Abstract
Murrel namely Channa striatus or haruan contains all essential elements to maintain good health and to recover the lost energy after prolonged illness. The fatty acid composition (% of total fatty acid) indicated the abundant presence of C16:0 fatty acid as 30% and the other major fatty acids were C22:6 (15%), C20:4 (19%), C18:1 (12%) and C18:0 (15%). Haruan contains arachidonic acid (C20:4) as 19.0%, a precursor for prostaglandin and thromboxane biosyntheses. Both fatty and amino acids are important components for wound healing processes. Both the fillet and mucus extracts of haruan were found to exhibit a concentration dependent antinociceptive activity. In vitro antioxidant activity was higher in Channa roe protein hydrolysate than in Labeo roe protein hydrolysate in both DPPH radical scavenging and ferric reducing power. Protein content of roe concentrates (RPC) was found to be 90.2% (Channa) and 82.5% (Lates). Water absorption, oil absorption, foam capacity, stability and emulsifying capacity were found to be higher in Channa RPC than in Lates RPC. Characterization of protein hydrolysates from muscle and myofibrillar samples of haruan showed different kinetic and proteolytic activities. The skin extract of haruan influences the serotonergic receptor system thus they can function as an anti-depressant. Thus, haruan is the best example for food as medicine.
Keywords: Channa striatus, Haruan, Antinociceptive, Antioxidant, Cardiological effects
1. Introduction
Fish are not only the healthy diet to eat and relish but are often used as medicine for various diseases. Till date, marine fish products such as cod liver oil capsule, shark cartilages, etc., are recommended for patients. Whereas, medicinal value of freshwater fishes is not much familiar among consumers. Freshwater fishes viz: Murrels, catfishes and eel cure various human ailments but not yet widely known.
Murrels, belonging to the family Channidae, are widely called snakeheads and among them, the striped murrel [Channa striatus (C. striatus)] is known as haruan in South-East Asia. C. striatus, or chevron snakehead murrel, is an obligate air-breathing freshwater fish which inhabits all types of water bodies from small ditches to ricefields, rivers and lakes across tropical and subtropical Asian countries from Pakistan and India to Southeast Asia and Southern China. In South East Asia, consumers believe that haruan contains all essential elements to maintain good health and to recover the lost energy after prolonged illness. Essence of the murrel with ginseng compound is used by patients during post-operative period as a remedy to promote wound healing especially after surgical intervention. Among caesarian mothers, it alleviates post-operative pain and discomfort and trauma[1],[2]. C. striatus features prominently in the local diet among the Malays, the Orang Asli of Peninsular Malaysia[3]. C. striatus has been used for centuries in Malaysia for reducing pain and inflammation by promoting wound healing.
2. Wound healing properties
Wound healing is a complex phenomenon that results in the treatment of anatomic continuity and function, accomplished by several processes involving the orderly sequence of biological events such as inflammation, cell migration, angiogenesis, provisional matrix synthesis, collagen deposition and re-epithelization[4],[5]. The wound healing properties of haruan are attributed to its fatty acid and amino acid composition. Haruan's mucus and tissue extracts are found to contain high amount of amino acids especially glycine and arachidonic acid. These two are reported to promote wound healing by initiating collagen synthesis and re-epithelialisation in damaged tissues. Hence, haruan extracts are recommended for post operative wound healing as well as post pregnancy rehabilitation[6]. Haruan is known to produce polyunsaturated fatty acids which regulate prostaglandin synthesis inducing wound healing[7].
The proximate analysis of C. striatus showed ample amount of crude protein (23%), crude fat (5.7%) and crude ash (1.8%). C. striatus is a low fat fish with an average fat less than 10%. The fatty acid composition (% of total fatty acid) indicated the abundant presence of C16:0 fatty acid as 30% and the other major fatty acids were C22:6 (15%), C20:4 (19%), C18:1 (12%) and C18:0 (15%) as shown in Table 1. Moreover, the ω-3:ω-6 ratio lower than 1 i.e., 0.55 and the PUFA/Saturated (P/S) ratio of 0.89 were obtained in the muscle tissue of haruan[8]. The ω-3:ω-6 ratio has been suggested as reliable indicator for comparing relative nutritional values of fish oils. It was suggested that the ratio of 1:1-1:1.5 constituted a healthy human diet[9].
Table 1. Fatty acid and amino acid composition of C. striatus[8].
| Fatty acid | % of total fatty acid | Amino acid | % of total protein |
| C14:0 (Myristic acid) | ND | Aspartic acid | 11.40±0.12 |
| C16:0 (Palmitic acid) | 30.39±0.23 | Glutamic acid | 21.7±0.9 |
| C18:0 (Stearic acid) | 15.18±0.15 | Serine | 4.80±0.03 |
| C20:0 (Arachidic acid) | ND | Glycine | 4.30±0.19 |
| C16:1 (Palmitoleic acid) | 2.98±0.07 | Histidine | 1.20±0.02 |
| C18:1 (Oleic acid) | 12.04±0.54 | Arginine | 5.90±0.15 |
| C18:2 (Linoleic acid) | 8.34±1.01 | Threonine | 4.20±0.06 |
| C18:3 (Linolenic acid) | ND | Alanine | 5.80±0.73 |
| C20:4 (Arachidonic acid) | 19.02±0.78 | Proline | 3.20±0.21 |
| C20:5 (Eicosapentaenoic acid) | ND | Tyrosine | 3.60±0.14 |
| C22:6 (Docosahexaenoic acid) | 15.18±1.12 | Valine | 4.20±0.06 |
| Methionine | 3.40±0.11 | ||
| Cystine | 0.90±0.15 | ||
| Isoleucine | 3.80±0.25 | ||
| Leucine | 7.50±0.85 | ||
| Phenylalanine | 4.3±1.2 | ||
| Lysine | 9.70±0.57 |
The amino acid composition (% of total protein) showed the major presence of glutamic acid (21.7%), aspartic acid (11.4%), and lysine (9.7%) as shown in Table 2. Haruan contains arachidonic acid (C20:4) as 19.0%, a precursor for prostaglandin and thromboxane biosyntheses interfering with blood clotting process and its attachment to endothelial cells promoting wound healing[10]–[12]. Both fatty and amino acids are important components for wound healing processes. Hence, deficiency in these essential components will hinder the recovery process[2]. Glycine (4.3%), a major component of human skin collagen, together with other essential amino acids such as alanine, proline, arginine, serine, isoleucine and phenylalanine form a polypeptide promoting re-growth and tissue healing[13].
