Abstract
Objective
To analyse qualitative and quantitative phytochemical and evaluate in vitro antioxidant properties of various alcoholic and aqueous extracts of leaf and root parts of Hypochaeris radicata.
Methods
Preliminary phytochemical analysis for alkaloids, cardiac glycosides, flavonoids, glycosides, phenols, resins, saponins, steroids, tannins, terpenoids and triterpenoids and quantitative phytochemical analysis for alkaloids, total phenolics, total flavonoids, tannins, saponins and ascorbic acid were made by following standard procedures. In vitro antioxidant properties were evaluated by assessing DPPH•, NO• and ABTS•+, radical scavenging abilities and assaying the reducing power, β-carotene and antihemolytic activities by adapting standard methods.
Results
The quantitative phytochemical analysis of this species exhibited the presence of alkaloids, total phenolics, total flavonoids, tannins, saponins and ascorbic acid in considerable quantity. The in vitro antioxidant activity of the species, Hypochaeris radicata clearly demonstrated that both the leaf and root parts have prominent antioxidant properties.
Conclusions
From this study, it can be concluded that the species is effective in scavenging free radicals and has the potential to be a powerful antioxidant.
Keywords: Hypochaeris radicata, Phytochemical analysis, In vitro antioxidant activities
1. Introduction
A free radical is defined as any atom or molecule possessing unpaired electrons. The reactive oxygen species are oxygen derived free radicals such as superoxide anion (O2), hydroxyl (OH•), hydroperoxyl (OOH•), peroxyl (ROO•) and alkoxyl (RO•) radicals and non free radicals such as hydrogen peroxide (H2O2), hypochorous acid (HOCl), ozone (O3) and singlet oxygen (O2)[1]. It can be formed in living organisms by both endogenously (respiration, peroxisomes stimulation of polymorphonuclear leucocytes and macrophages) and exogenously (ionizing radiation, tobacco smoke, pollutants, pesticides and organic solvents)[2]. These free radicals are produced by our body and to stabilize the body's natural function, but the excess amount could cause the cell and tissue damage[3]. It can also cause oxidative damage to proteins, lipids and DNA and chronic diseases such as cancer, diabetes, aging and other degenerative diseases in humans[4].
An antioxidant can be broadly defined as any substance that delays or inhibits oxidative damage to a target molecule[5]. The characteristic feature of an antioxidant is ability to scavenge the free radicals due to their redox hydrogen donators and singlet oxygen quencher[6],[7]. The free radicals can be scavenged by the natural (plants) and synthetic (butylated hydroxyl toluene, butylated hydroxyl anisol and tetra butyl hydro quinone) antioxidants[8]. But the usages of these synthetic antioxidants are now replaced because the natural antioxidants could be considered as safer without any side effects[9]. In recent decades, many researchers are interested in medicinal plants for evaluation of antioxidant phytochemicals such as phenols, flavonoids and tannins which have received more attention for their potential role in prevention of human diseases[10].
Hypochaeris radicata (H. radicata), belonging to the family Asteraceae, is an edible perennial herb, distributed in high hills of Nilgiris, the Western Ghats at 2 000 m above mean sea level. The whole plant is said to be medicinally important by having antiinflammatory, anticancer, antioxidant[11], antibacterial[12], antifungal[13] and antidiuretic properties. It is being used for the treatment of jaundice, rheumatism, dyspepsia, constipation, hypoglycemia and kidney related problems in traditional medicinal practice of Tamil Nadu, India[14]. However, no much scientific validation has been made for this species for its medicinal uses. To address this lacuna, the present study was carried out for qualitative and quantitative phytochemical analysis and in vitro antioxidant activities of leaf and root parts of H. radicata using various alcoholic (petroleum ether, chloroform, ethyl acetate and methanol) and aqueous extracts.
2. Materials and methods
2.1. Chemicals
In the present study, all the chemicals were purchased from HI-MEDIA Pvt. Ltd., Bombay. The chemicals used were of analytical grade.
2.2. Collection and identification of plant materials
The plant H. radicata was collected from Nilgiris, the Western Ghats, Tamil Nadu, India. The authenticity of the plant was confirmed in Botanical Survey of India, Southern Circle, Coimbatore by referring the deposited specimen. The voucher number of the specimen is BSI/SRC/5/23/2010-11/Tech.153. The fresh leaf and root parts of this species were washed under running tap water, shade dried at room temperature and powdered.
2.3. Extract preparation
The powdered plant samples (50 g/250 mL) were extracted successively with petroleum ether, chloroform, ethyl acetate, methanol and water using Soxhlet apparatus at 55-85 °C for 8-10 h in order to extract the polar and non-polar compounds[15]. For each solvent extraction, the powdered pack material was air dried and then used. The solvents of the respective extracts were reduced under room temperature and stored at 4 °C for further use. The dried plant extracts were then redissolved in dimethyl sulfoxide and to get the solution of 10 mg/10 mL for each extract which was subjected to analysis of in vitro antioxidant activities.
2.4. Preliminary qualitative phytochemical analysis
Preliminary qualitative phytochemical analysis was carried out to identify the secondary metabolites present in the various alcoholic and aqueous extracts of leaf and root parts of H. radicata[16],[17].
2.5. Quantitative estimation of chemical constituency
2.5.1. Determination of alkaloids
A total of 200 mL of 20% acetic acid was added to 5 g of leaf and root powders taken in a separate 250 mL beaker and covered to stand for 4 h. This mixture containing solution was filtered and the volume was reduced to one quarter using water bath. To this sample, concentrated ammonium hydroxide was added drop-wise until the precipitate was complete. The whole solution was allowed to settle and the precipitate was collected by filtration and weighed[18]. The percentage of total alkaloid content was calculated as:
Percentage of total alkaloids (%)=Weight of residue×100/Weight of sample taken
2.5.2. Total phenolics content
The total phenolics content of H. radicata was estimated using Folin-Ciocalteau reagent by the method of Sidduraju and Becker[19]. About 20 µg of leaf and root extracts were taken separately and it was made up to 1 mL with distilled water. Then 500 µL of diluted Folins-phenol reagent (1:1 ratio with water) and 2.5 mL of sodium carbonate Na2CO3 (20%) were added. The mixture was shaken well and incubated in dark condition for 40 min for the development of colour. After incubation, the absorbance was measured at 725 nm. A calibration curve of gallic acid was constructed and linearity was obtained in the range of 10-50 µg/mL. The total phenolics content in the plant extracts were expressed as mg of gallic acid equivalent (mg GAE/g extract) by using the standard curve.
