Abstract
Objective
To investigate effect of essential oils on Aspergillus spore germination, growth and mycotoxin production.
Method
In vitro antifungal and antiaflatoxigenic activity of essential oils was carried out using poisoned food techniques, spore germination assay, agar dilution assay, and aflatoxin arresting assay on toxigenic strains of Aspergillus species.
Results
Cymbopogon martinii, Foeniculum vulgare and Trachyspermum ammi (T. ammi) essential oils were tested against toxicogenic isolates of Aspergillus species. T. ammi oil showed highest antifungal activity. Absolute mycelial inhibition was recorded at 1 µl/mL by essential oils of T. ammi. The oil also showed, complete inhibition of spore germination at a concentration of 2 µl/mL. In addition, T. ammi oil showed significant antiaflatoxigenic potency by totally inhibiting aflatoxin production from Aspergillus niger and Aspergillus flavus at 0.5 and 0.75 µl/mL, respectively. Cymbopogon martinii, Foeniculum vulgare and T. ammi oils as antifungal were found superior over synthetic preservative. Moreover, a concentration of 5 336.297 µl/kg body weight was recorded for LC50 on mice indicating the low mammalian toxicity and strengthening its traditional reputations.
Conclusions
In conclusion, the essential oils from T. ammi can be a potential source of safe natural food preservative for food commodities contamination by storage fungi.
Keywords: Aspergillus species, Essential oils, Food spoilage, Mycotoxin, Preservatives
1. Introduction
Despite the advancement food science and the technology of food production, diseases caused by foodborne fungal pathogens are still the major public health problems and quarter of worlds' food commodities have been wasted due to the contamination by toxic fungi or by fungal metabolic products[1],[2]. Improper storage conditions offer favorable environment for the growth of Aspergillus spp and production of mycotoxins[2]. Consumptions of such contaminated food lead to serious cases of illness and mycotoxicoses[3],[4]. Among these, aflatoxicosis causes acute hemorrhage, acute liver damage, edema, and death and chronic toxicological effect of cancer, mutagenicity, immune suppression, birth defects, estrogenic, gastrointenial, urogenital, vascular, kidney and nervous system disorder[1],[2],[5]. In Africa particularly, in parts of sub-Saharan Africa about 250 000 hepatocarcinoma related deaths occur annually due to aflatoxin ingestion alone[1],[4].
Management of food stuffs contaminations are required to ensure that food commodities remain safe and uncontaminated throughout the supply chain (from ‘farm to plate’)[2]. Several synthetic preservatives have been effectively used in management of food contamination by Aspergillus spp. But their continuous application has led to the development of fungal resistance[6], number of environmental and health problems[7]–[9]; hormonal imbalance and spermatotoxicity and also some individuals produce allergic reactions to these substances[10],[11]. However, natural products could potentially serve as effective alternatives of synthetic chemicals for the control of food contamination by Aspergillus spp[12],[13]. Among natural products, essential oils (EOs) of aromatic plant are gaining interest as food additives and widely accepted by consumers because of their relatively low toxicity, high volatility, transient nature and biodegradability[14],[15]. European Union allowed the use of EOs in food and aromatherapy[16]. So, EOs with antimicrobial activity are possible candidates for the preservativation of food commodities against Aspergillus spp[17].
Cymbopogon martinii (C. martinii) L., Foeniculum vulgare (F. vulgare) Miller and Trachyspermum ammi (T. ammi) L. Sprague ex Turrill are medicinal aromatic plant of Ethiopia. They are used traditionally as food additives and also for the treatment of various diseases[18]. However, there is no reliable evidence that indicated these plants EOs have fungitoxic and antiaflatoxigenic potential against aflatoxigenic Aspergillus spp in Ethiopia. The aim of this study was to evaluate the effect of EOs on growth, spore and mycotoxin production of Aspergillus spp that could alternate synthetic chemical preservatives.
2. Materials and methods
2.1. Chemicals and media
Our media, chemical and solvents used in the study were obtained from different companies in different countries: Peptone Dextrose Agar was obtained from Himedia Laboratories Pvt. Ltd., India; Sabouraud Dextrose Agar from Oxoid Ltd., England; Sabouraud Dextrose Broth from Defco, Ltd., England; sucrose and yeast extract from Labort fine Chem Pvt. Ltd, India; Aflatoxin Mix Kit-M from Supelco, USA; anhydrous sodium sulphate, MgSO4.7H2O, potassium hydroxide, vanillin and silica gel 60 thin layer chromatography (TLC) plate 0.2 mm from Merck-Schuchardt, Germany; potasium nitrate from Rhone Poulenc, US; sodium benzoate from Codex® Farmacopia, Italy; thymol, chloroform, ethyl acetate, sulphuric acid, tween 20 and tween 80 from Sigma-Aldrich Chemie, Germany; acetone from Labort Fine Chem Pvt. Ltd, India; ethanol absolute and methanol from Finkem, India; and Tolune from AnalaR®, England.
2.2. Plant material collection identification and extraction
Different parts of the test plants of C. martinii (aerial part) was collected from the botanical garden of TMMRD, the Ethiopian Health and Nutrition Research Institute (EHNRI), Ethiopia; F. vulgare (leaf lamina and leaf sheath) were collected from Shashamane, Ethiopia; and T. ammi (fruits) was collected from Tepi, Ethiopia. Following collection, the identities of plant materials were confirmed by taxonomist and botanist in Traditional and Modern Drug Research Department of EHNRI. Fresh areal part of C. martinii, F. vulgare and dried fruits of T. ammi (250 g) were placed in a 5 L round-bottom distillation flask and the plant material was wetted with 3 L distilled water. The EOs were obtained by hydro-distillation using Clevenger-type apparatus for continuous 3 h. The volatile oil was taken from the upper layer. The excess aqueous layer was further portioned using dichloromethane to extract and enrich the EO from the water layer. The organic layer (dichloromethane extract) was filtered and dried with anhydrous sodium sulfate and concentrated using rotary evaporator to give the crude EO.
