[15]. Abdominal trauma, described as a cause of acute necrotizing pancreatitis in cat was ruled out[16], based on the anamnesis and the necropsy findings (i.e., the lack of haemorrhages or fractured bones). No obstruction was found in the pancreatic duct system, nor evident signs of ischemic injury from shock, thrombosis, embolism or vasculitis, as possible primary underlying causes[15].
Actually, very little is known about the pathology of palm civets and viverrids. A more wide veterinary and human literature was then considered to discuss the possible causes underlying the observed pathological features, also considering that inflammatory lesions of the exocrine pancreas are not as common in domestic animals as in humans[17]. Although pancreatitis has occasionally been reported in a number of animal species (most dogs and cats), information on causal factors is sparse and incompletely understood[15].
Some considerations were made about diet and the nutritional status of the animal, which showed abundant adipose tissue in the subcutis and in the peritoneal cavity. Obesity has been considered a predisposing factor for acute pancreatitis in both human and animals[15],[18], often associated with pancreatic steatosis and a worse prognosis[19],[20]. Recent studies in human medicine demonstrated that obesity can act creating an environment that favours the induction and the progression of pancreatic disease by a promotion of inflammation and inhibition or deregulation of autophagy[20]. Despite the relative frequency of acute pancreatitis in human beings, the precise underlying mechanisms are unknown. Experiments on animal models were indispensable in providing insight in pathophysiology and treatment of acute pancreatitis[21]. In this respect, particular attention should be paid to the diseases of these animals, since palm civet would represent a potential suitable model for the study of some important human conditions, such as diet-induced pancreatitis.
In viverrids in captivity, overweight and obesity are common problems because of lack of exercise and dietary errors[14]. Indeed, the diets used for these animals are not specific, often adapted from commercial diet designed for other species. In the present case, the civet was kept in a closed enclosure and mainly fed with dry cat food, with two times per week raw egg, and rarely chicks, mice and rats. Considering their natural habits[1],[13],[22], the diet of Asian palm civets should include a major percentage of fruits. Furthermore, hepatic lipidosis was also observed, a lesion described as a consequence of pancreatitis in cat and often related to a worse prognosis when associated with concurrent acute pancreatitis[16],[23]. Noteworthy, two animals were kept in the same environments, fed with the same food, but only one developed the disease. This would let to suppose that other concomitant risk factors and/or a specific predisposition could have played a role. Inherited alterations in genes encoding pancreatitis enzyme or their inhibitors, as described at the basis of the human hereditary or idiopathic pancreatitis, could have not been ruled out. A condition characterized by homozygosis for a mutation in the lipoprotein lipase gene and hyperlipoproteinemia has been recently described in young mink, representing a valid model for the study of the corresponding human hyperlipoproteinemia type I[17],[24].
Although the definitive cause of the pancreatitis remains undetermined, dietary indiscretion (high-fat, high-energy) and consequent overweight are considered possible important risk factors in determining the disease outcome, raising concerns on how these animals are kept in captivity.
Acknowledgments
The authors would like to thank Marina Baffoni for the technical support.
Comments
Background
Asian palm civet is an important animal as wild reservoir of many infectious diseases such as rabies, severe acute respiratory syndrome, avian influenza virus, parvovirosis and so on. However, there was little knowledge about histological and pathological changes of pancreatitis about it.
Research frontiers
This paper is the first case report on acute lethal pancreatitis in an Asian palm civet and the author described its necropsy and histological changes in detail. Meanwhile, it pointed out that dietary indiscretion and overweight might be the important risk factors.
Related reports
Asian palm civets normally live in primary forests, parks and suburban gardens with mature fruit trees. Takashi Matsumoto et al. (2011) reported that a novel sylvatic rabies virus variant was detected in a golden palm civet in Sri Lanka and the virus diverged from canine rabies viruses.
Innovations and breakthroughs
This is the first case report of acute lethal pancreatitis in an Asian palm civet and it could teach us to know some pathological knowledge on the civet.
Applications
From the paper, we could know something about lethal pancreatitis in a palm civet and it facilitated the diagnosis in the near future.