Table 2. Fatty acids composition of haruan mucus extract (% of total lipid, n=5)[14].
| Fatty acid | Mean | Range |
| 16:0 (Palmitic) | 16.65 | 11.71-20.56 |
| 18:0 (Stearic) | 16.27 | 11.08-20.23 |
| 18:1 (Oleic) | 21.25 | 18.41-25.79 |
| 18: 2 n-6 (Linoleic) | 22.47 | 18.63-30.15 |
| 18:3 n-3 (Linolenic) | 1.65 | 0.59-4.40 |
| 20:0 (Arachidic) | 2.47 | 1.76-4.15 |
| 20:1 (Gadoleic) | 3.18 | 2.14-6.02 |
| 20:4 n-6 (Arachidonic) | 11.53 | 10.33-13.43 |
| 22:1 (Erucic) | 3.60 | 1.49-9.57 |
| 22:6 n-3 (Docosahexaenoic) | 0.40 | 0.40 |
| 24:0 (Lignoceric) | 2.96 | 2.96 |
| 24:1 (Nervonic) | 1.27 | 1.27 |
Mucus membrane of murrel contains oleic acid, a mono-unsaturated omega-9 fatty acid and linoleic acid, an unsaturated omega-6 fatty acid[14]. Compared to its flesh, the murrel eggs contain even higher levels of these acids, thus an optimal amount of fatty acid can be obtained from the murrel eggs instead of consuming the fish itself.
Traditionally, wound treatment has been carried out by letting the wound dry to create a scab by itself. For the past three decades, wound treatment has undergone changes and new techniques have been introduced. One among them includes the use of artificial wound dressing to cover the wound to maintain high humidity level thus stimulating the epidermis to reproduce fast. Though a number of spray products or aerosols for wound dressings are available in the market, Febriyenti et al. have formulated an aerosol concentrate containing a mixture of haruan extract and a film-forming polymer[15]. The concentrate when sprayed on the wound formed a thin layer of dressing and the added haruan extract enhanced the healing process[16],[17].
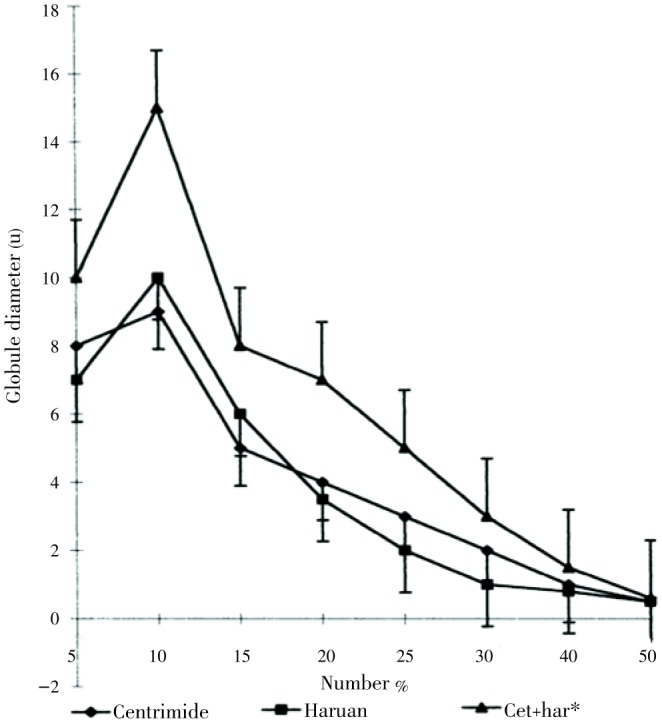
Baie and Sheikh studied the wound healing effect of C. striatus in Sprague-Dawley rats[16]. The cream formulations were prepared, stabilized and applied to wounds. The healing activity was characterized by an increase in the tensile strength of the skin on the 7th post-operative day (Table 3). They reported the increase in temperature from 25-45 °C enhanced the viscosity of cetrimide+haruan and haruan creams, but beyond 57 °C there was a marked decrease in viscosity of both formulations attributed to the Brownian movement of particles (Figure 1). They have also stated that creams also passed the freeze-thaw cycles until 3 months and were stable at room temperature for 6 months. The mean particle size of the creams is in the region of 1.82-3.90 mm (Figure 2). Similar distribution curves were obtained at intervals (every 15 d) up to 3 months.
Table 3. Tensile strength of healing wounds treated with different cream formulations[16].
| Formulations | Area of skin (cm2) | Force applied (N) | Tensile strength (N: cm2) |
| Cetrimide+haruan | 0.34 | 20.97±0.6 | 61.6±0.2 |
| Haruan | 0.33 | 17.44±0.3 | 52.8±0.3 |
| Cetrimide | 0.35 | 11.47±0.5 | 32.7±0.4 |
| Control | 0.30 | 5.09±0.7 | 14.9±0.4 |
Figure 1. Effect of shear rate on viscosity of cetrimide+haruan, haruan and cetrimide creams[16].