2.3. Phytochemical screening of the EOs
2.3.1. TLC analysis
Following the extraction of EOs that are intended for biological assay, a portion of EOs are subjected to TLC (pre coated silica gel G60 F254) finger print analyses for preliminary qualitative phytochemical screenings of the most active EOs for various secondary metabolites that have antimicrobial activity. Wagner & Bladts' procedures for plant drug analysis were used for the development of chromatogram and for the identification of major secondary metabolites responsible for biological activity[19].
2.3.2. Gas chromatographic (GC)-analysis
GC analysis of the oil of T. ammi was performed on a Shimadzu GC-2010 system, with split mode. The column used was a ZB-1MS equivalent to OV-1, fused silica capillary column 30 m×0.25 mm i.d., film thickness 0.25 µm, coated with 5% diphenyl-95% polydimethylsiloxane, operated with the following oven temperature programme: 50 °C, held for 2 min, rising at 3 °C to 210 °C/min. Injection temperature and volume, 250 °C and 1.0 µL, respectively; injection mode, split; split ratio, 10:1; carrier gas, nitrogen at 65.2 cm/s linear velocity and inlet pressure 100 KPa; detector temperature, 270 °C; nitrogen flow rate, 52.1 mL/min; air flow rate, 400 mL/min; make-up 32, (H2/air) flow rate 40 mL/min; sampling rate, 40 MS/s.
2.4. Test organisms
Aflatoxicogenic strains of Aspergillus flavus (A. flavus) and Aspergillus niger (A. niger) were selected for this study. The strains were isolated from food commodities that were collected from different local markets in Addis Ababa prior to the study. In addition, standard strains of A. flavus (ATCC 13697) and A. niger (ATCC 10535) were used in the study as a control. The standard strains were obtained from Microbiology Laboratory, Traditional and Modern Medicine Research Directorate, EHNRI.
2.5. Antifungal activity
2.5.1. Determination of sporicidal activity
Sporicidal activity of C. martinii, F. vulgare and T. ammi were conducted using spore germination assay according to standard reference methods[20]. The test organisms were grown on PDA medium for sporulation and spores were harvested when the cultures were fully sporulated, which was achieved after 10 d of incubation. Spores were collected by adding 5 mL of sterile water containing 0.1% (v/v) tween 80 (for better spore separation) to each Petri dish and rubbing the surface with a sterile L-shaped spreader (3 times). The suspension was collected and then centrifuged at room temperature at 2 000 r/min for 5 min. The supernatant was discarded and re-centrifuged until 1 mL of highly concentrated spore solution remained. A haemocytometer slide was used to count spore production to have approximately 108 spore/mL[21].
Various concentrations: 0.25 µl/mL, 0.5 µl/mL, 1 µl/mL, 2 µl/mL, 4 µl/mL and 8 µl/mL of both EOs were prepared in 5 mL of sabouraud dextrose broth in 100 mL flask and then 1 mL of the spore suspension were added to each flask. The flasks were then incubated for 24 h at 25 °C on a rotary shaker (60 r/min) as to evenly disperse the oil throughout the broth. At the end of the incubation period, germinated spores were observed using a light microscope at 400× magnification. Experiment was performed in triplicate and the extent of spore germination was assessed by looking for the presence of germ tubes. Results were expressed in terms of the percentage of spores germinated as compared to the control from the average of the triplicates. Percentage of spore germination inhibition is calculated according to the following formula:
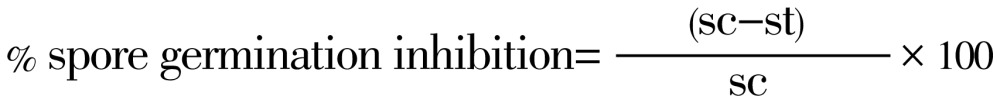 |
Where: sc, average number of spore germinated in control set; st, average number of spore germinated in test set.
2.5.2. Determination of minimum inhibitory concentration (MIC)
The MIC of C. martinii, F. vulgare and T. ammi EOs were determined using agar dilution methods[20],[22]. Twofold serial dilution of both oils in Sabouraud dextrose agar were made by adding two milliliter of each dilution of the desired concentrations of EOs into each 18 milliliter of agar in a test tube which was well mixed and poured into 90 mm Petri dish. The experiments were performed in triplicates. Control plates containing no EOs ran simultaneously. The agar surface of the plates containing the dilution of EOs and the control plate are inoculated five millimeter discs of the test fungi taken from advancing edge of 7-day-old cultures. The plate containing the lowest concentration of EOs was seeded first. Control plates were seeded last to insure that viable organisms were present throughout the procedure. Incubate the inoculated plates at (26±2) °C for 7 d before being read. End-points for each EOs are best determined by placing plates on a dark background and observing for the lowest concentration that inhibits visible growth, which is recorded as the MIC. The MIC of each antimicrobial agent is usually recorded in micro liter per milliliter.