2.5.3. Total flavonoids content
The total flavonoids content was estimated using the procedure described by Zhishen et al[20]. A total of 1 mL of plant extracts were diluted with 200 µL of distilled water separately followed by the addition of 150 µL of sodium nitrite (5%) solution. This mixture was incubated for 5 min and then 150 µL of aluminium chloride (10%) solution was added and allowed to stand for 6 min. Then 2 mL of sodium hydroxide (4%) solution was added and made up to 5 mL with distilled water. The mixture was shaken well and left it for 15 min at room temperature. The absorbance was measured at 510 nm. Appearance of pink colour showed the presence of flavonoids content. The total flavonoids content was expressed as rutin equivalent mg RE/g extract on a dry weight basis using the standard curve.
2.5.4. Estimation of tannins content
Tannins content of H. radicata was estimated by the method of Siddhuraj and Manian[21]. A total of 500 µL of the extracts were taken in test tube separately and treated with 100 mg of polyvinyl polypyrrolidone and 500 µL of distilled water. This solution was incubated at 4 °C for 4 h. Then the sample was centrifuged at 5 000 r/min for 5 min and 20 µL of the supernatant was taken. This supernatant has only simple phenolics free of tannins (the tannins would have been precipitated along with the polyvinyl polypyrrolidone). The phenolics content of the supernatant was measured at 725 nm and expressed as the content of free phenolics on a dry matter basis. From the above results, the tannins content of the extract was calculated as follows:
Tannins (mg GAE/g extract)=Total phenolics (mg GAE/g extract)-Free phenolics (mg GAE/g extract)
2.5.5. Estimation of total saponins content
Estimation of total saponins content was determined by the method described by Makkar et al. based on vanillin-sulphuric acid colorimetric reaction with some modifications[22]. About 50 µL of plant extract was added with 250 µL of distilled water. To this, about 250 µL of vanillin reagent (800 mg of vanillin in 10 mL of 99.5% ethanol) was added. Then 2.5 mL of 72% sulphuric acid was added and it was mixed well. This solution was kept in a water bath at 60 °C for 10 min. After 10 min, it was cooled in ice cold water and the absorbance was read at 544 nm. The values were expressed as diosgenin equivalents (mg DE/g extract) derived from a standard curve.
2.5.6. Ascorbic acid (vitamin C)
Ascorbic acid determination was done according to Klein and Perry[23]. About 10 mg of dried plant powder were re-extracted with 10 mL of 1% metaphosphoric acid. They were allowed to stand for 45 min at laboratory temperature and filtered through Whatman No. 4 filter paper. About 1 mL of filter was taken and it was mixed with 9 mL of 50 µmol/L 2,6-dichloroindophenol sodium salt hydrate and the absorbance was measured with 30 min at 515 nm. Ascorbic acid content was calculated on the basis of the calibration curve of authentic L-ascorbic acid and the results were expressed as mg of ascorbic acid equivalent (mg AE/g extract).
2.6. In vitro antioxidant activities
2.6.1. DPPH• radical scavenging activity
The ability of H. radicata extracts to scavenge the DPPH• radicals was assessed by using the method of Blois with some modifications[24]. About 0.2 mmol/L solution of DPPH• in methanol was prepared, and 500 µL of this solution was added to different concentrations of the extracts (50-250 µg/mL). The mixture was shaken vigorously and allowed to stand for 30 min at room temperature. Control was prepared as above but without the sample extracts and methanol was used for the baseline correction. Then changes in the absorbance of the plant samples were measured at 517 nm using spectrophotometer. A lower absorbance value indicates the higher radical scavenging activity. Results were compared with the standard antioxidants [rutin, quercetin, butylated hydroxylanisole (BHA) and butylated hydroxytoluene (BHT)]. The ability of DPPH• radical scavenging activity was calculated by using the following formula:
DPPH• scavenging effect (% of inhibition)=(A0-A1)×100/A0
Where, A0 is the absorbance of the control, and A1 is the absorbance of the sample extracts. The IC50 (the microgram of extract to scavenge 50% of the radicals) value was calculated using linear regression analysis. Lower IC50 value indicates greater antioxidant activity.
2.6.2. Nitric oxide radical scavenging activity
Nitric oxide radical scavenging activity was assessed by the method of Sreejayan and Rao[25]. Nitric oxide radicals were produced from sodium nitroprusside solution and measured by the Griess reagent. Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide radicals which interfere with oxygen to produce nitrite ions. Scavengers of nitric oxide act against oxygen, leading to reduce production of nitrite ions. About 3 mL of sodium nitro prusside (10 mmol/L) in phosphate buffer saline (0.2 mmol/L, pH 7.4) was added with various concentrations of the extracts (250-450 µg/mL) and it was incubated at 25 °C for 150 min. Then 500 µL of Griess reagent (1% sulphailamide, 2% orthophosphoric acide, 0.1% N-1-napthylethylenediamine dihydrochloride) was added. The absorbance values were measured at 546 nm and percentage of inhibition was calculated using the same formula as DPPH•. The decreasing of optical density values shows high nitric oxide radical scavenging activity. The IC50 value was calculated and it was compared with standard antioxidants, rutin, quercetin, BHA and BHT.
2.6.3. Reducing power activity
The reducing ability of H. radicata was determined by the method of Yildrim et al[26]. Various concentrations of plant extracts (300-700 µg/mL) were mixed with 1 mL of 0.2 mol/L sodium phosphate buffer (pH 6.6) and 1 mL of freshly prepared 1% potassium ferric cyanide. The mixture was incubated in water bath at 50 °C for 20 min. Then 1 mL of 10% of trichloro acetic acid was added and centrifuged at 3 000 r/min for 10 min. The supernatant (2 mL) was mixed with 2 mL of distilled water and 500 µL of 1% ferric chloride (freshly prepared). The absorbance was read at 700 nm. Higher absorbance of the reaction mixture indicates greater reducing power. The results were compared with that of the standard antioxidants, rutin, quercetin, BHA and BHT.
2.6.4. Total antioxidant activity by ABTS•+ radical cation decolorization assay
The total antioxidant activity of H. radicata was measured by decolorization of ABTS•+ radical cation using the method of Siddhuraju and Manina[21]. ABTS radical cation was generated by oxidation of ABTS•+ (7 mmol/L) with potassium persulfate (2.45 mmol/L) which was dissolved in 5 mL of distilled water. After incubation for 12-16 h at room temperature in dark condition, blue/green ABTS•+ chromophore was produced. The ABTS•+ solution was diluted with ethanol (1:89 v/v) and adjusted to equilibrate the absorbance of (0.700±0.001) at 734 nm. The generated ABTS•+ solution (2 mL) was mixed with 20 µL of sample extracts or trolox standards (0-15 µmol/L). The absorbance values were read at 734 nm exactly after 30 min. The total antioxidant activity unit was defined as the concentration of trolox having the equivalent antioxidant activity expressed as µmol/g sample extracts on dry weight basis.