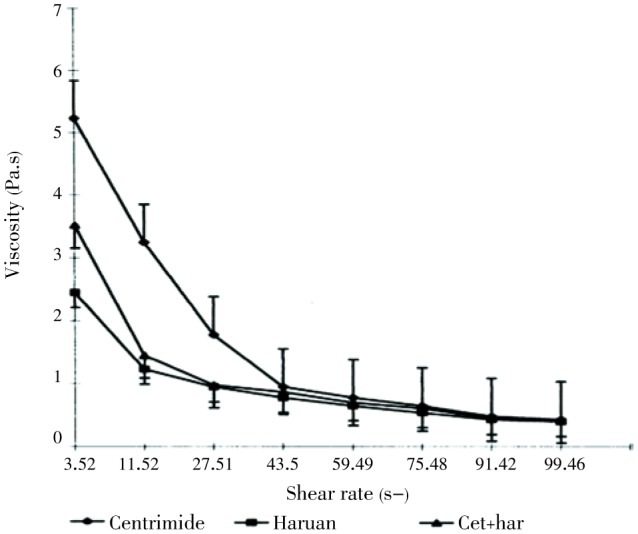
Figure 2. Particle size distribution curve of formulated creams[16].
They also stated that wounds treated with creams having cetrimide, C. striatus and cetrimide plus haruan showed a significant increase in tensile strength as compared to the control due to the polypeptide formation by the combination of glycine with aspartic and glutamic acid in the presence of leucine, methionine, alanine and arginine as described by Mat Jais et al[2]. Haruan treatment promoted remodeling of collagen, by the synthesis of inter- and intra-molecular protein cross linking and thus produced a marked increase in tensile strength as compared to the cetrimide treated group.
3. Antimicrobial properties
Though plants are still the centre of new screening for alternative agents to allopathy, due to problems of antibiotic resistance, side effects and limited efficacy[18],[19], scientists are looking into animal based resources and no doubt, Haruan is one of the promising candidates. As a part of the wound healing process, antimicrobial activity is equally important. The antimicrobial properties of the skin and intestinal mucus of different Channa sp. viz: C. striatus, Channa micropeltes, Channa marulius, Channa punctatus and Channa gachua have been studied by CARE research team[20]. The investigation showed a broad spectrum of antibacterial activity of skin mucus against Aeromonas hydrophila (A. hydrophila) [(19.5±2.0) mm], Pseudomonas aeruginosa (P. aeruginosa) [(28.0±2.9) mm] and Vibrio anguillarum [(28.0±2.9) mm] and intestinal mucus against A. hydrophila [(16.0±1.4) mm], P. aeruginosa [(29.0±3.2) mm] and Vibrio fischeri [(21.5±1.8) mm] as shown in Figures 3 & 4.
Figure 3. Antibacterial activity of fish mucus against P. aeruginosa[20].
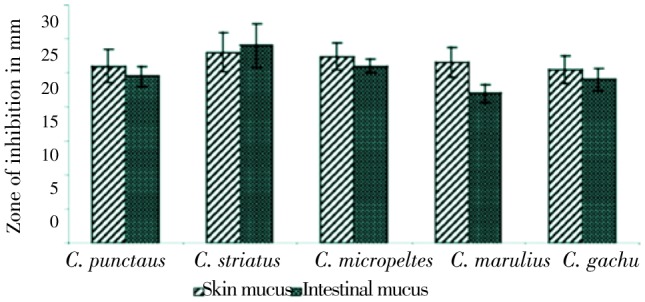
Figure 4. Antibacterial activity of fish mucus against A. hydrophila[20].

Mat and co-workers evaluated the antibacterial and antifungal property of haruan's extract and though the inhibition was not strong enough to kill the strain, the partial inhibition by an animal extract will be better for human consumption to avoid unnecessary repercussions[21]. Anti-fungal activities of haruan extract have only been demonstrated by an ethanolic fillet extract against Neurospora crassa, Aleurisma keratinophilum and Cordyceps militaris[21]. The same extract also inhibited Botrytis pyramidalis and Paecilomyces fumosa-roseus on a short-term basis.
4. Antinociceptive properties
Haruan is used to treat skin diseases like eczema which is refractory to standard medical treatment. Relief from pain and irritation is one of the reported benefits for those taking haruan fish. The analgesic tendencies of haruan were being studied only by a few researchers. For instance, Mat Jais et al. investigated the antinociceptive effects in mice with a view to establishing the scientific basis of pain-relieving activities[22], where the study showed that both the fillet and mucus extracts were found to exhibit a concentration dependent antinociceptive activity (Tables 4 and 5). Dambisya et al. studied the influence of temperature and pH on the antinociceptive activity and found it stable over a wide range of temperature and pH due to the presence of glycoprotein, polypeptide or carbohydrate[23]. They reported the general loss of inhibitory activity from 88.4% at 0 °C to 61.1% at 80-100 °C, with significance only at 80 °C. They also stated that lowering the mucus extract pH to 6.0 did not significantly affect its activity, while raising it to 8.0 attenuated its activity. There are evidences for arachidonic acid of haruan enhancing the activity of other antinociceptive agents such as morphine[22].
Table 4. Effects of 30 min haruan extract on abdominal constriction responses[22].
| Sample | N | No. of constrictions ±S.E.M | Inhibition (%) |
| Control | 44 | 27.5±1.8 | Control |
| 10% | 25 | 18.0±2.4 | 34.5 |
| 20% | 24 | 30.6±1.8 | 50.5 |
| 40% | 36 | 11.8±1.3 | 57.0 |
Table 5. Effects of mucus extract on abdominal constriction response[22].
| Sample | N | No. of constrictions ±S.E.M | Inhibition (%) |
| a) Undiluted extract | |||
| Saline control | 14 | 26.4±1.6 | - |
| Sample 1 | 13 | 7.4±1.3 | 72 |
| Sample 2 | 14 | 9.1±1.3 | 65.5 |
| Sample 3 | 14 | 8.4±1.3 | 68.2 |
| Sample 4 | 16 | 1.5±1.6 | 71.6 |
| Sample 5 | 14 | 8.8±1.1 | 66.7 |
| Average | 71 | 8.2±0.6 | 68.9 |
| b) Various concentrations of extract | |||
| Saline control | 10 | 26.8±2.6 | - |
| 12.5% | 14 | 19.6±2.1 | 26.9 |
| 25% | 13 | 13.8±1.2 | 48.5 |
| 50% | 13 | 11.2±1.5 | 58.2 |
5. Osteoarthritic treatment
Haruan were being used by people all over the world for arthritis treatment. One of the scientific report by Ganabadi is that haruan extract improved the density of PGP 9.5-immunoreactive nerve fibres in the synovial membrane in rat model and proved to be better than the other traditional fish Zingiber officinale[24].