2.5.3. Determination of mycelial dry weight
The effect of EOs on mycelial dry weight was evaluated in sabouraud dextrose broth[20]. Twofold serial dilution of each T. ammi EOs sabouraud dextrose broth was made by adding one part of the desired concentrations of EOs into each nine part of sabouraud dextrose broth in a 100 mL flask. The experiments were performed in triplicates. The flasks were aseptically inoculated with 0.36 mL spore suspensions (≈106 spore/mL). The flask containing the lowest concentration of EOs was seeded first. Control plates were seeded last to insure that viable organisms were present throughout the procedure. Incubate the inoculated plates at (26±2) °C for 7 d before being read. Flasks containing mycelia were be filtered through Whatman filter No. 1 and then were washed with distilled water. The mycelia were placed on pre-weighed Petri plates and were allowed to dry at 60 °C for 6 h and then at 40 °C overnight. The flasks containing dry mycelia were weighed. Growth inhibition percent on the basis of dry weight was calculated as:
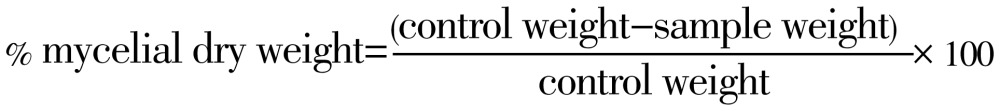 |
2.6. Efficacy of T. ammi oil in arresting aflatoxin elaboration
The methods that have been adopted by Kumar and his collogues[23], were followed to determine antiaflatoxigenic efficacy of EOs with lowest MIC on A. flavus using SMKY broth medium (sucrose, 200 g; MgSO4 7H2O, 0.5 g; KNO3, 0.3 g; yeast extract, 7.0 g; distilled water, 1 000 mL; pH, 5.6±0.2). Different concentrations of the oil (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0 µl/mL) were prepared separately by dissolving their requisite amount in 0.5 mL 5% tween-20 and then mixing it with 24.5 mL of SMKY medium in 100 mL erlenmeyer flask. The control sets were kept parallel to the treatment sets without EO. Then flasks were inoculated aseptically with 1 mL spore suspension (≈106 spores/mL) prepared in 0.1% tween-80 and incubated at (27±2) °C for 10 d. The content of each flask was filtered (Whatman filter paper No. 1). The filtrate was extracted with 20 mL chloroform in a separating funnel and the extracts were passed through anhydrous sodium sulphate kept in Whatman filter paper No. 42. The extracts were evaporated till dryness on water bath at 70 °C. Dry residues were dissolved in 1 mL chloroform and 50 µL of chloroform extract spotted on TLC plate [(20×20) cm2 of silica gel-G60 F254] then developed in chloroform: acetone [(9:1) v/v]. The intensity of aflatoxin was observed in ultra violate fluorescence analysis cabinet at an excitation wavelength of 366 nm.
2.7. Animal trials to determine safety limit of the oils
The safety limit of the best fungitoxic EOs were determined by recording LC50 value on mice following the protocol of Kumar and his collaborator[23]. Mice with an average weight and age (35 g, 3 months) were selected as test animal for the mammalian toxicity experiments. Requisite amount of EOs were mixed properly with tween 80 to prepare different solutions containing desired dose of EOs. The mice were administered 0.5 mL of each solution of EOs orally separately through a gavage syringe to each set containing 10 mice (equal proportion for gender). In control sets, equal volume of tween 80 was given to mice. After 72 h, the mortality of the animals was recorded and LC50 was calculated in terms of per kg body weight of mice using SPSS version 20.0 and Minitab version 16 computer software's by probit analysis.
2.8. Statistical analysis
All the measurements were replicated three times for each treatment and data were entered into Excel spreadsheet and are presented as mean±SE/SD. Significant differences between strains aflatoxin producer and non-producer were analyzed using statistical software (SPSS 20.0; Chicago, IL, USA) at 95% level of confidence by Chi-square analysis. Significant differences between treatment and sensitive strains were first tested for normality and then subjected to one-way analysis of variance using statistical software (SPSS 20.0; Chicago, IL, USA and Minitab 16.0, England). Significant differences between mean values were determined using Tukey's and Dunken's multiple range tests following one-way analysis of variance and P< 0.05 were considered as significant.
3. Results
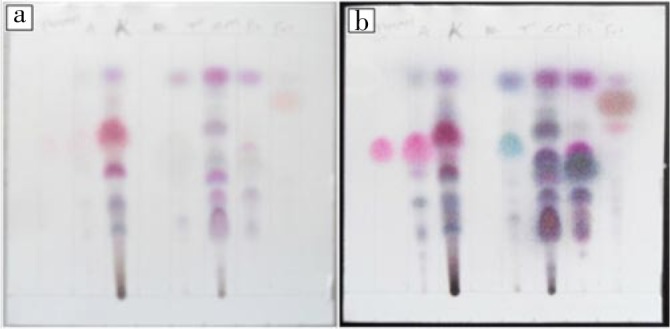
TLC fingerprint was used for screening of EOs as bioactive compounds were separated in a sequence of different zones and characterized by the value of retention factors (Rf) in toluene: ethyl acetate (9.3:0.7) solvent system and the color of zone they produce after being treated with detection reagent (vanillin-sulfuric acid). Each plant EOs have shown quite different TLC finger print (Table 1 and Figure 1). TLC screening indicated the presence of many terpenoids in the EO tested which was confirmed by the presence of different colored spots. Highest number of spots was obtained in the chromatogram of C. martinii EOs that showed distinctive 8 spot/bands, while F. vulgare and T. ammi EOs were separated in four different spot/bands when visually observed after treatment with vanillin-sulphuric acid reagent.
Table 1. TLC fingerprint of EOs of aromatic plants.
| Plant | Spot (bands) Rf value and corresponding colors |
|||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | |
| Thy | 0.51 (violet red) | - | - | - | - | - | - | - |
| Ta | 0.52 (violet red) | 0.45 (brown) | 0.35 (gray) | 0.23 (gray) | - | - | - | - |
| Cm | 0.96 (t violet) | 0.78 (blue) | 0.73 (gray) | 0.65 (blue) | 0.54 (violet) | 0.49 (blue) | 0.39 (blue) | 0.3 (blue) |
| Fv | 0.95 (blue violet) | 0.88 (violet) | 0.69 (gray) | 0.56 (violet) | - | - | - | - |
Thy: Thymol standard; Ta, T. ammi; Cm, C. martinii; Fv, F. vulgare.
Figure 1. TLC fingerprint (a; b=before and after heating at 110 °C for 5 min respectively) of thymol standard and EOs of T. ammi, Lippia adoensis Hochst. var. koseret, Ruta chalepensis, C. martinii, Rosmarinus officinal and F. vulgare, respectively.
