Abstract
Objective
To evaluate the effects of berberine (BBR) on the liver phosphatidate phosphohydrolase (PAP) and plasma lipids in rats fed on high lipogenic and normal diet.
Methods
Forty rats were randomly divided into 5 groups. Group I (control) received standard diet. Group II received standard diet plus 90 mg/kg BBR and Groups IV received lipogenic diet (containing sunflower oil, cholesterol and ethanol) without treatment. Groups III and V received lipogenic diet plus 90 mg/kg BBR and 30 mg/kg gemfibrozil, respectively. On Day 60 of the experiment, blood samples were collected and PAP, total cholesterol, triglyceride, low density lipoprotein cholesterol, high density lipoprotein cholesterol, very low density lipoprotein, malondialdehyde, plasma antioxidant, and liver histopathology assessments were conducted.
Results
PAP, plasma triglyceride, total cholesterol, very low density lipoprotein, and malondialdehyde levels decreased significantly (P<0.05) in Group III compared to Group IV (24.94%, 36.11%, 21.18%, 36.86% and 19.59%, respectively). The liver triglyceride and cholesterol in Groups III and V had a remarkable decrease (P<0.001) compared with Group IV (24.94% and 49.13%, respectively). There was a significant reduction (P<0.05) in atherogenic index in Groups III compared with Group IV.
Conclusions
These results clearly suggested that BBR could be effective in reducing liver PAP, lipid abnormality, liver triglyceride and lateral side effects of hyperlipidemia.
Keywords: Atherogenic index, Flavonoid, Fatty liver, Lipid profile, Lipogenic diet, Oxidative stress
1. Introduction
Berberine (BBR), an isoquinoline plant alkaloid, is a natural compound present in many herbs. It has been demonstrated that berberine has multiple pharmacological actions such as being active against hypertension, tumors, bacteria, inflammation, hyperlipidemia, and many other illnesses[1].
Phosphatidate phosphohydrolase (PAP, EC 3.1.3.4) catalyzes the dephosphorylation of phosphatidic acid to form inorganic phosphate and 1,2 diacylglycerol productions[2],[3]. In liver tissue, PAP is an important key regulatory enzyme in lipid metabolism pathways especially triacylglycerol and glycerophospholipids. The produced diacylglycerol from phosphatidic acid serves as a precursor for the synthesis of triglyceride (TG) and other phospholipids[3]. In organisms, TG is a critical storage molecule for periods of food deprivation. In human, the regulation of TG storage is very important because both excessive and inadequate fat storage are accompanied with dyslipidemia, insulin resistance, and diabetes[4]–[6]. Therefore, any alteration in PAP activity can influence lipid metabolism in the body.
A large body of evidence indicates that the incidence of hyperlipidemia and its complications are growing in the world. Hyperlipidemia develops the risk of many diseases such as coronary heart diseases, atherosclerosis, hypertension, and type 2 diabetes[7]. The antihyperlipidemic drugs such as fibrates, nicotinic acid, and bile acid sequestrants were used for many years. Nevertheless, the side effects of drugs led to the development of new oral antihyperlipidemic drugs including statins (HMG-CoA reductase inhibitors). Although the adverse reactions of statins are relatively low, they can result in rhabdomyolysis condition[8]. Therefore, the research for natural compounds with antihyperlipidemic properties and with less or no adverse reactions, especially herbal medicine, is warranted. These medicinal plants contain biological active substances including antioxidant, hypoglycemic and hypolipidemic compounds. Numerous reports have shown that BBR reduces hyperlipidemia[9]–[11]. Also, it has been reported that BBR stimulated AMP-activated protein kinase (AMPK) activity and fatty acid oxidation in HepG2 line cells and lowered hyperlipidemia in hamsters fed a high-fat diet[9]. In addition, BBR reduces blood cholesterol by stabilizing hepatic low density lipoprotein receptor in an extracellular signal-regulated kinase manner[12]. In spite of these findings, most of the previous studies on BBR focused less on the enzymes involving in TG metabolism, especially PAP enzyme, in details. Therefore, the aim of this study was to determine the effects of dietary supplementation with BBR on the liver PAP, plasma lipids, liver TG content, plasma antioxidant, and malondialdehyde levels in rats fed on high lipogenic and normal diet.