2.6.5. Inhibition of β-carotene bleaching assay
The antioxidant activity of H. radicata was evaluated by the β-carotene linoleate model system[27]. A solution of β-carotene-linoleic acid mixture was prepared by dissolving 1 mg of β-carotene in 10 mL of chloroform. To this, 20 µL of linoleic acid and 200 mg of Tween-40 emulsifier were added. Then the mixture containing chloroform solvent was completely removed by vacuum using a rotary vacuum evaporator at 45 °C. For the formation of emulsion, 50 mL of oxygenated distilled water was slowly added to this semi-solid residue and shaken vigorously. Aliquots (5 mL) of this emulsion were transferred into different test tubes containing 100 µg/mL of the sample extracts. An initial (0 time) absorbance at 470 nm was immediately recorded. Subsequent absorbance readings were recorded at 15 min intervals by keeping the sample tubes in a water bath at 50 °C until the visual of β-carotene in the control sample disappeared (about 120 min). Control samples (devoid of β-carotene) were used for the subtraction. Rutin, quercetin, BHA and BHT were used as standards. The antioxidant activity was expressed as per cent relative inhibition compared to control samples after 120 min and calculated as:
AA (%)= [1-(A0-A1)/(Z0-Z1)]×100
Where, A0 is the absorbance of sample at 0 min, A1 is the absorbance of sample at 120 min, Z0 is the absorbance of control at 0 min and Z1 is the absorbance of control at 120 min.
2.6.6. Antihemolytic activity
The inhibition of cow erythrocyte haemolysis by the extract was evaluated according to the method described by Naim et al[28]. The cow erythrocyte was performed with the help of H2O2 as free radical initiator. The erythrocytes from cow blood were separated by centrifugation at 2 000 r/min for 10 min and washed with phosphate buffer saline (0.9 g of sodium chloride dissolved in 100 mL of 0.2 mol/L phosphate buffer, pH 7.4) until the supernatant became colourless. The erythrocytes were then diluted with phosphate buffer saline to give 4% (v/v) suspension. A total of 500 µg samples and 1 mL phosphate buffer saline were added to 2 mL of 4% erythrocyte suspension and the volume was made up to 5 mL with phosphate buffer saline. This mixture was pre-incubated for 5 min and the 500 µL of H2O2 in the reaction mixture was adjusted as to bring about 90% hemolysis of blood cells after 240 min. After incubation, the mixture was centrifuged at 1 500 r/min for 10 min and measured at 540 nm. Natural and synthetic standards at the same concentrations as sample extracts were used for comparison. The percentage of hemolysis inhibition was calculated by using the same formula employed in DPPH• assay.
2.7. Statistical analysis
Statistical analysis was carried out by One-way analysis of variance (ANOVA) test using a statistical package program (SPSS 10.0) and the significance of the difference between means was determined by Duncan's multiple range test at (P<0.05) significant level. Analysis was carried out in triplicate and mean±SD of three parallel measurements.
3. Results
3.1. Preliminary qualitative phytochemical analysis
The present study revealed that the various alcoholic and aqueous extracts of leaf and root parts of H. radicata contained alkaloids, cardiac glycosides, flavonoids, glycosides, phenols, resins, saponins, steroids, tannins, terpenoids and triterpenoids (Table 1). However, phenols were detected only in methanolic extracts of both parts and the cardiac glycosides were found in root extracts of the solvents chloroform, ethyl acetate and methanol. Next to methanol extract, ethyl acetate extracts of both parts showed the presence of rich variety of secondary metabolites. Petroleum ether, chloroform and water extracts showed the less variety of these secondary metabolites. Compared to all other solvent extracts, methanolic leaf and root extracts had higher number of secondary metabolites with high degree of precipitation (+++). Triterpenoids and resins were determined to be present with lesser amount (+) only in all extracts.
Table 1. Preliminary qualitative phytochemical analysis of various alcoholic and aqueous extracts of leaf and root parts of H. radicata.
| Plant constituents | Petroleum ether |
Chloroform |
Ethyl acetate |
Methanol |
Water |
|||||
| L | R | L | R | L | R | L | R | L | R | |
| Alkaloids | - | - | + | +++ | - | - | +++ | ++ | + | - |
| Cardiac glycosides | + | - | - | +++ | - | +++ | + | +++ | + | - |
| Flavonoids | - | - | - | - | - | +++ | +++ | ++ | +++ | +++ |
| Glycosides | - | - | - | - | + | + | +++ | +++ | - | - |
| Phenols | - | - | - | - | - | - | +++ | +++ | - | - |
| Resins | - | - | + | + | + | - | + | + | + | - |
| Saponins | - | - | + | - | - | +++ | +++ | + | - | - |
| Steroids | - | +++ | - | ++ | + | +++ | +++ | +++ | ++ | ++ |
| Tannins | + | - | - | - | + | ++ | +++ | ++ | - | - |
| Terpenoids | - | ++ | - | ++ | + | +++ | +++ | +++ | ++ | ++ |
| Triterpenoids | - | ++ | + | +++ | ++ | + | + | + | ++ | - |
+++: highly present, ++: moderately present, +: Low, -: absent. L: leaf extract, R: root extract.
3.2. Quantitative determination of the chemical constituency
3.2.1. Extraction yield
Table 2 shows the percentage of yield of crude successive extracts (petroleum ether, chloroform, ethyl acetate, methanol and water) of leaf and root parts of H. radicata. Methanolic extracts of root exhibited higher yield (25.6%) followed by methanolic leaf extract (18.4%). The ethyl acetate leaf extract showed the lowest yield of 0.4%. Water extracts of root and leaf also showed lower yields (3.58% and 1.72% respectively) followed by petroleum ether leaf extracts (5%). Petroleum ether and chloroform root extracts determined to have the same quantity of yield of 2.8% each.