Ng Yeen et al. studied the effects of orally administered haruan extract in rabbits experimentally induced osteoarthritis[25]. Radiographic results revealed a significant reduction in soft tissues swelling for treated animals 9 weeks after treatment compared with the untreated group. They revealed that the distribution of PGP 9.5-immunoreactive nerve fibers detected in the sub-intimal layer of the synovial membrane was similar to that detected in the normal synovial membrane, though the density was lower than for normal synovial membrane (Table 6). There are significant reports explaining reduction of soft tissue swelling and synovial inflammation and significant improvement in the density of PGP 9.5-immunoreactive nerve fibres in the synovial membrane of the osteoarthritis joints in rats[26],[27].
Table 6. Density of PGP 9.5-immunoreactive fibers in the synovial membrane of New Zealand white rabbits[25].
| Joint | Density of PGP 9.5-immunoreactive fibers |
| Normal | ++++ |
| Saline treated arthritic joints | – |
| Arthritic joints treated with haruan extract | +++ |
++++ =Abundant immunoreactive fibers were present. Blood vessels completely surrounded by rich plexus of fibres and a large number of free nerve fibres were present; +++ =Blood vessels were only partially surrounded by thin plexus of immunoreactive fibers. Free nerve fibres were sparse; ++ =Few free nerve fibres and fibres associated with blood vessels present; + =Only one or two nerve fibres present in the entire synovial membrane; – =No nerve fibres were detected.
6. Antioxidant properties
Fish in general are an important source of antioxidant and haruan is one of the major freshwater fishes to have antioxidant activity, contributed by the amino acids and fatty acids (Table 1). Murrels produce more of lipophilic antioxidants which effectively act against the oxidation of omega 3[28]. The amino acids are known to have significant antioxidant properties as synergists or primary antioxidants and are believed to be important metal chelators with significant potential in linoleic acid and methyl esters of linoleic acid system.
Narsing et al. evaluated the functional and in vitro antioxidant properties on the bioactive roe protein hydrolysates prepared from C. striatus (CRPH) and Labeo rohita (LRPH)[29]. The degree of hydrolysis was 28.41% at 60 min in Channa and 18.85% in Labeo roe concentrates at 90 min. The yields of protein hydrolysates were 24.15% and 12.45% for Channa and Labeo roe protein concentrates, respectively. The protein content was identical (58%) in both roe protein hydrolysates. Protein solubility in Channa was higher (90.48%) when compared to Labeo (50.6%) at pH 12. Higher oil absorption capacity and foam stability were observed in CRPH and higher emulsifying capacity was found in LRPH. Smaller peptides of 12 kDa were noted in both CRPH and LRPH. In vitro antioxidant activity was higher in CRPH than in LRPH as seen from DPPH radical scavenging and ferric reducing power as in Table 7.
Table 7. In vitro antioxidant activity of roe protein hydrolysates[29].
| Concentration of roe protein (mg/mL) | DPPH, % inhibition |
Concentration of roe protein (mg/mL) |
Ferric reducing power (optical density) |
||
| CRPH | LRPH | CRPH | LRPH | ||
| 1 | 22.94±0.68 | 16.31±0.52 | 0.1 | 0.08±0.002 | 0.006±0.0001 |
| 2 | 27.59±0.82 | 23.08±0.17 | 0.2 | 0.13±0.011 | 0.009±0.0003 |
| 3 | 30.24±0.57 | 31.02±0.15 | 0.3 | 0.19±0.003 | 0.013±0.0012 |
| 4 | 35.01±0.19 | 37.40±0.46 | 0.4 | 0.21±0.026 | 0.019±0.0860 |
| 5 | 63.26±1.22 | 41.22±0.43 | 0.5 | 0.33±0.034 | 0.025±0.0043 |
In another study, Narsing et al. reported that C. striatus roe protein concentrate (CRPC) and Lates calcarifer roe protein concentrates (LRPC) yielded 20.7% and 22.5% respectively[30]. Protein content of concentrates was found to be 90.2% (Channa) and 82.5% (Lates) as shown in Table 8. Protein solubility was 3.93-54.6% and 1.6-55.5% in Channa and Lates concentrates. SDS-PAGE revealed that protein bands at 95 and ∼30 kDa were predominant. Sorption isotherms indicated both roe protein concentrates are hygroscopic. Water absorption, oil absorption, foam capacity, stability and emulsifying capacity were found to be higher in CRPC than in LRPC as in Table 9. Antioxidant activity determined by the radical scavenging activity and ferric reducing power was higher in CRPC (Table 10).