2. Materials and methods
2.1. Chemicals
Phosphatidic acid (sodium salts), dithiothreitol, phenylmethylsulfonyl fluoride, and 2, 4, 6-Tripyridyl-s-Triazine and berberine chloride were purchased from Sigma (St. Louis, MO). Tris-HCl, sodium acetate, bovine serum albumin, ferrous sulphate (FeSO4.7H2O), ferric chloride (FeCl3.6H2O), ethylenediaminetetraacetic acid (EDTA) and ethylene glycol-bis (beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid were obtained from Merck (Darmstadt, Germany). All other chemicals were of the highest quality available.
2.2. Experimental animals and diets
Male Wistar rats (150-200 g) were housed in the colony room with a 12-h light and dark cycle at (21±2) °C and had free access to water and food. Rats were randomly assigned to one of 5 diet groups (n=8) as below:
Group I, normal control rats, received standard pellet diet (Pars Dam, Tehran, Iran). This group received 0.5 mL distillated water by gavage to produce injection shock similar to other groups. Group II, animal rats were fed with a standard pellet diet plus 90 mg/kg body weight/day BBR by gavage. Groups III, IV, and V, the rats were fed with a lipogenic diet containing standard pellet diet supplemented with 0.5% (w/w) cholic acid, 20% (w/w) sunflower oil and 2% (w/w) cholesterol for at least two weeks to produce hyperlipidemia. Additionally, these groups drank water containing 3% (v/v) ethanol[13]. After 2 weeks of lipogenic diet feeding, Group III orally received 90 mg/kg body weight/day BBR accompanied with lipogenic diet for 45 days by gavage. The rats in Group IV were maintained on lipogenic diet (hyperlipidemic control group) without treatment throughout the experiment and received 0.5 mL distillated water by gavage to produce equal injection shock similar to other groups. The rats in Group V after 2 weeks were treated by 30 mg/kg body weight/day gemfibrozil through gavage[14]. On Day 60 of the experiment, fasted animals were anesthetized with chloroform. Blood samples were collected into test tubes containing EDTA through cardiac puncture. The plasma samples were separated by low speed centrifugation (2000 r/min) for 10 min and were stored at -80 °C until they were analyzed. All animal procedures were performed with regard to Iranian Animal Ethics Society and local university rules.
2.3. Lipid analysis
Plasma levels of TG, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), glutamic oxaloacetic transaminase, and glutamic pyruvic transaminase were calculated by enzymatic method (Pars Azmun kit, Tehran, Iran) by using autoanalyzer (BT 3000, Biotecnica Instruments, SpA Rome, Italy). Very low density lipoprotein cholesterol (VLDL-C) concentrations were determined with Fridewald formula[15]. Finally, liver TG and cholesterol were extracted from liver tissue by the method of Folch, et al.[16] and determined by enzymatic method (Pars Azmun kit, Tehran, Iran).
2.4. Preparation of rat liver homogenate
The rat liver was removed and a piece of the liver was homogenized in 4 volumes of ice-cold homogenate buffer containing 50 mmol/L of Tris-HCl (pH 7.4) buffer, 0.25 mol/L sucrose, 0.1 mmol/L EDTA, and 1 mmol/L Phenylmethanesulfonyl fluoride at 8000 r/min at 4 °C for 6 min[13]. The homogenate was then initially centrifuged at 4500 r/min at 4 °C for 10 min, resulting in a nuclear pellet and then, the supernatant was kept for the enzyme assay.
2.4.1. PAP activity assay
PAP activity was determined by the method of Yanagita, et al.[17] with slight modification. In brief, PAP activity was measured in the assay buffer containing 50 mmol/L Tris-HCl (pH 7.4), 1 mmol/L of phosphatidate, 1.25 mmol/L magnesium chloride, and 50 to 100 µg of liver enzyme solution in a final assay volume of 0.2 mL. The mixture was incubated for 15 min at 37 °C. The reaction was stopped by the addition of 0.8 mL of a solution containing 0.13% sodium dodecyl sulfate, 1.25% ascorbic acid, 0.32% ammonium molybdate-4H2O, and 380 mmol/L H2SO4. Then, phosphomolybdate color due to liberated inorganic phosphate was developed at 45 °C for 20 min, and the absorbance was measured at 820 nm. Non-enzymatic phosphate release was determined by inactivating the enzymes through boiling for 1 min without substrate. The enzyme activity was expressed as phosphate nanomoles in one minute per milligram of protein. All assays were linear in relation to the incubation time and the protein concentrations used in them. One unit (U) of PAP activity was defined as the amount of enzyme that catalyzes the release of 1 µmole of phosphate per min under the standard assay conditions. Specific activity was considered as units per mg protein. Protein concentration was measured by the Bradford method with bovine serum albumin as the standard[18].