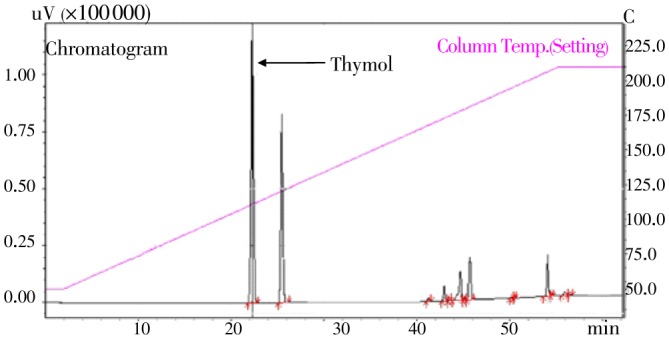
Our GC analysis of T. ammi EO showed the presence of 14 components accounting for 100% of the total amount (Figure 2). It is clearly seen from the chromatogram that EOs have two major peaks. Thymol (51.5%) was found as a major component of T. ammi EO. Moreover, the GC analyses of T. ammi EO showed the presence of other 12 minor components.
Figure 2. GC chromatograms of T. ammi fruit EO.
Each tested concentration of EOs showed notable inhibition of A. flavus spore germination. T. ammi EOs have pronounced spore germination inhibition effect on tested organisms. Absolute spore germination was recorded for EOs of T. ammi, C. martinii and F. vulgare at a concentration of 1 µl/mL, 2 µl/mL and 4 µl/mL, respectively. Moreover, the amount of EOs tested have significant spore inhibition effect (P <0.05) (Table 2).
Table 2. Percent of spore germination inhibition of toxigenic A. flavus by EOs, using fungal spore germination assay.
| Concentration | Palmarosa | Fennel | Ajowan |
| 0.25 µl/mL | 49.57±0.02c | 32.57±1.20d | 88.14±0.34c |
| 0.5 µl/mL | 75.14±0.01b | 45.71±1.93c | 94.86±0.34b |
| 1 µl/mL | 98.57±0.00a | 81.14±1.50b | 100.00±0.00a |
| 2 µl/mL | 100.00±0.00a | 99.80±0.00a | 100.00±0.00a |
| 4 µl/mL | 100.00±0.00a | 100.00±0.00a | 100.00±0.00a |
Values are expressed as mean±SD. Mean value with different letters in the same column are significantly different (P<0.05).
The mean score for antifungal activity of EOs are revealed by Table 3. A remarkable antifungal activity against the growth of aflatoxigenic strains of A. flavus was recorded after the treatment with different concentration of T. ammi EO, followed by C. martinii EO and F. vulgare EO. Increased mycelial expansions were observed at lower concentration of EOs while absolute inhibitions of mycelial expansion were observed at higher concentration indicating dose dependent activities. Interestingly, the MIC was recorded at 1 µl/mL for T. ammi and 4 µl/mL for both Cymbopogon mertinii and F. vulgare. At these points, the plant EO completely inhibited the growth of all isolated fungal strains A. flavus (Table 3).
Table 3. MIC of EOs against the toxigenic strain of A. flavus using agar dilution technique.
| Concentration | Palmarosa | Fennel | Ajowan |
| 0.25 µl/mL | 23.05±0.47a | 23.09±0.42a | 22.71±0.45a |
| 0.5 µl/mL | 21.19±0.66a | 21.62±0.46a | 17.28±0.34b |
| 1 µl/mL | 18.52±0.49b | 19.10±0.38b | 0.00±0.00c |
| 2 µl/mL | 16.76±0.57b | 17.14±0.66b | 0.00±0.00c |
| 4 µl/mL | 0.00±0.00c | 0.00±0.00b | 0.00±0.00c |
| 8 µl/mL | 0.00±0.00c | 0.00±0.00c | 0.00±0.00c |
Values are expressed as mean±SD. Mean value with different letters in the same column are significantly different (P<0.05).
All the three EOs were documented to have better fungitoxic activity against aflatoxigenic Aspergillus spp when compared to synthetic chemical preservative sodium benzoate (Table 4). Statistical results showed that both the kind and concentration of EO have significant effect (P<0.05). The most promising MIC was recorded by T. ammi at 1 µl/mL against A. flavus and A. niger.
Table 4. Comparative antifungal activity of EOs with sodium benzoate.
|
Aspergillus species |
MIC activity (mg/mL) |
|||
| Cm | Fv | Ta | Sb | |
| AFST | 4.00 | 8.00 | 1.00 | >16.00 |
| AF001 | 4.00 | 8.00 | 1.00 | >16.00 |
| AF006 | 4.00 | 8.00 | 1.00 | >16.00 |
| AF009 | 4.00 | 8.00 | 1.00 | >16.00 |
| AF019 | 4.00 | 8.00 | 1.00 | >16.00 |
| AF027 | 4.00 | 8.00 | 1.00 | >16.00 |
| AF037 | 4.00 | 8.00 | 1.00 | >16.00 |
| ANST | 2.00 | 8.00 | 1.00 | 16.00 |
| AN002 | 2.00 | 8.00 | 1.00 | 16.00 |
AFST, Aspergillus flavus (ATCC 13697); AF, Aspergillus flavus; ANST, Aspergillus niger (ATCC 10535); AN, Aspergillus niger; Cm, Cymbopogon martinii; Fv, Foeniculum vulgare; Sb, Sodium benzoate; Ta, Trachyspermum ammi.
The effect of T. ammi EO on the dry mycelial weight of Aspergillus spp in sabouraud dextrose broth is presented in Table 5. Results of statistical analysis showed each tested concentration of EOs have significantly different mycelial dry weight inhibition (P<0.05). It can be clearly seen that a complete inhibition of mycelial dry weight at a concentration of 1, 2 and 4 µl/mL. At least a 12, 43 and 71% of dry mycelia biomass weight suppression were recorded at 0.25, 0.5 and 0.75 µl/mL respectively against Aspergillus spp. A dose dependent suppression of mycelial growth of Aspergillus spp was observed; as the higher concentration of the T. ammi EO inhibited hundred percent of the mycelial growth.