Table 8. Physcio-chemical composition and colour readings of roe protein concentrates[30].
| Parameter | CRPC | LRPC |
| Yield (wt%) | 20.7±0.64 | 22.5±0.44 |
| Protein content (%) | 90.2±0.61 | 82.5±0.56 |
| Moisture (%) | 5.60±0.24 | 5.30±0.20 |
| Total ash (%) | 2.62±0.35 | 2.14±0.22 |
| Bulk density (g/mL) | 0.27±0.01 | 0.77±0.44 |
| Tintometer colour units Red | 1.10±0.17 | 2.00±0.26 |
| Yellow | 2.00±0.10 | 4.00±0.26 |
Table 9. Functional properties of roe protein concentrates[30].
| Parameter | CRPC | LRPC |
| Water absorption (%) | 315±1.73 | 198±2.28 |
| Oil absorption (%) | 241±1.0 | 172.0±2.00 |
| Emulsifying capacity (mL/g) | 56±2.64 | 12±1.00 |
| Foam captivity (mL) | 67±1.73 | 52±1.00 |
| Foam stability (mL), after 15 min | 62±2.00 | 36±2.00 |
| Foam stability (mL), after 30 min | 58±2.00 | 32±2.00 |
| Foam stability (mL), after 45 min | 51±1.00 | 28±3.00 |
| Foam stability (mL), after 60 min | 50±1.50 | 24±1.73 |
| Foam stability (mL), after 90 min | 42±1.00 | 18±2.64 |
Table 10. Antioxidant activity of roe protein concentrates[30].
| Weight of roe protein concentrate (mg) | CRPC |
LRPC |
||
| DPPH (Inhibition, %) | Ferric reducing power (OD) | DPPH (Inhibition, %) | Ferric reducing power (OD) | |
| 2 | 18.5±0.08 | 0.15±0.01 | 4.6±0.08 | 0.12±0.01 |
| 4 | 29.4±0.61 | 0.24±0.02 | 6.0±0.10 | 0.18±0.01 |
| 6 | 38.4±0.98 | 0.29±0.03 | 11.3±0.20 | 0.22±0.03 |
| 8 | 50.4±1.73 | 0.37±0.04 | 12.1±0.46 | 0.26±0.05 |
| 10 | 56.7±1.21 | 0.43±0.03 | 13.2±0.35 | 0.32±0.02 |
7. Cardiological effects of haruan
The skin extract called shol fish skin extract (SFSE) has been found to contain potent active compound, cardiotoxic factor II (CTF-II)[31], with hypotensive effect and cardiotoxic property that influence the increase in cardiac marker enzyme creatine phosphokinase and creatine phosphokinase-MB values as in Tables 11 and 12[32]. Characterization of protein hydrolysates from muscle and myofibrillar samples of haruan showed different kinetic and proteolytic activities[33], and the results led to isolation of angiotensin converting enzyme (ACE) inhibitor peptides with high ACE- inhibitory activity, further supporting the use of haruan as a functional food and preventative medicine for hypertensive patients[34].
Table 11. Effect of SFSE on haematological parameters of male albino mice[31].
| Hemogram of mice | Control (0 h) | SFSE (5 mg/20 g, po) |
||||
| 1 h | 2 h | 4 h | 8 h | 12 h | ||
| Hb (gm%) | 13.78±1.12 | 11.55±0.74+ | 7.82±0.38* | 5.94±1.25* | 7.07±0.97* | 6.50±0.17* |
| HCT (%) | 55.27±3.73 | 45.30±2.24+ | 30.37±5.96* | 27.17±5.28* | 32.48±1.64* | 25.75±1.56* |
| RBC (Million/mm3) | 9.54±0.79 | 8.09±0.24+ | 5.57±0.89* | 4.33±0.97* | 5.63±7.01* | 4.01±0.42* |
| WBC (Thousand/mm3) | 5.77±0.56 | 4.85±0.49+ | 2.27±0.20+ | 4.85±1.39+ | 8.66±1.48+ | 9.60±0.89+ |
Significant changes were observed at 2 h of SFSE treatment. Each values shown as mean±SE with analysis of variance (One way ANOVA) n=4; P<0.05 considered significant. Here *P<0.05, +P>0.05 (non-significant).
Table 12. Effect of CSS-CTF II on biochemical parameters of male albino mice[32].
| Parameters | Control (0.9% saline) | CSS-CTF II (100 µg/20 g, ip/day for 10 d) |
| Sugar (mg/dL) | 83.52±1.13 | 75.14±1.49 ** |
| Urea (mg/dL) | 18.94±0.59 | 22.27±0.97 * |
| Creatinine (mg/dL) | 0.44±0.02 | 1.64±0.05 *** |
| Albumin (g/dL) | 4.76±0.07 | 3.65±0.02 *** |
| Cholesterol (mg/dL) | 75.38±2.14 | 76.12±1.86 |
| SGPT (U/L) | 41.82±1.98 | 46.95±1.06 |
| SGOT (U/L) | 13.98±0.50 | 30.37±0.21 *** |
| Alkaline phosphatase (U/L) | 68.27±0.72 | 77.75±1.26 *** |
| Sodium (mEq/L) | 134.96±0.68 | 133.60±0.82 |
| Potassium (mEq/L) | 4.40±0.14 | 3.29±0.12 *** |
| Calcium (mEq/L) | 8.96±0.24 | 7.35±0.10 *** |
| CPK (U/L) | 133.67±2.69 | 233.21±3.11 *** |
| CPK-MB (U/L) | 18.57±0.14 | 19.37±0.16 ** |
*P<0.05, **P<0.01, ***P<0.001.
Fish oil supplementation is now widely regarded as an effective preventive measure against cardiovascular problems. Italian researchers, Calo et al. have reported that fish oil supplementation could be useful in preventing post-operative atria fibrillation[35]. Arachidonic acid (19.02%) present in murrels reduces coronary heart disease considerably. Docosahexaenoic acid and eicosapentaenoic acid exert preventive effects on human coronary artery disease[36]. Calo et al. first demonstrated that PUFA administration during hospitalization in patients undergoing CABG substantially reduced the incidence of postoperative atrial fibrillation (54.4%) and was associated with a shorter hospital stay[35]. They reported that postoperative AF developed in 27 patients of the control group (33.3%) and in 12 patients of the PUFA group (15.2%) (P=0.013). There was no significant difference in the incidence of nonfatal postoperative complications, and postoperative mortality was similar in the PUFA-treated patients (1.3%) vs control (2.5%). After CABG, the PUFA patients were hospitalized for significantly fewer days than control [(7.3 +/- 2.1) d vs. (8.2 +/- 2.6) d, P=0.017)]. Epidemiological surveys suggest that fish oil consumption may reduce the risk of fatal ventricular arrhythmias, consistently supporting an anti-arrhythmic effect of PUFAs.