2.5. Measurement of malondialdehyde
The plasma malondialdehyde level was measured by high performance liquid chromatography method with the thiobarbituric acid described by Agarwal and Chase[19]. The measurements were done in triplicates and the results were expressed in µmol/L. Malondialdehyde standards were prepared from 1, 1, 3, 3-tetraethoxypropane.
2.6. Ferric reducing/antioxidant (FRAP) power assay
FRAP of each sample was determined according to the procedure described by Benzie and Strain[20]. In this method, the complex between Fe2+ and 2, 4, 6-tri(2-pyridyl)-1, 3, 5-triazine gives a blue color with absorbance at 593 nm. FeSO4 was used as a standard of FRAP assay at a concentration range between 100 to 1 000 µmol/L.
2.7. Histopathological studies
Immediately after sacrificing rats, a piece of each liver was fixed in 10% formalin. After processing [dehydrating in gradual ethanol (50 to 100%) and clearing in xylene], the livers were embedded in paraffin wax and sectioned into 5 µm thickness using microtome. The sections were stained with hematoxylin and eosin for photomicroscopic observation, especially fatty droplets[21].
2.8. Statistical analysis
All data were expressed as mean±SD. They were analyzed by SPSS software (version 17, Chicago, IL). For statistical analysis of the data, group means were compared by One-way analysis of variance followed by Tukey's post hoc test for multiple comparison. A significant difference was considered at the level of P<0.05.
3. Results
3.1. Effects of BBR on the liver PAP activity and the liver TG and cholesterol
Table 1 shows the effects of BBR on the liver PAP activity, the liver TG and cholesterol. Groups II, III, IV, and V had a conspicuous decrease (P<0.01) in the liver PAP activity compared to Group I (19.77%, 19.70%, 25.63% and 19.90%, respectively). On the other hand, Group IV showed a remarkable reduction in the liver PAP activity compared to Groups III and V (7.37% and 8.32%, respectively). Also, no considerable difference (P>0.05) was seen between Groups II and III.
Table 1. The effects of BBR on liver PAP, triglyceride, and cholesterol content in experimental groups.
| Groups | PAP activity (nmol phosphate/min/mg protein) | Liver TG (mg/g wet tissue) | Liver cholesterol (mg/g wet tissue) |
| Group I (control) | 15.02±0.63 | 3.91±0.29 | 2.22±0.35 |
| Group II | 12.05±0.41* | 2.59±0.33* | 1.64±0.22* |
| Group III | 12.06±0.48*† | 8.28±0.41#*† | 4.13±0.25*† |
| Group IV | 11.17±0.65* | 10.86±0.26* | 8.12±0.72* |
| Group V | 12.13±0.46*† | 6.65±0.59#*† | 4.43±0.85*† |
The data were expressed as Mean±SD; n=8 in each group. Group I: Normal diet; Group II: Normal diet supplemented with 90 mg/kg body weight BBR; Group III: Hyperlipidemic rats treated with 90 mg/kg body weight BBR; Group IV: Hyperlipidemic rats without treatment; Group V: Hyperlipidemic rats treated with 30 mg/kg body weight gemfibrozyl. *: P<0.05 compared with the corresponding value for Group I; †: P<0.05 compared with the corresponding value for Group IV; #: P<0.05 compared with the corresponding value for Group V.
Group II showed a salient decrease (P<0.01) in the liver TG and cholesterol compared with Group I (33.75% and 26.13%, respectively). Also, the liver TG and cholesterol in Groups III and V had a significant decrease (P<0.01) compared with Group IV (24.94% and 49.13%, respectively). The liver TG in Group V significantly declined (19.68%, P<0.01) with respect to Group III whereas no significant change (P>0.05) was observed in the liver cholesterol between Groups III and V.