Table 5. Growth inhibition percent of Aspergillus spp on the basis of dry weight after treatments with different concentration of T. ammi EOs.
| Aspergillus species | Growth inhibition percent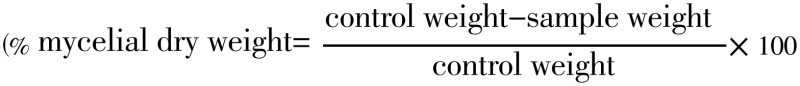 |
|||||
| 0.25 µl/mL | 0.5 µl/mL | 0.75 µl/mL | 1 µl/mL | 2 µl/mL | 4 µl/mL | |
| AFST | 11.93 | 43.07 | 71.18 | 100.00 | 100.00 | 100.00 |
| AF001 | 24.23 | 51.06 | 73.05 | 100.00 | 100.00 | 100.00 |
| AF006 | 30.65 | 42.69 | 70.15 | 100.00 | 100.00 | 100.00 |
| AF009 | 22.11 | 64.09 | 80.92 | 100.00 | 100.00 | 100.00 |
| AF019 | 30.71 | 65.26 | 81.30 | 100.00 | 100.00 | 100.00 |
| AF027 | 28.87 | 65.89 | 81.83 | 100.00 | 100.00 | 100.00 |
| AF037 | 21.53 | 58.20 | 72.07 | 100.00 | 100.00 | 100.00 |
| ANST | 29.34 | 72.62 | 91.85 | 100.00 | 100.00 | 100.00 |
| AN002 | 26.37 | 75.66 | 92.48 | 100.00 | 100.00 | 100.00 |
AFST, Aspergillus flavus (ATCC 13697); AF, Aspergillus flavus; ANST, Aspergillus niger (ATCC 10535); AN, Aspergillus niger.
Table 6 shows antiaflatoxigenic potentials of T. ammi EO against aflatoxigenic strains of Aspergillus spp (A. flavus and A. niger). It has been documented from this study's chromatogram that the aflatoxin production in SMKY liquid medium was reduced by the EOs of T. ammi in dose dependent manner. Aflatoxin production was completely inhibited at a concentration of 0.50 µl/mL for strains of A. niger and at the concentration of 0.75 µl/mL for A. flavus. These concentrations were less than that are recorded for MIC and absolute mycelial dry weight inhibiting concentration (1 µl/mL). In all untreated control (tween 20 5%), high level of aflatoxin production was observed in all aflatoxigenic Aspergillus spp.
Table 6. Antiaflatoxigenic activity of T. ammi EOs at a concentration of 0.00, 0.25, 0.5, 0.75, 1 and 2 µl/mL against toxigenic Aspergillus spp.
|
Aspergillus |
Aflatoxin production (fluorescence under UV 366λ) |
|||||
| species | C* | 0.25 µl/mL | 0.5 µl/mL | 0.75 µl/mL | 1 µl/mL | 2 µl/mL |
| AFST | + | + | + | ND | ND | ND |
| AF001 | + | + | + | ND | ND | ND |
| AF006′ | + | + | + | ND | ND | ND |
| AF009 | + | + | + | ND | ND | ND |
| AF019 | + | + | + | ND | ND | ND |
| AF027 | + | + | + | ND | ND | ND |
| AF037 | + | + | + | ND | ND | ND |
| ANST | + | + | ND | ND | ND | ND |
| AN002 | + | + | ND | ND | ND | ND |
AFST, A. flavus(ATCC 13697); AF, A. flavus; ANST, A. niger (ATCC 10535); AN, A. niger; +, aflatoxin production detected; ND, aflatoxin production not detected; C*, tween 20 (5%).
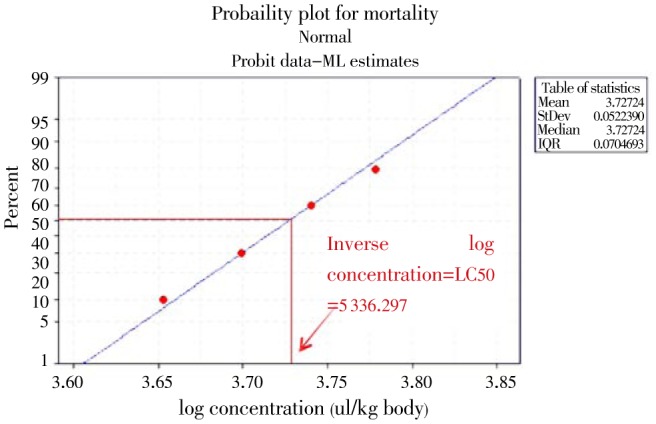
During the safety limit tests, all the test animals were observed closely for up to 14 d; symptoms of toxicity, recovery and death were noted. No sign of toxicity was recorded for the mice in group of control, 3 000, 3 500 and 4 000 µl/kg body weight. The majority of mice from group 4 500, 5 000 and 5 500 have showed hypo-activity (decreased motility, debiting effect) and decreased feed intake. Mice from group 6 000 and 7 000 µl/kg have showed the sign of prostration, anaesthesia and muscle spasm; within 30 min and followed by deaths within the 24 h. The mice that have showed hypo-activity and decreased feed intake have recovered within 24 h.
As it can be observed from Figure 3, there is no mortality at the log concentration of 3 000 through 4 000 µl/kg while there was one death at 4 500, two mortality at a dose of 5 000, four mortality at 5 500 and seven mortality at 6 000 and 10 mortality from 7 000. The LC50 was determined by drawing a vertical line on the X-axis from the point of the straight line the 50% mortality taken (Figure 3) and by calculating the inverse log of the value found on X-axis. The LC50 of the EOs was thus 5 336.297 µl/kg body weight.
Figure 3. Probit transformed responses of mice treated with a different concentrations of T. ammi EOs after oral administration.
4. Discussion
In this millennium, infection from Aspergillus species become the major public health problem of modern mycology[24]. They have a capability to cause directly infection and indirectly mycotoxicosis especially upon the consumption of food contaminated with Aspergillus species. Many chemical preservative have been used for the control of Aspergillus food contamination[25]. The widespread use of chemical preservative has significant drawbacks including increased cost, handling hazards, concern about pesticide residues on food, and threat to human health and environment[10]. Public awareness of these risks has increased interest in finding safer alternatives natural products to replace currently used synthetic chemical preservatives to control Aspergillus food contamination. One such alternative is the use of EOs with antifungal and antiaflatoxigenic activity, since they tend to have low mammalian toxicity, less environmental effects and wide public acceptance[17]. C. martinii, F. vulgare and T. ammi are common economic food spices in Ethiopia. Thus, it is an advantage to develop safe botanical food preservative against toxigenic Aspergillus species that have strong affinity to colonize various food commodities due to its secretion of hydrolytic enzymes[18].