8. Neurological effects
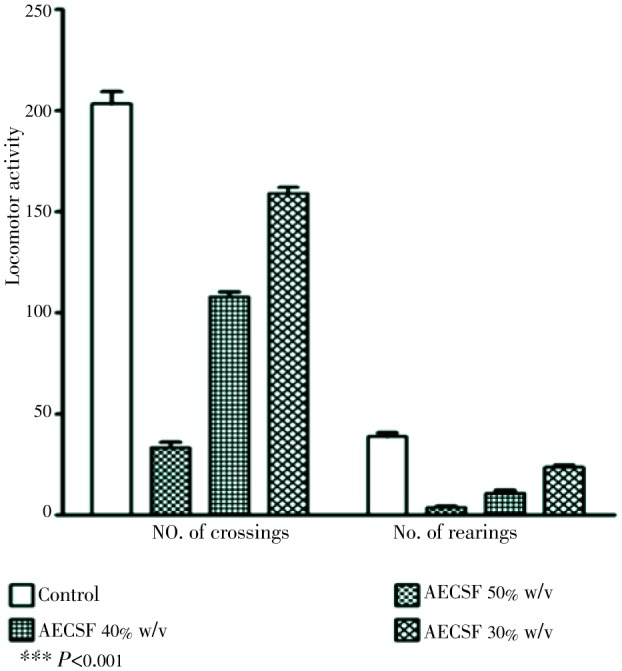
The skin extract of haruan influences the serotonergic receptor system thus they can function as an anti-depressant. Saleem et al. stated that different concentration of haruan fillet extract (30%, 40% and 50% w/v) significantly reduced the immobility time in forced swimming test and tail suspension test (Figure 5)[37]. A dose-dependent significant reduction in locomotor activity during open field test was also reported (Figure 6). The skin extract of C. striatus (SFSE) could initiate apnoea and irreversible blockade of nerve-muscle preparation and influence the serotonergic receptor system[29], as an anti-depressant[37],[38]. It is also able to exert positive changes in the regenerative potential of neurons involved in traumatic injury as observed by neurite outgrowth and multipolarity of cells which took place in phaechromocytoma PC12 cells treated with haruan therapeutic extract[39]. These findings open up the possibility of using haruan extract as a regenerative and restorative agent for treating damage to many types of organs. Reports on encapsulation of haruan extract reveal a new medicine in future for treatment various ailments in one capsule[40].
Figure 5. Effect of aqueous extract of C. striatus fillet (AECSF) and amitriptyline (10 mg/kg) in forced swimming test (FST) and tail suspension test (TST) in male ICR mice[37].
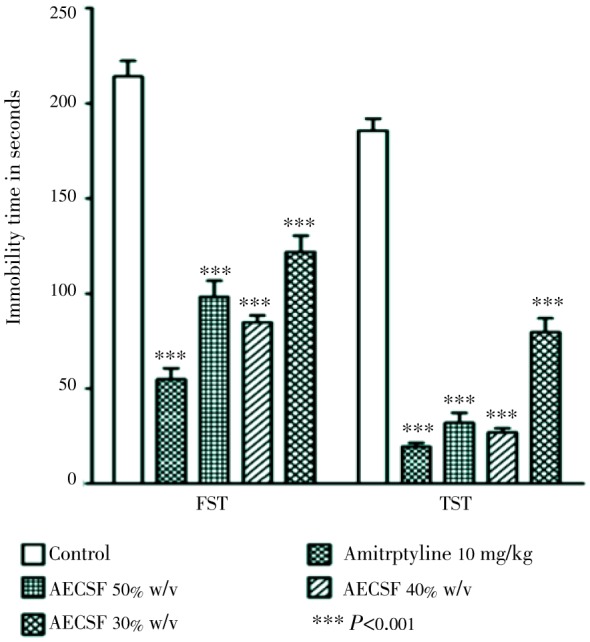
Figure 6. Effect of aqueous extract of C. striatus fillet (AECSF) in open-field test in male ICR mice[37].
9. Conclusion
Haruan is a freshwater medicinal fish inhabitant in several Asian countries and used as medication to treat wounds, alleviate pain and boost energy. Research findings have put a light on its traditional uses by elucidating possible compounds that may give rise to the observable therapeutic effects and confirming these effects through in vitro as well as in vivo studies. C. striatus extract may also have a role in other non-traditional uses such as in treating neurological diseases and in inducing regenerative potential of organs and cells. Future work on this therapeutic fish must maintain the vigor in studies on haruan extracts that are not confined and limited to its previously known, traditional uses.
Acknowledgments
We acknowledge the financial assistance received from Indian Council of Agricultural Research – National Agricultural Innovation Project, New Delhi, India (Grant No. 1(5)/2007-NAIP dt. 22 August 2008).
Comments
Background
C. striatus or haruan is a freshwater medicinal fish inhabiting in several Asian countries and used as medication to treat wounds, alleviate pain and provide instant energy. The skin extract of haruan influences the serotonergic receptor system thus they can function as an anti-depressant. Therefore there is need to know about haruan and its medical properties.
Research frontiers
The present research emphasizes the therapeutic effects through in vitro as well as in vivo studies. C. striatus extract may also have a role in other uses such as in treating neurological diseases and in inducing regenerative potential of organs and cells.
Related reports
Haruan is one of the important species. The antimicrobial properties of the skin and intestinal mucus of different Channa sp. viz: C. striatus, Channa micropeltes, Channa marulius, Channa punctatus and Channa gachua have been already studied.