Table 2. Percentage yield, total phenolics, total flavonoids, tannins and vitamin C contents of various alcoholic and aqueous extracts of leaf and root parts of H. radicata.
| Sample | Percentage yield (w/w) |
Total phenolics1 |
Total flavonoids2 |
Tannins1 |
Vitamin C3 |
Saponins4 |
||||||
| L | R | L | R | L | R | L | R | L | R | L | R | |
| PE | 5.00 | 2.80 | 0.77±0.01a | 0.46±0.01a | - | - | 0.17±0.01 | 0.06±0.02b | 42.30±0.44c | 24.89±0.07a | 15.73±0.30bc | 13.02±0.06b |
| CH | 2.20 | 2.80 | 0.45±0.07c | 0.71±0.02a | - | 14.31±0.03a | 0.12±0.01 | 0.29±0.03b | 19.32±0.06b | 23.35±0.07a | 14.01±0.02b | 12.86±0.02a |
| EA | 0.40 | 1.60 | 0.11±0.04b | 0.44±0.05bc | 17.79±0.09a | 14.28±0.06b | 0.03±0.01 | 0.18±0.01a | 3.75±0.01a | 13.35±0.18b | 16.67±0.01c | 16.69±0.02c |
| ME | 18.40 | 25.60 | 3.75±0.05b | 5.04±0.03b | 8.84±0.08a | 6.36±0.07bc | 1.03±0.17 | 1.61±0.09c | 162.44±1.28d | 213.92±1.17c | 13.46±0.02b | 16.25±0.02c |
| WA | 1.72 | 3.58 | 0.32±0.01a | 0.70±0.01a | 6.68±0.11b | 03.61±0.05b | 0.07±0.01 | 0.18±0.03b | 15.00±0.08b | 31.71±0.16b | 11.96±0.02a | 13.40±0.03b |
Values were performed in triplicates and represented as mean±SD. PE: petroleum ether, CH: chloroform, EA: ethyl acetate, ME: methanol, WA: water, L: leaf, R: root, -: not detected. 1: mg GAE/100 g extract, 2: mg RE/100 g extract, 3: mg AE/100 g extract, 4: mg DE/100 g extract. Mean values followed by different superscript in a column are significantly different (P<0.05).
3.2.2. Determination of alkaloids
The gravimetric analysis for total alkaloid contents in leaf and root parts of H. radicata exhibited that higher alkaloid contents were present in leaf powder (4 560.21 mg/100 g sample) than that of the root powder (3 800.83 mg/100 g sample).
3.2.3. Total phenolics content
Total phenolics content of various extracts of leaf and root parts of H. radicata was varying widely between 0.32 to 5.04 mg GAE/100 g extract (Table 2). Methanolic extract of leaf and root parts were demonstrating higher total phenolics content (3.75 and 5.04 mg GAE/100 g extracts respectively) than that of the other solvent extracts.
3.2.4. Total flavonoids content
The total flavonoids content was high in ethyl acetate leaf extract (17.79 mg RE/100 g extract) followed by root chloroform and ethyl acetate extracts (14.31 and 14.28 mg RE/100 g extract respectively) (Table 2). In addition, it has noted that the flavonoids content were not deducted in petroleum ether leaf and root extracts and chloroform leaf extracts.
3.2.5. Estimation of tannins content
The tannins content of the various extracts of leaf and root parts of H. radicata was determined to be ranged between 0.03 and 1.62 mg GAE/100 g extract (Table 2). Among the solvents used the methanolic leaf and root extracts were registered high amount of tannins, 1.03 and 1.61 mg GAE/100 g extract respectively.
3.2.6. Estimation of saponins content
The total saponins contents of both leaf and root parts of H. radicata were ranging between 11.96 and 16.69 mg DE/100 g extract across the various solvent extracts studied (Table 2). The ethyl acetate extract of leaf and root parts depicted high content of saponins 16.67 and 16.69 mg DE/100 g extract respectively.
3.2.7. Ascorbic acid (vitamin C)
The ascorbic acid content of both parts of H. radicata was found to be present between 3.75 and 213.92 mg AE/100 g extract (Table 2). Among the solvents used, the methanol has drawn high amount of ascorbic acid content, 162.44 and 213.92 mg AE/100 g extracts respectively from leaf and root parts. On the other hand, ethyl acetate leaf extract contained lower amount of ascorbic acid (3.75 mg AE/100 g extract).
3.3. In vitro antioxidant activity
3.3.1. DPPH• radical scavenging activity
The data on DPPH• radical scavenging activity of leaf and root parts of H. radicata along with the best known natural and synthetic antioxidant standards, viz., rutin, quercetin, BHA and BHT are presented in Table 3. The mean IC50 values of ethyl acetate leaf and root extracts were lower which showed that the radical scavenging activity was shown to be effective in this solvent extracts (125.94 and 122.54 µg/mL respectively). Further, the IC50 values of the leaf and root extracts were comparable to that of the natural and synthetic standard antioxidants. Next to methanolic leaf (130.54 µg/mL) and root (134.40 µg/mL) extracts, root chloroform extract showed significant activity. Among the all extracts, water leaf extract had higher IC50 value (595.23 µg/mL) which indicated its poor scavenging activity.
Table 3. DPPH•, nitric oxide (NO•) and ABTS•+ radical scavenging activity of various alcoholic and aqueous extracts of leaf and root parts of H. radicata.
| Sample extracts | IC50 values (µg/mL) |
ABTS•+ radical scavenging activity (µmol of TE/g DW) |
||||
| DPPH• radical scavenging activity |
NO• radical scavenging activity |
|||||
| L | R | L | R | L | R | |
| PE | 228.31±0.04a | 328.94±0.03b | 276.24±4.58c | 714.29±3.97e | 988.9±264.9b | 1 110.4±341.5a |
| CH | 199.20±0.02a | 135.87±0.04b | 114.94±0.15a | 193.80±0.43b | 2 031.7±606.8d | 3 766.5±792.3d |
| EA | 125.94±0.12c | 122.54±0.01a | 263.15±2.38b | 357.14±1.48d | 2 639.2±314.7c | 3 675.4±1 643.8e |
| ME | 130.54±0.23c | 134.40±0.21c | 289.02±1.95c | 91.07±0.04a | 1 778.6±150.3a | 2 001.4±592.7c |
| WA | 595.23±0.07b | 292.39±0.07c | 310.56±0.04d | 304.88±2.14c | 2 143.1±276.6b | 2 072.2±412.3b |
| RU | 15.75±0.01a | 42.07±0.00b | ||||
| QE | 20.72±0.05b | 50.82±4.00c | - | |||
| BHA | 21.42±0.11c | 52.97±8.23d | ||||
| BHT | 34.74±0.26d | 38.47±1.03a | ||||
Values were performed in triplicates and represented as mean±SD. Mean values followed by different superscript in a column are significantly different (P<0.05). PE: petroleum ether, CH: chloroform, EA: ethyl acetate, ME: methanol, WA: water, L: leaf, R: root. RU: rutin, QE: quercetin, BHA: butylated hydroxylanisole and BHT: butylated hydroxyltoluene, TE: trolox equivalent, DW: dry weight.