Our TLC analysis confirmed the presence of various components of EOs which were characterized by the distance they travel in a particular TLC system and their appearance (color) after visualization of the spots. EOs are very complex natural mixtures which can contain about 20-60 components at quite different concentrations. They are characterized by two or three major components at fairly high concentrations (20-70%) compared to other components present in trace amounts[26]. This chromatogram developed from EOs with the distinctive spot Rf and color were due to the presence of major component of EOs. i) Alcohols: geraniol in C. martini; ii) Phenols: thymol and carvacrol in T. ammi; iii) Aldehydes: anisaldchyde in F. vulgare; iv) Ketones: fenchone in F. vulgare; v) Esters: geranyl acetate in C. martinii; and vi) Phenylpropanoids: anethole in F. vulgare EOs[19]. And in our GC analysis, we confirmed the presence of thymol (51.5%) and carvacrol (28.5%) as a major component. TLC and GC finger print is the most common chromatographic technique widely available for phytochemical analysis of plant EOs. They are used in standardizing the constituents of EOs that are classified as generally recognized as safe by FDA for their use as food additives in controlling food spoilage[17].
Another important finding of our research was that EOs have remarkable sporicidal activity against toxigenic strains of organisms tested. Hundred percent inhibitions of spore germination were recorded at 4 µl/mL for EO of F. vulgare and at 2 µl/mL for EOs of C. martinii and T. ammi. Similarly, previous studies reported mycosporicidal activity of EOs from Cymbopogon mertinii[27]; antimicrobial activity of T. ammi EOs and antifungal effects of F. vulgare EOs[24],[28],[29]. The impacts of EO on sporulation may be due to denaturation of the enzymes responsible for spore germination or interferenced with the aminoacid involved in germination[30]. The vapor action exerted by volatile constituents of this EO on surface mycelial development and/or the transduction of signals involved in the switch from vegetative to reproductive development could also be responsible for the spore germination inhibition activity[30],[31].
EOs had a clear dose-dependent antifungal activity on A. fluvus at the concentration tested in our agar dilution assay to determine MIC. Our finding suggests the increment of dose to 2, 4 and 8 µl/mL have a significant effect on the inhibition of fungal in reduction of fungal growth while all tested doses have significant effect in inhibition of spore germination. Absolute inhibition of fungal growth were seen at a concentration of 2 µl/mL, 4 µl/mL and 8 µl/mL for the EOs of T. ammi, C. martinii and F. vulgare, respectively and the concentration were recorded as MIC. Many have reported antifungal property of EOs; certainly, differences in major and minor constituents of the oils that are responsible for their biological activity by geographical location and seasons of collection, and the difference in test organisms used in the study could contribute to the difference in MIC of C. martinii[32], F. vulgare[33] and T. ammi[34]. Volatile constituent of the EOs create vapor action that could be responsible for the activity.
In our study, we tried to compare the preservative potentials of plant EOs with the prevalent synthetic preservative by using their MIC value. For easy of comparison, we took the weight of each EOs. For easy of comparison, we took the weight of 1 µL of each EO and found to be 1.10 mg, 1.04 mg and 1.09 mg for C. martinii, F. vulgare and T. ammi, respectively. All EOs have better fungitoxic activity than synthetic preservative are in accordance with the finding of previous study[26]. The inferior activity could be due to nature of the chemicals since it was reported that sodium benzoate were highly active at pH 3.5. Weak acid compounds are more lipophilic in their non-dissociated form which enables them to cross the cell membrane that led to pH lowering of cytoplamic cell with rupture of certain metabolic reactions of the microorganism, permeabilization of the cytoplasmic membrane and cell death. Other authors' demonstrated benzoic acid has membrane-perturing potentials. In addition, these acids induce loss of mitochondrial function, and one possibility that we entertained could be the result of mitochondrial autophagy[35].
Moreover, EOs are complex mixtures of numerous molecules, and one might wonder if their biological effects are the result of a synergism of all molecules or reflect only those of the main molecules present at the highest levels. In the literature in most cases, only the main constituents of certain EOs like thymol, carvacrol, carvone, geraniol, were analyzed[26]. Thus, synergistic functions of the various molecules contained in an EO, in comparison to the action of one or two main components of the oil, seem questionable. However, it is possible that the activity of the main components is modulated by other minor molecules[26]. Moreover, it is likely that several components of the EOs play a role in cell penetration, lipophilic or hydrophilic attraction and fixation on cell walls and membranes, and cellular distribution[26].
Moreover, our study also suggested that T. ammi EOs have pronounced activity in reducing mycelial biomass. It can be clearly seen that the effect are dose dependent, as up on the increment of the concentration of the oils, the mycelia dry weights of the tested Aspergillus spp were recorded. As shown in our result, a hundred percent mycelia growth was inhibited at concentration similar to the 1 µl/mL. The reduction in fungal mycelia biomass may be due to the presence of phenolic compounds in the EOs. Our study that showed this EOs have the ability to reduce mycelial dry weight is in line study of Ahmed and his collogues[36]. It was documented these author that at low dose, phenols affected enzyme activity, especially of those enzymes associated with energy production, while at greater concentrations, caused protein denaturation as reported by Ahmed and his collogues[36]. In addition, lypophilic nature of the EOs helps to cross cell membrane of the fungal cell interacting with the enzymes and proteins of the membrane, so producing a flux of protons towards the cell exterior which induces changes in the cells and, ultimately leading to death.