Innovations and breakthroughs
Fish in general are an important source of antioxidant and haruan is one of the major freshwater fishes to have antioxidant activity. In the present study, authors have demonstrated wound healing properties, antimicrobial properties, and antinociceptive properties.
Applications
From the literature survey, it has been found that haruan is safe to humans. This scientific study supports and suggests the use of this fish for reducing pain and inflammation by promoting wound healing.
Peer review
This is an important research work in which authors have demonstrated the wound healing properties, antimicrobial properties, and antinociceptive properties of hatuan. The activity was assessed based on biochemical parameters and haruan is one of the major freshwater fishes to have antioxidant activity, contributed by the amino acids and fatty acids.
Footnotes
Foundation Project: Supported by Indian Council of Agricultural Research–National Agricultural Innovation Project, New Delhi, India (Grant No. 1(5)/2007-NAIP dt. 22 August 2008).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Mohd SM, Abdul Manan MJ. Therapeutic potential of the haruan (Channa striatus): from food to medicinal uses. Malays J Nutr. 2012;18(1):125–136. [PubMed] [Google Scholar]
- 2.Jais AM, McCulloch R, Croft K. Fatty acid and amino acid composition in haruan as a potential role in wound healing. Gen Pharmacol. 1994;25:947–950. doi: 10.1016/0306-3623(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 3.Haemamalar K, Jr, Zalilah MS, Neng Azhanie A. Nutritional status of Orang Asli (Che Wong tribe) adults in Krau wildlife reserve, Pahang. Malays J Nutr. 2010;16(1):55–68. [PubMed] [Google Scholar]
- 4.Pierce GF, Mustoe TA. Pharmacologic enhancement of wound healing. Annu Rev Med. 1995;46:467–481. doi: 10.1146/annurev.med.46.1.467. [DOI] [PubMed] [Google Scholar]
- 5.Parthiba V, Gupta PD. Cutaneous wound healing: Significance of proteoglycans in scar formation. Curr Sci. 2000;78(6):697–701. [Google Scholar]
- 6.Marimuthu K, Haniffa MA, Muruganandam M, Arockia Raj AJ. Low cost murrel seed production technique for fish farmers. Naga. 2001;24:21–22. [Google Scholar]
- 7.Gibson RA. Australian fish-An excellent source of both arachidonic acid and ω-3 polyunsaturated fatty acids. Lipids. 1983;18:743–752. doi: 10.1007/BF02534631. [DOI] [PubMed] [Google Scholar]
- 8.Zuraini A, Somchit MN, Solihah MH, Goh YM, Arifah AK, Zakaria MS, et al. Fatty acid and amino acid composition of three local Malaysian Channa spp. fish. Food Chem. 2006;97:674–678. [Google Scholar]
- 9.Osman H, Suriah AR, Law EC. Fatty acid composition and cholesterol content of selected marine fish in Malaysian waters. Food Chem. 2001;73:55–60. [Google Scholar]
- 10.Pompeia C, Freitas JJ, Kim JS, Zyngier SB, Curi R. Arachidonic acid cytotoxicity in leukocytes: implications of oxidative stress and eicosanoid synthesis. Biol Cell. 2002;94(4–5):251–265. doi: 10.1016/s0248-4900(02)01200-5. [DOI] [PubMed] [Google Scholar]
- 11.Mohsin AK, Ambak MA. Kuala Lumpur: Universiti Pertanian Malaysia Press; 1983. Freshwater fishes of peninsular Malaysia; p. 284. [Google Scholar]
- 12.Rahnan SA, Huah TS, Nassan O, Daud NM. Fatty acid composition of some Malaysian freshwater fish. Food Chem. 1995;54:45–49. [Google Scholar]
- 13.Witte MB, Thornton FJ, Tantry U, Barbul A. L-Arginine supplementation enhances diabetic wound healing: involvement of the nitric oxide synthase and arginase pathways. Metabolism. 2002;51(10):1269–1273. doi: 10.1053/meta.2002.35185. [DOI] [PubMed] [Google Scholar]
- 14.Jais AM, Matori MF, Kittakoop P, Sowanborirux K. Fatty acid compositions in mucus and roe of haruan, Channa striatus, for wound healing. Gen Pharmacol. 1998;30(4):561–563. doi: 10.1016/s0306-3623(97)00305-4. [DOI] [PubMed] [Google Scholar]
- 15.Febriyenti, Noor AM, Baie S. Formulation of aerosol concentrates containing haruan (Channa striatus) for wound dressing. Malaysian J Pharm Sci. 2008;6(1):43–58. [Google Scholar]
- 16.Baie SH, Sheikh KA. The wound healing properties of Channa striatus-cetrimide cream – tensile strength measurement. J Ethnopharmacol. 2000;71:93–100. doi: 10.1016/s0378-8741(99)00184-1. [DOI] [PubMed] [Google Scholar]
- 17.Baie SH, Sheikh KA. The wound healing properties of Channa striatus-cetrimide cream wound contraction and glycosaminoglycan measurement. J Ethnopharmacol. 2000;73(1–2):15–30. doi: 10.1016/s0378-8741(00)00253-1. [DOI] [PubMed] [Google Scholar]
- 18.Nitta T, Arai T, Takamatsu H, Inatomi Y, Murata H, Linuma M, et al. Antibacterial activity of extracts prepared from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus. J Health Sci. 2002;48:273–276. [Google Scholar]
- 19.Souza LK, de Oliveira CM, Ferri PH, de Oliveira Júnior JG, de Souza Júnior AH, Fernandes Ode F, et al. Antimicrobial activity of Hyptis ovalifolia towards dermatophytes. Mem Inst Oswaldo Cruz. 2003;98:963–965. doi: 10.1590/s0074-02762003000700018. [DOI] [PubMed] [Google Scholar]