3.3.2. Nitric oxide radical scavenging activity
The nitric oxide scavenging activity of the leaf and root extracts of H. radicata was found to be strong IC50 values of 114.94 µg/mL for chloroform leaf extract and 91.07 µg/mL for methanolic root extract (Table 3). The IC50 values of the extracts were comparable to the standards used.
3.3.3. Total antioxidant activity by ABTS•+ radical cation decolorization assay
The ethyl acetate extracts of root and leaf parts registered better activity (3 675.4 and 2 639 µmol trolox equivalent/g extract respectively) than the other solvents performed. Similarly, the root chloroform extract had more pronounced ABTS•+ radical cation scavenging activity (3 766.5 µmol trolox equivalent/g extract) (Table 3).
3.3.4. Reducing power activity
The reducing capacity of leaf and root parts of H. radicata was much varied across the solvents used for extraction (Table 4). The reducing power activity increased with the increase in concentration of extract. In the present study, ethyl acetate leaf extract showed the highest reducing ability (absorbance 2.142 at 700 nm). Chloroform, ethyl acetate and methanol extracts of both leaf and root parts exhibited high reducing power activity than that of the petroleum ether and water extracts. This reducing power activity was comparable to that of the standard antioxidants (Table 5).
Table 4. Reducing capability of various alcoholic and aqueous extracts of leaf and root parts of Hypochaeris radicata.
| Sample concentration (µg/mL) | PE |
CH |
EA |
ME |
WA |
|||||
| L | R | L | R | L | R | L | R | L | R | |
| 300 | 0.726±0.010a | 0.451±0.430d | 0.934±0.230b | 0.813±0.220d | 1.424±0.220c | 1.076±0.430c | 0.722±0.070d | 0.751±0.040b | 0.572±0.020a | 0.503±0.220c |
| 400 | 0.788±0.030b | 0.498±0.030b | 1.053±0.560c | 0.932±0.760e | 1.733±0.010a | 1.397±0.110a | 0.858±0.330e | 0.881±0.160c | 0.607±0.050b | 0.534±0.340d |
| 500 | 0.836±0.020b | 0.506±0.120c | 1.122±0.090b | 1.137±0.020b | 1.805±0.090b | 1.506±0.750d | 1.044±0.030a | 1.068±0.060 | 0.701±0.340c | 0.554±0.020a |
| 600 | 0.850±0.120c | 0.545±0.010a | 1.278±0.050a | 1.248±0.050c | 2.065±0.770e | 1.657±0.220b | 1.120±0.060c | 1.191±0.020a | 0.708±0.450c | 0.599±0.120b |
| 700 | 0.870±0.130c | 0.578±0.030b | 1.296±0.070a | 1.361±0.010a | 2.142±0.650d | 1.340±0.410c | 1.267±0.040b | 1.305±0.030a | 0.786±0.350b | 0.637±0.110b |
Values were performed in triplicates and represented as mean±SD. Mean values followed by different superscript in a column are significantly different (P<0.05). PE: petroleum ether, CH: chloroform, EA: ethyl acetate, ME: methanol, WA: water, L: leaf, R: root.
Table 5. Reducing power activity of standard antioxidants.
| Sample concentration (µg/mL) | RU | QE | BHA | BHT |
| 20 | 0.238±0.003a | 0.359±0.012a | 0.236±0.010b | 0.224±0.001a |
| 40 | 0.350±0.013c | 0.632±0.023c | 0.396±0.017c | 0.368±0.009b |
| 60 | 0.408±0.013c | 0.718±0.019b | 0.496±0.028d | 0.478±0.013c |
| 80 | 0.476±0.006b | 0.833±0.044e | 0.593±0.008a | 0.517±0.017d |
| 100 | 0.557±0.014c | 0.973±0.029d | 0.644±0.011b | 0.584±0.012c |
Values were performed in triplicates and represented as mean±SD. Mean values followed by different superscript in a column are significantly different (P<0.05). RU: rutin, QE: quercetin, BHA: butylated hydroxylanisole, BHT: butylated hydroxyltoluene.
3.3.5. Inhibition of β-carotene bleaching assay
Figure 1 depicts the inhibition of β-carotene bleached by the leaf and root parts of various alcoholic and aqueous extracts of H. radicata. In this β-carotene bleaching system, the highest antioxidant activity was exhibited by leaf methanol extract (90.43%) and root water extracts (90.34%). These values were comparably higher than that of the natural and synthetic antioxidants used.
Figure 1. Inhibition of β-carotene bleaching assay for various alcoholic and aqueous extracts of leaf and root parts of H. radicata.
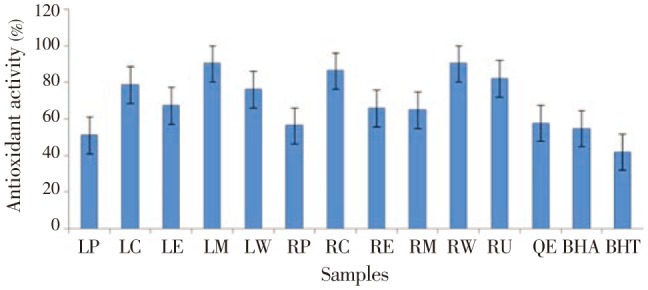
Values were presented as the mean±SD of three independent experiments. Vertical bars labeled with different letters are significantly different (P<0.05).LP: leaf-petroleum ether, LC: leaf-chloroform, LE: leaf-ethyl acetate, LM: leaf-methanol, LW: leaf-water, RP: root-petroleum ether, RC: root-chloroform, RE: root-ethyl acetate, RM: root-methanol, RW: root-water. RU: rutin, QE: quercetin, BHA: butylated hydroxylanisole, BHT: butylated hydroxyltoluene.
3.3.6. Antihemolytic activity
Figure 2 shows the antihemolytic activity of various alcoholic and aqueous extracts of H. radicata. The highest antihemolytic activity was found in water root extract (68.45%). It is nearly equal to that of the rutin and quercetin of natural antioxidant standards. The anithemolytic activity of petroleum ether root extract and methanolic leaf extract was significantly higher (46.75% and 34.98% respectively) than the other solvent extracts.
Figure 2. Antihemolytic activity of various alcoholic and aqueous extracts of leaf and root parts of H. radicata.