3.2. Effect of BBR on plasma lipid profile
Table 2 shows the atherogenic index and plasma lipoproteins profile of the rats fed with BBR. The levels of plasma TC, TG, LDL-C and VLDL-C in Group IV (consuming oil and cholesterol diet) significantly elevated (P<0.05) compared to Group I (108.82%, 117.53%, 84.94% and 117.93%, respectively). Additionally, the level of plasma TC, TG, and VLDL in Groups III and V showed a noticeable reduction (P<0.01) compared to Group IV. On the other hand, TG in Groups III and IV showed a significant elevation (P<0.05) compared to Group I but TG in Group V had a remarkable reduction compared to Groups III and IV (34.87% and 58.39%, respectively). Group V (treated hyperlipidemic rats with gemfibrozil) was associated with a conspicuous (P<0.05) decrease in plasma TC, TG, and VLDL concentrations compared to Group IV (30.52%, 58.39% and 58.74%, respectively). On the other hand, gemfibrozil administration led to a significant reduction in VLDL and atherogenic index in Group V compared to Group IV. HDL level in Groups III and IV showed a significant reduction (P<0.05) compared to Group I (23.40% and 26.70%, respectively) whereas, HDL level in Group V showed a noticeable increase compared to Group III and IV (72.02% and 38.24%, respectively). HDL-C reduced slightly in Group II compared to Group I (not significant, P>0.05). LDL-C in Groups III, IV, and V were remarkably (P<0.05) higher than Group I (79.07%, 84.94% and 75.86%, respectively). Moreover, no significant difference (P>0.05) was seen for LDL-C between Groups III, IV, and V. There was a significant reduction (P<0.05) in atherogenic index in Groups III compared with Group IV. Moreover, no salient change was seen in atherogenic index between Groups I and II. On the other hand, atherogenic index in Group V showed a significant reduction (P<0.05) compared to Groups IV and III.
Table 2. Comparison the effects of BBR on plasma TC, TG, LDL-C, HDL-C, VLDL-C level, and atherogenic index in hyperlipidemic rats.
| Groups | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) | TC/HDL-C | LDL/HDL-C |
| Group I | 59.62±4.50 | 60.62±10.58 | 40.82±2.54 | 29.29±2.12 | 12.10±2.12 | 1.48±0.15 | 0.64±0.08 |
| Group II | 52.37±6.02 | 53.87±5.93 | 39.82±2.48 | 31.16±2.36 | 10.77±0.58 | 1.45±0.13 | 0.78±0.07 |
| Group III | 98.12±10.26*† | 84.25±7.41#*† | 31.17±2.16#* | 52.45±7.42* | 16.85±1.46#*† | 3.32±0.22#*† | 1.61±0.21*† |
| Group IV | 124.50±10. 73#* | 131.88±9. 20#* | 29.92±2.45#* | 54.17±8.75* | 26.37±1.84*# | 4.10±0.32*# | 1.92±0.23*# |
| Group V | 86.50±12.88 | 54.87±4.91 | 53.62±5.42* | 51.51±6.21* | 10.80±1.23 | 1.61±0.10 | 0.97±0.12* |
The data were expressed as Mean±SD; n=8 in each group. Group I: Normal diet; Group II: Normal diet supplemented with 90 mg/kg body weight BBR; Group III: Hyperlipidemic rats treated with 90 mg/kg body weight BBR; Group IV: Hyperlipidemic rats without treatment; Group V: Hyperlipidemic rats treated with 30 mg/kg body weight gemfibrozyl. *: P<0.05 compared with the corresponding value for Group I; †: P<0.05 compared with the corresponding value for Group IV; #: P<0.05 compared with the corresponding value for Group V.
3.3. Effect of BBR on the plasma level of malondialdehyde
Figure 1 shows that after the consumption of lipogenic diet, a significant (P<0.05) elevation in plasma malondialdehyde level was observed in Group IV (the group consumed oil and cholesterol diet) when compared with other groups. On the other hand, in Group II the consumption of BBR led to an important (P<0.05) reduction of plasma malondialdehyde (8.23%) in comparison with Group I. Also, a significant reduction (P<0.01) of plasma malondialdehyde was seen in Group III, fed with a lipogenic diet supplemented with BBR, as compared with Groups IV and V (19.56% and 11.93%, respectively).
Figure 1. Plasma malondialdehyde level in experimental groups.