Another crucial aim of this study was confirmed by our result that have shown us the aflatoxin arresting potentials of EOs extracted from T. ammi against aflatoxicogenic Aspergillus spp tested in our study. Aflatoxin can be produced by Aspergillus spp but the fungus may no longer be present in the food, hence preservative used for the control of aflatoxin should act on both fungus and the mycotoxin they produce. Interestingly, the productions of aflatoxin were inhibited by T. ammi EO at concentrations lower than recorded for MIC, spore germination inhibition and mycelial dry weight. Thus, the inhibition of aflatoxin production cannot be completely attributed to reduced fungal growth, but may be because of inhibition of carbohydrate catabolism in Aspergillus spp by acting on some key enzymes, reducing its ability to produce aflatoxins. Our finding that T. ammi has aflatoxin arresting potential replicates the finding of Hajare and his collaborators on the aqueous extract of T. ammi seed having aflatoxin inactivation potential[37]. Once again, as mycelial growth and spore germination inhibition, aflatoxin production inhibition could be due to the presence of thymol and carvacrol (phenolic OH group) that form hydrogen bonds with target enzyme active site[38].
Our result of safety limit on mice shows that T. ammi is essential unlikely to present acute toxicity supporting the consumption of this plant seed as spice in Ethiopia. All preservatives used against food spoilage moulds should not be harmful for human beings upon consumption. The safety limit of the T. ammi EO was also determined through its oral administration (acute oral toxicity) on mice and its LC50 value was found to be 5 336.297 µl/kg body weight and classified using WHO recommended classification in the U group[39]. The high value of LC50 is a symbol of the non-mammalian toxicity of the T. ammi EO. Hence it may be recommended as a safe preservative of foods, as 5 000.00 µL of the test substance/kg body weight is the practical upper limit for the amount of test material that can be administered in one oral gavages dose to a rodent. Now a day's using EOs as food additives is common throughout the world[17].
Result of our study indicated EOs of plants possess antifungal property against toxigenic strains of Aspergillus spp. We found EOs of C. martinii, F. vulgare and T. ammi have fungitoxic potential. Moreover, EOs of T. ammi have antiaflatoxigenic potentials and no mammalian toxicity on mice. Therefore, EOs of T. ammi could be recommended as best safe botanical food preservative as it has antifungal as well as antiaflatoxigenic activity, superiority over synthetic fungicides and non-mammalian toxicity. In addition, this EO has practical applicability as fumigant of food commodities due to their aromatic volatility nature. Moreover, we can also minimize the residual effect of this plant in food commodities by drying food staffs using sun light before consumption. As a result of these finding and opportunities we suggest T. ammi EO as a potential source of safe botanical food preservative that inhibits Aspergillus spore germination, growth and mycotoxin production inhibition. However, further studies should be conducted to explore large scale utilization and also exploring the efficacy of T. ammi EOs using other toxigenic organisms that contaminate food commodities.
Acknowledgments
Financial supports for this study were provided by Addis Ababa University and by Ministry of Finance and Economic Development through EHNRI (project number 342/02/04/01/012). We are thankful the staff of the Traditional and Modern Medicine Directorate, EHNRI for their help and support during our study.
We would like to thank Mr. Awoke Kebede for his generous permission to use his aflatoxin standard (Aflatoxin Mix Kit-M). The authors are grateful to Department of Microbiology, Immunology and Parasitology (DMIP), College of Health Science, AAU, and EHNRI for their facility and financial support.
Comments
Background
Food contamination with spoilage fungi presents a global concern. Growth and metabolism of these organisms can cause serious food-borne intoxications and rapid spoilage of food products and ultimately economic loss. Aspergillus species, a group of opportunistic fungi are the leading cause of infection and mycotoxicosis. Their mycotoxins cause cancer and exert toxic effects on gastrointestinal, urogenital, vascular, kidney and nervous systems.
Research frontiers
This study shows the in vitro antifungal and antiaflatoxigenic activity of essential oils from C. martinii, F. vulgare and T. ammi against toxicogenic strains of Aspergillus species by use of various bioassay techniques.
Related reports
Synthetic food preservatives cause various environmental and health hazards. In this study, essential oils have shown interesting bioactivity against toxygenic Aspergillus spp. Essential oils from various medicinal plants can be used as green preservative. Concordant findings were reported by Koul et al. 2008 and Kumar et al. 2008.
Innovations and breakthroughs
Medicinal plants are rich source of variety of secondary metabolites with diverse chemical structure and biological activities. In this paper, the authors evaluated botanical preservative effect of essential oils against aflatoxigenic Aspergillus spp in Ethiopia.
Applications
With the increasing problem with the use of chemical preservatives, there is urgent need to find efficacious affordable and environmentally friendly alternatives from natural product with minimal side effects. Available literatures indicate essential oils of plants used in this study have wider safety margins and can be used as alternative for preservation of food commodities.
Peer review
This study is an excellent piece of work where the authors investigated the preservative effect of essential oils against food contaminating toxigenic Aspergillus fungi. The in vitro antifungal and antiaflatoxigenic activity of essential oils was evaluated using poisoned food techniques, spore germination assay, agar dilution assay, and aflatoxin arresting assay on toxigenic strains of Aspergillus species.
Footnotes
Foundation Project: Provided by Addis Ababa University and by Ministry of Finance and Economic Development through EHNRI (Project number 342/02/04/01/012).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.DeWaal CS, Robert N. Global and local: food safety around the world. Washington: Center for Science in the Public Interest; 2005. [Google Scholar]
- 2.Leslie JF, Bandyopadhyay R, Visconti A. Mycotoxins-Detection Methods, Management, Public Health and Agricultural Trade, CAB International, UK. 2008.