Peer review
The work sounds like interesting and it was basic work for the research. We could learn the pathological changes about pancreatitis in a palm civet from the paper. Moreover, the author thought diet and overweight might be the key causes for the disease. The research deserved our attentions.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Denver M. Procyonidae and viverridae. In: Fowler M, Miller RE, editors. Zoo and wild animal medicine. Philadelphia, Pennsylvania: W.B. Saunders; 2003. pp. 516–523. [Google Scholar]
- 2.Deem SL, Spelman LH, Yates RA, Montali RJ. Canine distemper in terrestrial carnivores: a review. J Zoo Wildl Med. 2000;31:441–451. doi: 10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Kapil S, Yeary TJ. Canine distemper spillover in domestic dogs from urban wildlife. Vet Clin North Am Small Anim Pract. 2011;41(6):1069–1086. doi: 10.1016/j.cvsm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King AA, Meredith CD, Thomson GR. Canid and viverrid rabies viruses in South Africa. Onderstepoort J Vet Res. 1993;60(4):295–299. [PubMed] [Google Scholar]
- 5.Nandi S, Kumar M. Development in immunoprophylaxis against rabies for animals and humans. Avicenna J Med Biotechnol. 2010;2(1):3–21. [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto T, Ahmed K, Wimalaratne O, Nanayakkara S, Perera D, Karunanayake D, et al. Novel sylvatic rabies virus variant in endangered golden palm civet, Sri Lanka. Emerg Infect Dis. 2011;17(12):2346–2349. doi: 10.3201/eid1712.110811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Z, Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008;133(1):74–87. doi: 10.1016/j.virusres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata N, Iwata-Yoshikawa N, Taguchi F. Studies of severe acute respiratory syndrome coronavirus pathology in human cases and animal models. Vet Pathol. 2010;47:881–892. doi: 10.1177/0300985810378760. [DOI] [PubMed] [Google Scholar]
- 9.Roberton SI, Bell DJ, Smith GJ, Nicholls JM, Chan KH, Nguyen DT, et al. Avian influenza H5N1 in viverrids: implications for wildlife health and conservation. Proc Biol Sci. 2006;273(1595):1729–1732. doi: 10.1098/rspb.2006.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen XY, Xie ZJ, Zhao ZP, Jiang SJ, Zhao HK, Zhu YL, et al. Genetic diversity of parvovirus isolates from dogs and wild animals in China. J Wildl Dis. 2011;47(4):1036–1039. doi: 10.7589/0090-3558-47.4.1036. [DOI] [PubMed] [Google Scholar]
- 11.Sato S, Kabeya H, Shigematsu Y, Sentsui H, Une Y, Minami M, et al. Small Indian mongooses and masked palm civets serve as new reservoirs of Bartonella henselae and potential sources of infection for humans. Clin Microbiol Infect. 2013;19(12):1181–1187. doi: 10.1111/1469-0691.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassman LI. Movements and fruit selection of two Paradoxurinae species in a dry evergreen forest in southern Thailand. Small Carnivore Conserv. 1998;19:25–29. [Google Scholar]
- 13.Nakabayashi M, Bernard H, Nakashima Y. An observation of several common palm civets Paradoxurus hermaphroditus at a fruting tree of Endospermum diadenum in Tabin Wildlife Reserve, Sabah, Malaysia: comparing feeding patterns of frugivorous carnivorans. Small Carnivore Conserv. 2012;47:42–45. [Google Scholar]
- 14.Wemmer C. Civets and genets. In: Macdonald DW, editor. The encyclopaedia of mammals. Oxford, UK: Oxford University Press; 1984. pp. 136–143. [Google Scholar]
- 15.Charles JA. Pancreas. In: Maxie MG, editor. Jubb, Kennedy and Palmer's pathology of domestic animals. Philadelphia, Pennsylvania: Elsevier, Saunders; 2007. pp. 398–403. [Google Scholar]
- 16.Armstrong PJ, Williams DA. Pancreatitis in cats. Top Companion Anim Med. 2012;27(3):140–147. doi: 10.1053/j.tcam.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordstoga K, Sørby R, Olivecrona G, Smith AJ, Christophersen B. Pancreatitis in hyperlipemic mink (Mustela vison) Vet Pathol. 2012;49(3):557–561. doi: 10.1177/0300985811417248. [DOI] [PubMed] [Google Scholar]
- 18.Chen SM, Xiong GS, Wu SM. Is obesity an indicator of complications and mortality in acute pancreatitis? An updated meta-analysis. J Dig Dis. 2012;13(5):244–251. doi: 10.1111/j.1751-2980.2012.00587.x. [DOI] [PubMed] [Google Scholar]
- 19.Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8(3):169–177. doi: 10.1038/nrgastro.2011.4. [DOI] [PubMed] [Google Scholar]
- 20.Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1199–1209. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford) 2006;8:264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jothish PS. Diet of the common palm civet Paradoxurus hermaphroditus in a rural habitat in Kerala, India, and its possible role in seed dispersal. Small Carnivore Conserv. 2011;45:14–17. [Google Scholar]
- 23.Akol KG, Washabau RJ, Saunders HM, Hendrick MJ. Acute pancreatitis in cats with hepatic lipidosis. J Vet Intern Med. 1993;7(4):205–209. doi: 10.1111/j.1939-1676.1993.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 24.Nordstoga K, Christophersen B, Ytrehus B, Espenes A, Osmundsen H, Landsverk T, et al. Pancreatitis associated with hyperlipoproteinaemia type I in mink (Mustela vison): earliest detectable changes occur in mitochondria of exocrine cells. J Comp Pathol. 2006;134(4):320–328. doi: 10.1016/j.jcpa.2006.01.003. [DOI] [PubMed] [Google Scholar]