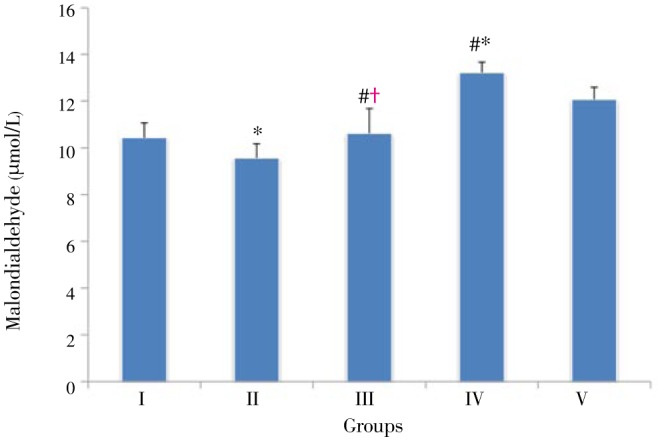
The data were expressed as Mean±SD; n=8 in each group. Group I: Normal diet; Group II: Normal diet supplemented with 90 mg/kg body weight BBR; Group III: Hyperlipidemic rats treated with 90 mg/kg body weight BBR; Group IV: Hyperlipidemic rats without treatment; Group V: Hyperlipidemic rats treated with 30 mg/kg body weight gemfibrozyl. *: P<0.05 compared with the corresponding value for Group I; †: P<0.05 compared with the corresponding value for Group IV; #: P<0.05 compared with the corresponding value for Group V.
3.4. Effect of BBR on the plasma level of antioxidant power
Figure 2 shows the plasma level of antioxidant power in each experimental animal group. BBR supplementation resulted in a significant elevation (P<0.01) of FRAP in Group II as compared to the other Groups. FRAP in Group IV showed a noticeable reduction (P<0.01) compared to Group I (42.65%). There was a significant (P<0.01) elevation in plasma level antioxidant power in Groups III and V compared to Group IV (68.96% and 71.27%, respectively). No significant change was observed in plasma level antioxidant power between Group III and Group V (P>0.05).
Figure 2. Plasma FRAP in experimental groups.
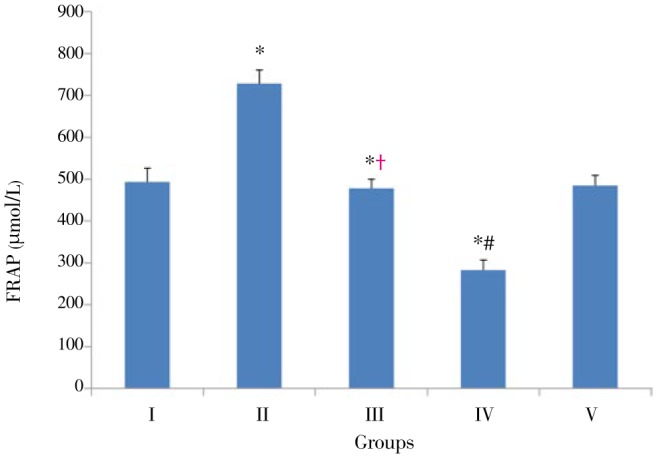
The data were expressed as Mean±SD; n=8 in each group. Group I: Normal diet; Group II: Normal diet supplemented with 90 mg/kg body weight BBR; Group III: Hyperlipidemic rats treated with 90 mg/kg body weight BBR; Group IV: Hyperlipidemic rats without treatment; Group V: Hyperlipidemic rats treated with 30 mg/kg body weight gemfibrozyl. *: P<0.05 compared with the corresponding value for Group I; †: P<0.05 compared with the corresponding value for Group IV; #: P<0.05 compared with the corresponding value for Group V.
3.5. Histopathological findings
The histological findings of liver sections are shown in Figure 3. The photomicrograph of the livers in control group and rats treated with normal diet plus BBR indicates a normal hepatic structure with well-preserved cytoplasm and prominent nucleus (Figures 3A and 3B). The histological liver in hyperlipidemic rats without treatment (Figure 3D) shows an accumulation of numerous spherical vacuoles of fat droplets, whereas Figure 3C shows a remarkable reduction of hepatocyte fat droplets in hyperlipidemic rats treated with BBR. Also, Figure 3F shows positive effects of silymarin on the hepatic histological structure of the liver in hyperlipidemic rats treated with silymarine. The livers of all treated hyperlipidemic groups with BBR and silymarine were improved and appeared with less fatty infiltration of hepatocytes as compared with untreated group.
Figure 3. Effects of high-fat feeding and berberine traetment on liver histology of the experimental groups.