- 3.Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagacha JM, Muthomi JW. Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int J Food Microbiol. 2008;124:1–12. doi: 10.1016/j.ijfoodmicro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Zain ME. Impact of mycotoxins on humans and animals. J Saudi Chem Soc. 2011;15:129–144. [Google Scholar]
- 6.Brul S, Coote P. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Int J Food Microbiol. 1999;50:1–17. doi: 10.1016/s0168-1605(99)00072-0. [DOI] [PubMed] [Google Scholar]
- 7.Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 2011;8:1402–1419. doi: 10.3390/ijerph8051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin AL. Mammalian toxicity of microbial pest control agents. In: Krieger RI, Krieger WC, editors. Handbook of pesticide toxicology principle. 2nd ed. USA: Acadamic Press; 2001. pp. 859–871. [Google Scholar]
- 9.Havelaar AH, Brul S, de Jong A, de Jonge R, Zwietering MH, Ter Kuile BH. Future challenges to microbial food safety. Int J Food Microbiol. 2010;139(Suppl. 1):S79–S94. doi: 10.1016/j.ijfoodmicro.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Nollet LM, Rathore HS. Handbook of pesticides: methods of pesticide residues analysis. USA: Taylor and Francis; 2010. p. 608. [Google Scholar]
- 11.Cardinale F, Mangini F, Berardi M, Sterpeta Loffredo M, Chinellato I, Dellino A, et al. [Intolerance to food additives: an update] [Article in Italian] Minerva Pediatr. 2008;60:1401–1409. [PubMed] [Google Scholar]
- 12.Gonzalez-Coloma A, Reina M, Diaz CE, Fraga BM. Natural product-based biopesticides for insect control. Comprehensive Nat Prod. 2010;3:237–268. [Google Scholar]
- 13.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Sharma S, Naik SN. Biopesticidal value of selected essential oils against pathogenic fungus, termites, and nematodes. Int Biodeterior Biodegrad. 2011;65:703–707. [Google Scholar]
- 15.Alizadeh A, Zamani E, Sharaifi R, Javan-Nikkhah M, Nazari S. Antifungal activity of some essential oils against toxigenic Aspergillus species. Commun Agric Appl Biol Sci. 2010;75(4):761–767. [PubMed] [Google Scholar]
- 16.Ipsilantis I, Samourelis C, Karpouzas DG. The impact of biological pesticides on arbuscular mycorrhizal fungi. Soil Biol Biochem. 2012;45:147–155. [Google Scholar]
- 17.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rezaee MB, Jaimand K, Alinezhad S, Saberi R, et al. Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Control. 2009;20:1018–1024. [Google Scholar]
- 18.Tadeg H. Phytopharmaceutical studies of some selected medicinal plants locally used in the treatment of skin disorders. Ethiopia: MSc thesis, Addis Ababa University; 2004. [Google Scholar]
- 19.Wagner H, Bladt S. Plant drug analysis: a thin layer chromatography atlas. 2nd ed. New York: Springer-Verlag Berlin Heidelberg; 1996. [Google Scholar]
- 20.Das K, Tiwari RK, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J Med Plants Res. 2010;4:104–111. [Google Scholar]
- 21.Uldahl SA, Knutsen G. Spore swelling and germination as a bioassay for the rapid screening of crude biological extracts for antifungal activity. J Microbiol Methods. 2009;79:82–88. doi: 10.1016/j.mimet.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Balows A. Manual of clinical microbiology. 5th ed. USA: ASM; 1991. [Google Scholar]
- 23.Kumar A, Shukla R, Singh P, Prasad CS, Dubey NK. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innovat Food Sci Emerg Tech. 2008;9:575–580. [Google Scholar]
- 24.Pitt JI, Hocking AD. Fungi and food spoilage. 3rd ed. New York: Springer Science; 2009. [Google Scholar]
- 25.Maier E, Kurz K, Jenny M, Schennach H, Ueberall F, Fuchs D. Food preservatives sodium benzoate and propionic acid and colorant curcumin suppress Th1-type immune response in vitro. Food Chem Toxicol. 2010;48:1950–1956. doi: 10.1016/j.fct.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils--a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 27.Sivamani P, Hameed AS. Mycosporicidal activity of essential oils from selected herbals against isolates from HIV/AIDS patients. J Pharm Res. 2010;3:679–683. [Google Scholar]
- 28.Paul S, Dubey RC, Maheswari DK, Kang SC. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control. 2011;22:725–731. [Google Scholar]
- 29.Uniyal V, Bhatt RP, Saxena S, Talwar A. Antifungal activity of essential oils and their volatile constituents against respiratory tract pathogens causing Aspergilloma and Aspergillosis by gaseous contact. J Appl Nat Sci. 2012;4(1):65–70. [Google Scholar]
- 30.Nychas GJ. Natural antimicrobials from plants. In: Gould GW, editor. New methods of food preservation. London: Blackie Academic; 1995. pp. 58–89. [Google Scholar]
- 31.Tian J, Ban X, Zeng H, He J, Chen Y, Wang Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One. 2012;7:e30147. doi: 10.1371/journal.pone.0030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bansod S, Rai M. Antifungal activity of essential oils from Indian medicinal plants against human pathogenic Aspergillus fumigatus and A. niger. World J Med Sci. 2008;3:81–88. [Google Scholar]
- 33.Marei GI, Rasoul MA, Abdelgaleil SA. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic Biochem Physiol. 2012;103:56–61. [Google Scholar]
- 34.Javed S, Shahid AA, Haider MS, Umeera A, Ahmad R, Mushtaq S. Nutritional, phytochemical potential and pharmacological evaluation of Nigella sativa (Kalonji) and Trachyspermum ammi (Ajwain) J Med Plants Res. 2012;6:768–775. [Google Scholar]
- 35.Hazan R, Levine A, Abeliovich H. Benzoic acid, a weak organic acid food preservative, exerts specific effects on intracellular membrane trafficking pathways in Saccharomyces cerevisiae. Appl Environ Microbiol. 2004;70:4449–4457. doi: 10.1128/AEM.70.8.4449-4457.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad A, Khan A, Yousuf S, Khan LA, Manzoor N. Proton translocating ATPase mediated fungicidal activity of eugenol and thymol. Fitoterapia. 2010;81:1157–1162. doi: 10.1016/j.fitote.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Hajare SS, Hajare SN, Sharma A. Aflatoxin inactivation using aqueous extract of ajowan (Trachyspermum ammi) seeds. J Food Sci. 2005;70:C29–C34. [Google Scholar]
- 38.Tian J, Ban X, Zeng H, He J, Huang B, Wang Y. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int J Food Microbiol. 2011;145:464–470. doi: 10.1016/j.ijfoodmicro.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 39.WHO . The WHO recommended classification of pesticides by hazard and guidelines to classification 2009. WHO; 2010. p. 78. [Google Scholar]