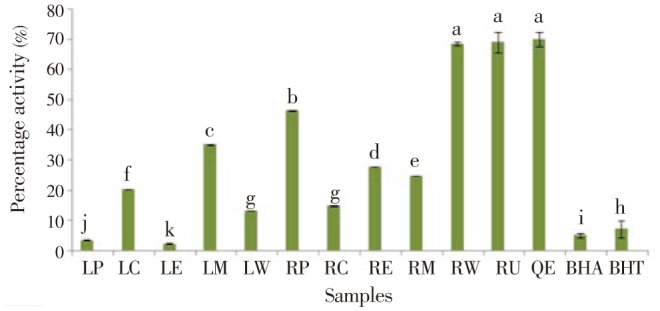
Values were presented as the mean±SD of three independent experiments. Vertical bars labeled with different letters are significantly different (P<0.05).LP: leaf-petroleum ether, LC: leaf-chloroform, LE: leaf-ethyl acetate, LM: leaf-methanol, LW: leaf-water, RP: root-petroleum ether, RC: root-chloroform, RE: root-ethyl acetate, RM: root-methanol, RW: root-water. RU: rutin, QE: quercetin, BHA: butylated hydroxylanisole, BHT: butylated hydroxyltoluene.
4. Discussion
Preliminary qualitative phytochemical analysis made for the leaf and root parts of H. radicata revealed the presence of alkaloids, cardiac glycosides, flavonoids, glycosides, phenols, resins, saponins, steroids, tannins, terpenoids and triterpenoids. These secondary metabolites are reported to have many biological and therapeutic properties[29]–[32], so this species is expected to have many medicinal uses. The extraction yield calculated for petroleum ether, chloroform, ethyl acetate, methanol and water extracts of both parts of H. radicata showed that methanol extract registered higher percentage of yield. It may be due to high polarity of methanolic solvent which can draw high variety of plant constituents than the other solvents did[33].
Generally, majority of the secondary metabolites studied and ascorbic acid in leaf and root parts of H. radicata have present with higher amount in methanolic extract than that of the other alcoholic and aqueous solvents. However, flavonoids and saponins were rich in ethyl acetate extracts. It is explained that the polarity level and species nature are playing major role in extracting the secondary metabolites[34].
The biological property, antioxidant activity was determined to be effective through various assays for the leaf and root parts of H. radicata.
The concentration of antioxidant compounds are needed to decrease the DPPH• radicals by IC50, which is a parameter widely used to estimate the antioxidant activity[35]. DPPH• is a stable free radical and that can accept an electron or hydrogen radical to become a stable diamagnetic molecule[36]. A freshly prepared DPPH• solution is of deep purple colour with absorption maximum at 517 nm and in the presence of antioxidant, this colour disappears due to quenching of DPPH• free radicals and converting them into a colourless product 2,2-dipenyl-1-picryl hydrazine[37]. Hence DPPH• is usually used as a substance to evaluate the antioxidant activity[38]. In the present study, the extracts had significant scavenging effects on the DPPH• radical which was increasing with the increase in the concentration of the sample from 50-250 µg/mL. Similar trend of DPPH• free radical scavenging activity was already documented well[29],[39]. Among the various solvent extracts tested, the ethyl acetate extracts of leaf and root parts of H. radicata exhibited higher DPPH• radical scavenging activity. This might be due to the presence of higher flavonoids content, the most required biocompounds for scavenging activity in this extracts. Next to ethyl acetate, the methanolic extract of both parts of H. radicata showed effective DPPH• scavenging activity which may be attributed to the presence of greater amounts of phenolics and tannins in this solvent extracts.
Nitric oxide (NO•) is an important chemical mediator generated by endothelial cells, macrophages, neurons, etc. and is involved in the regulation of various physiological process[40]. Nitric oxides formed during their reduction with oxygen or with superoxides such as NO2, N2O4, N3O4 are very reactive. The excess concentration of NO• will cause diseases in human beings which can alter the structural and functional behavior of many cellular components. Nitrite ions react with Griess reagent and form a purple azo dye. The decrease in the formation of purple azo dye reflects the presence of scavengers in the test compounds. The methanolic extracts of leaf and root parts of H. radicata are found to be an efficient scavenger of nitric oxide radicals in sodium nitroprusside. It clearly indicates that this plant extract has a noticeable effect as scavenging nitric oxide radicals. Among the samples of two parts evaluated, the methanolic root extract of H. radicata exhibited more activity, which may be due to the presence of water-soluble compounds like phenolics with potent free radical-scavenging effects.
The reduction of the 2,2′azinobis [3-ethylbenzothiazoline sulphonate] radical cation [ABTS•+] has been widely used to measure the antioxidant capacity of natural extracts[41]. ABTS•+, a stable free radical with the characteristic absorbance at 734 nm, was used to study the radicals scavenging effect of extracts of H. radicata. The presence of bioactive chemical compounds in the tested extracts that inhibit the potassium persulfate activity may reduce the production of ABTS•+. The study revealed that the chloroform and ethyl acetate extracts of root part of H. radicata exhibited higher ABTS•+ radical scavenging activity. On the other hand, in the leaf extract, the activity was decreasing in the order of ethyl acetate>water>chloroform>methanol>petroleum ether. This assay was calibrated with the water soluble trolox.
The reducing power of the extracts may serve as a significant indicator of its potential antioxidant activity[42]. In this assay, the yellow colour of the test solution changes to various shades of green and blue, depending on the reducing power of test specimen. The presence of reductones, which have been shown to be an impart antioxidant action by breaking the free radical chain by donating a hydrogen atom. The presence of reductones (i.e. antioxidants) in the sample extracts might cause the reduction of Fe3+/Ferric cyanide complex to ferrous form which can be monitored by spectrophotometrically at 700 nm[43]. The reducing power increased with the increase in the extract concentrations. This may be served as significant indicator of its potential antioxidant activity. Hence, this study presumed that the ethyl acetate extracts of leaf and root parts of H. radicata may have high amount of reductones and hence the antioxidant property.
In the β-carotene bleaching assay, linoleic acid produces hydroperoxides as free radicals during incubation at 50 °C. The presence of antioxidants in the extract will minimize the oxidation of β-carotene by hydroperoxides. Hydroperoxides formed in this system will be neutralized by the antioxidants present in the extracts[44]. Therefore, the degradation of β-carotene linolate depends on the antioxidant activity of the extracts. In the present study, significant positive correlation between degradation rate of β-carotene linolate and phenolics, flavonoids and tannins has obtained which exhibited the highest antioxidant activity due to these secondary metabolites. Among the samples evaluated, leaf methanol and root water extracts have more pronounced activity (90.43% for leaf methanol and 90.34% for root water extracts). Further, the activity of all the extracts was comparable to that of natural and synthetic antioxidant standards used.