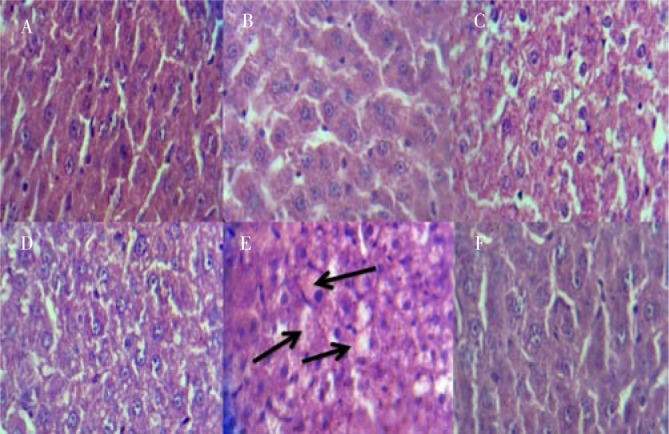
A: Control group; B: Rats treated with normal diet and 90 mg/kg body weight BBR; C: Hyperlipidemic rats treated with 90 mg/kg body weight BBR; D and E: Hyperlipidemic rats without treatment (arrows show lipid droplets); F: Hyperlipidemic rats treated with 30 mg/kg body weight gemfibrozyl.
4. Discussion
Nowadays, hyperlipidemia, especially hypercholesterolemia is correlated with a risk for the incidence of coronary heart disease and fatty liver[22]. Many new synthetic oral antihyperlipidemic drugs such as fibrates and bile acid sequestrants are available but they have adverse side effects such as myopathy, increase in hepatic aminotransferases and rhabdomyolysis condition[7],[23]. In recent years, many studies have focused on the therapeutic potential of flavonoids for treating many important common diseases especially obesity and its complications[13],[24],[25]. In previous studies, BBR is introduced as lipid-lowering therapeutic agent[9],[11],[12]. It is reported that BBR down-regulated the expression of genes involved in lipogenesis and up-regulated those involved in energy expenditure in adipose and muscle tissues such as glycerol kinase and acyl-CoA dehydrogenase[26]. Also, this lipid-lowering effect of BBR has been reported by other investigators which shows that BBR can reduce metabolic disorders[9],[11],[12], including obesity, insulin resistance and hyperlipidemia by stimulating AMPK activity in both in vivo and ex vivo experiments[9],[27]. Nevertheless, to the best of our knowledge, there has been no study investigating the effect of BBR on PAP in hypercholesterolemic animals or humans. In this study, the ingestion of the lipogenic diet led to the elevation of the plasma levels of cholesterol, VLDL, LDL and liver TG in Group IV (hyperlipidemic animals) whereas, in Groups II and III (rats treated with BBR) the plasma levels of cholesterol, VLDL, LDL, liver TG, and liver cholesterol decreased compared to Groups I and IV, respectively. In our study, It is well determined that an additional mechanism for the hypolipidemic effects of BBR is due to reduction of PAP activity. Recently, the effect of garlic has been reported on the liver PAP activity in normal and hyperlipidemic rats[13]. The administration of garlic, as a traditional herbal medicine, led to the decline of liver PAP activity and liver TG level. In present study, BBR supplementation gives rise to the reduction of PAP activity in Group II as opposed to Group I (control). On the other hand, in Groups III and IV (animals fed by lipogenic regime) PAP activity reduced with respect to control group but the liver TG and cholesterol levels elevated in these groups. Previous studies showed that excessive intake of fatty acids led to accumulation of TG in fat tissue and other non-adipose tissues especially liver tissue[28]. Also, fatty acid esters result in the inactivation of PAP by a negative allosteric interaction. Therefore, the complex of PAP-fatty acid (or acyl-CoA esters) leads to the inactivation of PAP[29]. Thus, reduced PAP activity in groups fed with high cholesterol diet enriched with fat (Groups III and IV) seems to be caused by the accumulation of TG, fatty acids or acyl-CoA esters in the liver. Nevertheless, the reduction of PAP activity in Groups III and IV (animals fed by lipogenic regime) can probably act, at least in part, as a defense mechanism of liver for reducing the production of endogenous liver TG. Thereby, serum and liver TG will decline and probably decrease the risk of liver injury such as fatty liver and cirrhosis. Also, results in this study showed that elevated liver TG and cholesterol in Group III had significantly reduction compared to Group IV through supplementation with BBR. Overall, BBR can be useful in lowering the liver content of TG and cholesterol and treating fatty liver in hyperlipidemic regime. Also, another positive effect of reduced liver PAP activity by BBR is reduction in atherogenic index. Therefore, consumption of BBR can be useful to reduce the risk of cardiovascular diseases, fatty liver, and atherosclerosis. On the other hand, both gemfibrozil and BBR significantly decrease TG, TC, VLDL, and atherogenic index without adversely affecting LDL-cholesterol. From this perspective they are effective in the management of hyperlipidemia but BBR has less effect than gemfibrozil on VLDL, TG, and TC. This different effects between gemfibrozil and BBR, at least in part in this study, may be due to their doses.