- 20.Haniffa M, Dhanaraj M, Arun SS, Muthu RC, Manikandaraja D, Milton JM. Antibacterial activity of skin and intestinal mucus of five different freshwater fish species viz., Channa striatus, C. micropeltes, C. marulius, C. punctatus and C. gachua. Malaysian J Sci. 2009;28(3):257–262. [Google Scholar]
- 21.Mat Jais AM, Zakaria ZA, Luo A, Song YX. Antifungal activity of Channa striatus (haruan) crude extracts. Int J Trop Med. 2008;3(3):43–48. [Google Scholar]
- 22.Mat Jais AM, Dambisya YM, Lee TL. Antinociceptive activity of Channa striatus extracts in mice. J Ethnopharmacol. 1997;57:125–130. doi: 10.1016/s0378-8741(97)00057-3. [DOI] [PubMed] [Google Scholar]
- 23.Dambisya YM, Lee TL, Sathivulu V, Mat Jais AM. Influence of temperature, pH, and naloxone on the antinociceptive activity of Channa striatus (haruan) extracts in mice. J Ethnopharmacol. 1999;66:181–186. doi: 10.1016/s0378-8741(98)00169-x. [DOI] [PubMed] [Google Scholar]
- 24.Ganabadi S. Channa striatus extract supplementation significantly increased protein gene product 9.5-immunoreactive nerve fibres compared to Zingiber officinale extract in collegenase induced osteoarthritis. Osteoarthr Cartil. 2009;17(1):S281–S282. [Google Scholar]
- 25.Michelle YT, Shanthi G, Loqman MY. Effect of orally administered Channa striatus extract against experimentally induced osteoarthritis in rabbits. Intern J Appl Res Vet Med. 2004;2(3):171–175. [Google Scholar]
- 26.Al-Saffar FJ, Ganabadi S, Fakuraz S. Response of Channa striatus extract against monosodium iodoacetate induced osteoarthritis in rats. J Anim Vet Adv. 2011;10(4):460–469. [Google Scholar]
- 27.Al-Saffar FJ, Ganabadi S, Fakuraz S, Yaakub H. Zerumbone significantly improved immuno-reactivity in the synovium compared to Channa striatus extract in monosodium iodoacetate (MIA)- induced knee osteoarthritis in rat. J Med Plant Res. 2011;5(9):1701–1710. [Google Scholar]
- 28.Dahlan-Daud CK, Mat Jais AM, Ahmad Z, Md Akim A, Adam A. Amino and fatty acids composition in haruan traditional extract. Boletin Latino-americano y del Caribe de Plantas Medicinales y Aromaticas. 2010;9:414–429. [Google Scholar]
- 29.Galla NR, Pamidighantam PR, Akula S, Karakala B. Functional properties and in vitro antioxidant activity of roe protein hydrolysates of Channa striatus and Labeo hohita. Food Chem. 2012;135(3):1479–1484. doi: 10.1016/j.foodchem.2012.05.098. [DOI] [PubMed] [Google Scholar]
- 30.Narsing RG, Balaswamy K, Satyanarayana A, Prabhakara RP. Physico-chemical, amino acid composition, functional and antioxidant properties of roe protein concentrates obtained from Channa striatus and Lates calcarifer. Food Chem. 2012;132(3):1171–1176. doi: 10.1016/j.foodchem.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 31.Karmakar S, Dasgupta SC, Gomes A. Pharmacological and haematological study of shol fish (Channa striatus) skin extract on experimental animals. Indian J Exp Biol. 2002;40(1):115–118. [PubMed] [Google Scholar]
- 32.Karmakar S, Das T, Ghosh Dasgupta SC, Biswas AK, Gomes A. Isolation and partial structural evaluation of a cardiotoxic factor from Indian common murrel (Channa striatus L.) skin extract. Indian J Exp Biol. 2004;42(3):271–278. [PubMed] [Google Scholar]
- 33.Ghassem M, Fern SS, Said M, Mohd Ali Z, Ibrahim S, Babji AS. Kinetic characterisation of Channa striatus muscle sarcoplasmic and myofibrillar protein hydrolysates. J Food Sci Technol. 2014;51:467–475. doi: 10.1007/s13197-011-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghassem M, Arihara K, Babji AS, Said M, Ibrahim S. Purification and identification of ACE inhibitory peptides from haruan (Channa striatus) myofibrillar protein hydrolysate using HPLC-ESI-TOF MS/MS. Food Chem. 2011;129(4):1770–1777. [Google Scholar]
- 35.Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, De Ruvo E, et al. N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 36.Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988;318:549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- 37.Saleem AM, Hidayat MT, Mat Jais AM, Fakurazi S, Mohamad Moklas MA, Sulaiman MR, et al. Antidepressant-like effect of aqueous extract of Channa striatus fillet in mice models of depression. Eur Rev Med Pharmacol Sci. 2011;15:795–802. [PubMed] [Google Scholar]
- 38.Saleem AM, Hidayat MT, Mat Jais AM, Fakurazi S, Mohamad Moklas MA, Sulaiman MR, et al. Universiti Putra Malaysia, Malaysia: 25th Scientific Meeting of the Malaysian Society of Pharmacology and Physiology; 2010. Evidence for the involvement of monoaminergic system in the antidepressant activity of haruan extract in mice; p. 108. [Google Scholar]
- 39.Mohd Shafri MA, Mat Jais AM, Kyu MK. Neuroregenerative properties of haruan (Channa striatus spp.) traditional extract. J Intelek. 2011;6:77–83. [Google Scholar]
- 40.Hui LY, Mat Jais AM, Krishnaiah D, Sundang M, Ismail NM, Hong TL, et al. Encapsulation of Channa striatus extract by spray drying process. J Appl Sci. 2010;10(21):2499–2507. [Google Scholar]