Erythrocytes are considered as prime targets for free radical attack owing to the presence of both membrane concentration of polyunsaturated fatty acids and the O2 transport associated with redox active haemoglobin molecules, which are potent promoters of reactive O2 species. H2O2 mediated oxidation of lipid in cow blood erythrocytes membrane induces membrane damage and subsequently it leads to hemolysis. It is a peroxyl radical initiator that generates free radicals by its thermal decomposition and will attack erythrocytes to induce the chain oxidation of lipid and protein, disturbing the membrane organization and eventually leading to hemolysis. The root water extracts of H. radicata possessed effective antihemolytic activity (68.45%). The more pronounced antioxidant activity of high polar solvent extracts may be involved in the activation of lipid free radicals or prevented decomposition of hydroperoxides into free radicals. This high activity is due to the presence of secondary metabolites in this plant extracts[45].
On the basis of response in terms of scavenging radicals, reducing power, reduction of radical cation, β-carotene bleaching and antihemolytic, it is concluded that the species, H. radicata possessed potential antioxidant activity. It may be due to the presence of respective secondary metabolites such as phenolics, flavonoids, tannins etc. in the plant species. The strong correlation between the contents of total phenolics, flavonoids and tannins and radical scavenging activity indicates that these phytochemical constituents are major contributors to the antioxidant potential of this species. Therefore, this species can be attempted to derive the drugs of antioxidant properties. However, further studies by in vivo models are still needed to confirm this property.
Acknowledgments
The authors are acknowledging Dr. M. Aruchami Research Foundation, Coimbatore for financial support to carry out the work (Grant No. ARF/RA-2012/018 dt. 12.02.2012).
Comments
Background
Antioxidants are involved in the prevention of cellular damage, which is common pathway for cancer, aging and variety of diseases. The scientific community has begun to interest in the identification, recovery and enhanced performance of natural antioxidant principles from plant sources. The purpose of this present study is also guide to the invention of new antioxidant sources from H. radicata.
Research frontiers
The present research work clearly revealed the preliminary phytochemical investigation on H. radicata, an invasive plant of Nilgiris and its antioxidant, radical scavenging and reducing potency of aqueous and different solvent extracts. The results are compared with standard drugs in each assay.
Related reports
The antioxidant, radical scavenging and reducing principles and their methods are well elaborated. The results are discussed with adequate references and well justified in discussion part.
Innovations and breakthroughs
H. radicata already have explored for hepatoprotective, antioxidant, anti-inflammatory, antimicrobial and anticancer models. The present study is investigated for radical scavenging and reducing activities first time.
Applications
The selected plant has varieties of phytochemicals such as phenolics, flavonoids and tannins and possesses various activities such as radical scavenging, antioxidant and reducing activities. Therefore, this plant species may be attempted to derive the drugs of antioxidant properties.
Peer review
The research work is highly valuable for identification of newer antioxidant principles. The plant possesses significant antioxidant, radical scavenging and reducing potency and also the plant has reported vitamin C which usually presents in fruits, but the plant sample reported here is something interesting. The more numbers of photochemical observed in methanol extract as usual that is high polarity solvent. The experimental data were analyzed with appropriate manner and well discussed.
Footnotes
Foundation Project: Supported by Dr. M. Aruchami Research Foundation, Coimbatore (Grant No. ARF/RA-2012/018 dt. 12.02.2012).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Halliwell B, Gutteridge J. Free radicals in biology and medicine. 3rd ed. Oxford: Oxford University Press; 1999. pp. 23–27. [Google Scholar]
- 2.Irshad M, Chaudhuri PS. Oxidant - antioxidant system: role and significance in human body. Indian J Exp Biol. 2002;40:1233–1239. [PubMed] [Google Scholar]
- 3.Sen S, Chakaraborty R, Sridhar C, Reddy Y, Biplab D. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int J Pharm Sci Rev Res. 2010;3(1):91–100. [Google Scholar]
- 4.Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med. 2010;10:21. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamagishi S, Matsui T. Nitric oxide, a Janus-faced therapeutic target for diabetic microangiopathy - friend or foe? Pharmacol Res. 2011;64:187–194. doi: 10.1016/j.phrs.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Wu YY, Li W, Xu Y, Jin EH, Tu YY. Evaluation of the antioxidant effects of four main thaeflavin derivative through chemiluminescence and DNA damage analyses. J Zhejiang Univ Sci B. 2011;12(9):744–751. doi: 10.1631/jzus.B1100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anokwuru CP, Esiaba I, Ajibaye O, Adesuyi AO. Polyphenolic content and antioxidant activity of Hibiscus sabdariffa calyx. Res J Med Plant. 2011;5:557–566. [Google Scholar]
- 8.Mbaebe BO, Edeoga HO, Afolayan AJ. Phytochemical analysis and antioxidants activities of aqueous stem bark extract of Schotia latifolia Jacq. Asian Pac J Trop Biomed. 2012;2(2):118–124. doi: 10.1016/S2221-1691(11)60204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meenakshi S, Umayaparvathi S, Arumugam M, Balasubramanian T. In vitro antioxidant properties of FTIR analysis of two sea weeds of Gulf of Mannar. Asian Pac J Trop Biomed. 2011;1(Suppl 1):S66–S70. [Google Scholar]
- 10.Upadhyay NK, Kumar MS, Gupta A. Antioxidant, cytoprotective and antibacterial effect of sea buckthorn (Hippohae rhamnoides L.) leaves. Food Chem Toxicol. 2010;48:3443–3448. doi: 10.1016/j.fct.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Jamuna S, Paulsamy S, Karthika K. Screening of in vitro antioxidant activity of methanolic leaf and root extracts of Hypochaeris radicata L. (Asteraceae) J Appl Pharm Sci. 2012;2(7):149–154. [Google Scholar]