We did not assess the effects of BBR on other enzymes involving in the lipid metabolism, especially glucose-6-phosphate dehydrogenase (G6PDH) and malic enzyme. These enzymes generate NADPH employed for fatty acid and cholesterol syntheses. Thus, we suggest that future studies focus on other possible mechanisms of the TG-lowering action of the BBR on PAP, G6PDH, and malic enzyme.
Oxidative stress is accompanied with elevated malondialdehyde, a biomarker of lipid peroxidation, in experimental animals fed on high cholesterol diet[13]. Also, oxidative stress in hypercholesterolemic individuals has been reported[30]. On the other hand, there are data suggesting the ability of phenolics to modulate and positively affect lipoprotein metabolism[31]. Medicinal plants have phenolic materials which are related to their antioxidant activity. BBR is an isoquinoline plant alkaloid with potent antioxidant effects. In this research, BBR supplementation led to a significant reduction in plasma lipid peroxidation together with an increase in plasma antioxidant power (Figures 1 and 2). Therefore, on the basis of our results, BBR can probably play, at least in part, an anti-lipid peroxidation role in hyperlipidemic diets.
These results clearly suggested that BBR can be effective in reducing liver PAP activity and liver TG. Also, BBR has blood lipid-reducing, anti-fatty liver, and antioxidant effects in hyperlipidemic regimes, probably due to its polyphenolic properties.
Acknowledgments
This study was funded by Shahrekord University of Medical Sciences (Grant No. 812), Shahrekord, Iran.
Comments
Background
A large body of evidence indicates that the incidence of hyperlipidemia and its complications are growing in the world. Hyperlipidemia with serum-elevated concentrations of cholesterol and triacylglycerol is considered to be the cause of cardiovascular diseases and fatty liver. There is experimental evidence indicating that fruit and vegetables have chemopreventive and flavoring agents which act against various diseases especially hyperlipidemia and its complications. Therefore, a research for natural compounds, especially medicinal plants, with lipid-lowering properties and with less or no adverse effects is warranted. This study was to determine the effects of BBR, an isoquinoline plant alkaloid, on liver phosphatidate phosphohydrolase which is a key regulatory enzyme in lipid metabolism pathways in rats fed on high lipogenic diet.
Research frontiers
The present study were to evaluate the effects of BBR on the activity of liver phosphatidate phosphohydrolase, liver TG and cholesterol, oxidative stress, atherogenic index, and plasma lipids in rats fed on high lipogenic and normal diet.
Related reports
Earlier studies on BBR have shown that this flavonoid stimulates AMPK activity and fatty acid oxidation in HepG2 line cells and lowers hyperlipidemia in experimental animals. Results of the present work showed that BBR can be effective in reducing liver phosphatidate phosphohydrolase activity, liver TG (fatty liver), serum TG and cholesterol.
Innovations and breakthroughs
This study showed that an additional mechanism for the hypolipidemic effects of BBR could be through reducing liver phosphatidate phosphohydrolase.
Applications
BBR is a potent antioxidant and can be effective in reducing liver phosphatidate phosphohydrolase activity, lipid abnormality, liver TG and lateral side effects of hyperlipidemia.
Peer review
This is a good study in which the authors have shown that one of the mechanisms of the hypolipidemic effects of berberine can be through reducing liver phosphatidate phosphohydrolase.
Footnotes
Foundation Project: Supported by Shahrekord University of Medical Sciences (Grant No. 812), Shahrekord, Iran.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ji HF, Shen L. Berberine: a potential multipotent natural product to combat Alzheimer's disease. Molecules. 2011;16(8):6732–6740. doi: 10.3390/molecules16086732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carman GM, Han GS. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem Sci. 2006;31(12):694–699. doi: 10.1016/j.tibs.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidarian E, Haghighi B. Enzymological characteristic of plasma membrane phosphatidate phosphohydrolase (PAP2) from rat liver. Iran J Sci Technol Trans A Sci. 2008;32:117–127. [Google Scholar]
- 4.Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidarian E, Soofiniya Y. Hypolipidemic and hypoglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. J Med Plant Res. 2011;5(13):2717–2723. [Google Scholar]
- 6.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152(4):673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Saito I. Epidemiological evidence of type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disease in Japan. Circ J. 2012;76(5):1066–1073. doi: 10.1253/circj.cj-11-1519. [DOI] [PubMed] [Google Scholar]
- 8.Ling H, Burns TL, Hilleman DE. Novel strategies for managing dyslipidemia: treatment beyond statins. Postgrad Med. 2012;124(6):43–54. doi: 10.3810/pgm.2012.11.2612. [DOI] [PubMed] [Google Scholar]
- 9.Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296(4):E812–E819. doi: 10.1152/ajpendo.90710.2008. [DOI] [PubMed] [Google Scholar]
- 10.Derosa G, Maffioli P, Cicero AF. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther. 2012;12(8):1113–1124. doi: 10.1517/14712598.2012.704014. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Xiao X, Feng K, Wang T, Li W, Yuan T, et al. Berberine moderates glucose and lipid metabolism through multipathway mechanism. Evid Based Complement Alternat Med. 2011 doi: 10.1155/2011/924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm Sin B. 2012;2(4):327–334. [Google Scholar]
- 13.Heidarian E, Jafari-Dehkordi E, Seidkhani-Nahal A. Effect of garlic on liver phosphatidate phosphohydrolase and plasma lipid levels in hyperlipidemic rats. Food Chem Toxicol. 2011;49(5):1110–1114. doi: 10.1016/j.fct.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Umeda Y, Kako Y, Mizutani K, Iikura Y, Kawamura M, Seishima M, et al. Inhibitory action of gemfibrozil on cholesterol absorption in rat intestine. J Lipid Res. 2001;42(8):1214–1219. [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 17.Yanagita T, Han SY, Wang YM, Tsuruta Y, Anno T. Cycloalliin, a cyclic sulfur imino acid, reduces serum triacylglycerol in rats. Nutrition. 2003;19(2):140–143. doi: 10.1016/s0899-9007(02)00857-2. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R, Chase SD. Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;775(1):121–126. doi: 10.1016/s1570-0232(02)00273-8. [DOI] [PubMed] [Google Scholar]
- 20.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21.Bancroft JD, Gamble M. Microorganisms. In: Bartlett JH, editor. Theory and practice of histological techniques. 5th ed. Philadelphia, USA: Churchill Livingstone Elsevier; 2002. pp. 325–344. [Google Scholar]
- 22.Yadav R, France M, Younis N, Hama S, Ammori BJ, Kwok S, et al. Extended-release niacin with laropiprant: a review on efficacy, clinical effectiveness and safety. Expert Opin Pharmacother. 2012;13(9):1345–1362. doi: 10.1517/14656566.2012.690395. [DOI] [PubMed] [Google Scholar]
- 23.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53(9):1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen C, Rayalam S, Della-Fera MA, Baile CA. Phytochemicals and adipogenesis. Biofactors. 2010;36(6):415–422. doi: 10.1002/biof.115. [DOI] [PubMed] [Google Scholar]
- 25.Heidarian E, Jafari-Dehkordi E, Seidkhani-Nahal A. Lipid-lowering effect of artichoke on liver phosphatidate phosphohydrolase and plasma lipids in hyperlipidemic rats. J Med Plant Res. 2011;5:4918–4924. doi: 10.1016/j.fct.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou MH. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS One. 2011;6(9):e25436. doi: 10.1371/journal.pone.0025436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YX, Kong WJ, Li YH, Tang S, Li Z, Li YB, et al. Synthesis and structure-activity relationship of berberine analogues in LDLR up-regulation and AMPK activation. Bioorg Med Chem. 2012;20(22):6552–6558. doi: 10.1016/j.bmc.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 28.van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94(2):231–241. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 29.Elabbadi N, Day CP, Gamouh A, Zyad A, Yeaman SJ. Relationship between the inhibition of phosphatidic acid phosphohydrolase-1 by oleate and oleoyl-CoA ester and its apparent translocation. Biochimie. 2005;87(5):437–443. doi: 10.1016/j.biochi.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Ondrejovičová I, Muchová J, Mišľanová C, Nagyová Z, Duračková Z. Hypercholesterolemia, oxidative stress and gender dependence in children. Prague Med Rep. 2010;111(4):300–312. [PubMed] [Google Scholar]
- 31.Daleprane JB, Abdalla DS. Emerging roles of propolis: antioxidant, cardioprotective, and antiangiogenic actions. Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/175135. [DOI] [PMC free article] [PubMed] [Google Scholar]