- 12.Jamuna S, Paulsamy S, Karthika K. In-vitro antibacterial activity of leaf and root extracts of Hypochaeris radicata L. (Asteraceae) - a medicinal plant species inhabiting the high hills of Nilgiris, the Western Ghats. Int J Pharm Pharm Sci. 2013;5(1):175–178. [Google Scholar]
- 13.Jamuna S, Paulsamy S, Karthika K. In vitro antifungal activity of leaf and root extracts of the medicinal plant, Hypochaeris radicata L. Int J Pharm Pharm Sci. 2013;5(3):758–761. [Google Scholar]
- 14.Pullaiah T. Encyclopedia of world medicinal plants. New Delhi: Regency publication; 2006. pp. 1–525. [Google Scholar]
- 15.Elgorashi EE, Van Staden J. Pharmacological screening of six Amaryllidaceae species. J Ethnopharmacol. 2004;90:27–32. doi: 10.1016/j.jep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Trease G, Evans SM. Pharmacognosy. 15th ed. London: Bailer Tindal; 2002. pp. 23–67. [Google Scholar]
- 17.Harborne JB. Phytochemical methods - a guide to modern techniques of plant analysis. 2nd ed. London: Chapman and Hall; 1984. pp. 4–16. [Google Scholar]
- 18.Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of the crude extracts of some homeostatic plants in Edo and Delta States of Nigeria. Glob J Pure Appl Sci. 2001;8(2):203–208. [Google Scholar]
- 19.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51(8):2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 20.Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- 21.Siddhuraju P, Manian S. The antioxidant activity and free radical scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds. Food Chem. 2007;105(3):950–958. [Google Scholar]
- 22.Makkar HP, Siddhuraju P, Becker K. Methods in molecular biology: plant secondary metabolites. Totowa: Human Press; 2007. pp. 93–100. [DOI] [PubMed] [Google Scholar]
- 23.Klein BP, Perry AK. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J Food Sci. 1982;47:941–945. [Google Scholar]
- 24.Blois MS. Antioxidant determination by the use of a stable free radical nature. Nature. 1958;181:1199–1200. [Google Scholar]
- 25.Sreejayan N, Rao MN. Nitric oxide scavenging activity by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 26.Yildirim A, Mani A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49(8):4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 27.Taga MS, Miller EE, Pratt DE. Chia seeds as a source of natural lipid antioxidants. J Am Oil Chem Soc. 1984;61:928–931. [Google Scholar]
- 28.Naim M, Gestener B, Bondi A, Birk Y. Antioxidative and antihemolytic activities of soyabean isoflavones. J Agric Food Chem. 1976;24:1174–1177. doi: 10.1021/jf60208a029. [DOI] [PubMed] [Google Scholar]
- 29.Vishnu R, Nisha R, Jamuna S, Paulsamy S. Quantification of total phenolics and flavonoids and evaluation of in vitro antioxidant properties of methanolic leaf extract of Tarenna asiatica - an endemic medicinal plant species of Maruthamali hills, Western Ghats, Tami Nadu. J Res Plant Sci. 2013;2(2):196–204. [Google Scholar]
- 30.Benedec D, Vlase L, Oniga I, Mot AC, Damian G, Hanganu D, et al. Polyphenolic composition, antioxidant and antibacterial activities for two Romanian subspecies of Achillea distans Waldst. et Kit. ex Wild. Molecules. 2013;18:8725–8739. doi: 10.3390/molecules18088725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charalampos P, Konstantina L, Olga KM, Panagiotis Z, Vassileia JS. Antioxidant capacity of selected plant extracts and their essential oils. Antioxidants. 2013;2:11–22. doi: 10.3390/antiox2010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narender PD, Ganga R, Sambasiva E, Mallikarjuna T, Praneeth VS. Quantification of phytochemical constituents and in vitro antioxidant activity of Mesua ferrea leaves. Asian Pac J Trop Biomed. 2012;2(Suppl 2):S539–S542. [Google Scholar]
- 33.Paulsamy S, Jeeshna MV. Preliminary phytochemistry and antimicrobial studies of an endangered medicinal herb Exacum bicolor Roxb. Res J Pharm Biol Chem Sci. 2011;2(4):447–457. [Google Scholar]
- 34.Ghasemzadeh A, Jaafar H, Rahmat A. Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (Zingiber officinale Roscoe.) extracts. J Med Plant Res. 2011;5(7):1147–1154. [Google Scholar]
- 35.Krishna TM, Shiva D, Keerthi D, Nandini A, Aswaq A, Reddy T, et al. In vitro evaluation of antioxidant properties of Cucumis melo L. extracts of leaves and fruit. Int J Pharm Bio Sci. 2013;4(1):705–712. [Google Scholar]
- 36.Bijaya LM, Bikash B. Antioxidant capacity and phenolics content of some Nepalese medicinal plants. Am J Plant Sci. 2013;4:1660–1665. [Google Scholar]
- 37.Sumathy R, Sankaranarayanan S, Bama P, Ramachandran J, Vijayalakshmi M, Deecaraman M. Antioxidant and antihemolytic activity of flavonoids extract from fruit peel of Punica granatum. Asian J Pharm Clin Res. 2013;6(2):211–214. [Google Scholar]
- 38.Shah R, Kathad H, Sheth R, Sheth N. In vitro antioxidant activity of roots of Tephrosia purpurea Linn. Int J Pharm Pharm Sci. 2010;2(3):30–33. [Google Scholar]
- 39.Thambiraj J, Paulsamy S. In vitro antioxidant potential of methanol extract of the medicinal plant, Acacia caesia (L.) Wild. Asian Pac J Trop Biomed. 2012;2(Suppl 2):S732–S736. [Google Scholar]
- 40.Saurabh G, Kumar M, Duraiswamy B, Mahavir C, Atika C. In vitro antioxidant and free radical scavenging activities of Ocimum sanctum. World J Pharm Res. 2012;1(1):78–94. [Google Scholar]
- 41.Ali M, Mehdi AT, Nooshim M, Reza GS. Antioxidant, antimicrobial and antimutogenic potential of 4 Iranian medicinal plants. Life Sci J. 2013;10(7):1085–1091. [Google Scholar]
- 42.Oliveira I, Sousa A, Ferreira I, Bento A, Estevinho L, Pereira JA. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol. 2008;46:2326–2331. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Nair VD, Paneerselvam R, Gopi R. Studies on methanolic extract of Rawolfia species from Southern Western Ghats of India - In vitro antioxidant properties, characterization of nutrients and phytochemicals. Ind Crop Prod. 2012;39:17–25. [Google Scholar]
- 44.Govindappa M, Bharath N, Shruthi HB, Sadananda TS, Sharanappa P. Antimicrobial, antioxidant and in vitro anti-inflammatory activity and phytochemical screening of Crotalaria pallida Aiton. Afr J Pharm Pharmacol. 2011;5(21):2359–2371. [Google Scholar]
- 45.Karuppusamy S, Muthuraja G, Rajasekaran KM. Antioxidant activity of selected lesser known edible fruits from Western Ghats of India. Indian J Nat Prod Resour. 2011;2(2):174–178. [Google Scholar]


