Abstract
Objective
To evaluate the antivenom potential of ethanolic extract of bark of Cordia macleodii against Naja venom induced pharmacological effects such as lethality, hemorrhagic lesion, necrotizing lesion, edema, cardiotoxicity and neurotoxicity.
Methods
Wistar strain rats were challenged with Naja venom and treated with the ethanolic extract of Cordia macleodii bark. The effectiveness of the extract to neutralize the lethalities of Naja venom was investigated as recommended by WHO.
Results
At the dose of 400 and 800 mg/kg ethanolic extract of Cordia macleodii bark significantly inhibited the Naja venom induced lethality, hemorrhagic lesion, necrotizing lesion and edema in rats. Ethanolic extract of Cordia macleodii bark was effective in neutralizing the coagulant and defibrinogenating activity of Naja venom. The cardiotoxic effects in isolated frog heart and neurotoxic activity studies on frog rectus abdominus muscle were also antagonized by ethanolic extract of Cordia macleodii bark.
Conclusions
It is concluded that the protective effect of extract of Cordia macleodii against Naja venom poisoning may be mediated by the cardiotonic, proteolysin neutralization, anti-inflammatory, antiserotonic and antihistaminic activity. It is possible that the protective effect may also be due to precipitation of active venom constituents.
Keywords: Naja venom, Cordia macleodii, Hemorrhagic activity, Necrotizing activity, Cardiotoxicity, Neurotoxicity
1. Introduction
Venomous snakebite is a WHO identified common acute medical emergency in most rural areas of the tropical and subtropical countries like India. Approximately 35 000–50 000 deaths are reported due to snakebite each year[1]. India has rich assortment of snake fauna, of which 242 species have been identified including 57 poisonous or harmful species. The four major ubiquitous species of venomous snakes in India, known as “big four” are considered responsible for life-threatening envenomation around the country. They include Indian cobra (Naja naja), the common krait (Bungarus caeruleus), the Russell's viper (Daboia russelii) and the saw-scaled viper (Echis carinatus[2]. Many plants viz. Mimosa pudica[3], Mucuna pruriens[4],[5], Andrographis paniculata and Aristolochia indica[6]–[8], Acalypha indica[9]–[12] are reported to inhibit krait and cobra venom activity. Cordia macleodii (Ehretiacae), known as Dahiman in Hindi is a rare medicinal and timber plant found in moist, dry deciduous forests and successfully used for the treatment of snakebites by traditional healers in Chhattisgarh State of India. The leaves of the plant has been reported to have anti-inflammatory activity[13]. The present study was aimed to examine the snake venom neutralization potential of ethanolic extract of Cordia macleodii bark to scientifically validate the traditional claim.
2. Materials and methods
2.1. Plant material and preparation of extract
The bark of Cordia macleodii was collected during the month of December 2009 from Marwahi Forest Division, Pendra Road, Bilaspur (Voucher number: SLT/Med. Plant/2009/15). The plant material was taxonomically identified and authenticated by Dr. Manoj Singh, Assistant Professor, Tulsi Mahavidyalaya, Anuppur (M.P). The shade dried coarsely powdered plant material (1.5 kg) was extracted with 70% ethanol in a Soxhlet apparatus. The extract was dried under vacuum (yield 8.7%). Preliminary phytochemical screening was carried out to detect the presence of steroids, carbohydrates, flavonoids, alkaloids, etc.
2.2. Experimental animals
Healthy adult male Wistar albino rats weighing about 180-250 g and Swiss albino mice weighing about 20-25 g between 2 and 3 months of age were used for the study. Animals were housed individually in polypropylene cages, maintained under standard conditions [12 h light and 12 h dark cycle; (25±3) °C]. The animals were fed with standard rat pellet diet (Pranav Agro Industries Ltd, Baroda, India) and water ad libitum. The study was approved by Institutional Animal Ethics Committee of the institute (Registration no. 994/ac/06/CPCSEA).
2.3. Venom
The Naja (Naja naja) venom was obtained from Kanan Pendari Zoo, Bilaspur, Chhattisgarh, India and was preserved at 4 °C. Before use, the venom was dissolved in saline, centrifuged at 2 000 r/min for 10 min and the supernatant was used for antivenom studies. Venom concentration was expressed in term of dry weight.
2.4. Snake venom antiserum
Liquid snake antivenin (polyvalent) IP, Biological E. Ltd., Hyderabad (Batch no.: 90, Mfg Date: March 2009) was obtained from Primary Health Center, Pendra Road, Bilaspur.
2.5. Acute toxicity studies
The acute toxicity study was carried out as per OECD 423 guidelines. The limit test for acute toxicity was carried out at 2 g/kg oral dose. Nulliparous and non-pregnant female rats were used for the study and were observed continuously for 24 h for behavioral, neurological and autonomic profiles and, after a period of 24 and 72 h, for any lethality, morbidity state or death[14].
2.6. Neutralization of lethal venom effect
The toxicity of Naja venom was assessed by i.p. administration of different concentrations of venom dissolved in 0.2 mL of physiological saline to groups (n=6) of Wistar albino rats (180–250 g). The median lethal dose (LD50) of venom was determined 24 h later by the method of Theakston and Reid 1983[15]. The antivenom potential of ethanolic extract of bark of Cordia macleodii (CME) was assessed by i.p. administration of LD50 dose of venom into groups of rats (n=6), followed by i.p. administration of different doses of the plant extract. The standard reference group was administered snake venom antiserum after administration of LD50 dose of venom.
2.7. Neutralization of hemorrhagic activity
The minimum haemorrhagic dose is defined as the least amount of venom which when injected intradermally into rats results in a haemorrhagic lesion of 10 mm diameter in 24 h[15]. Neutralization of the haemorrhagic activity was estimated by mixing a fixed amount of venom with different amounts of CME. The CME–venom mixture was incubated at 37 °C for 1 h and 0.1 mL of the mixture was injected intradermally into mice. The haemorrhagic lesion was estimated after 24 h[4].
2.8. Neutralization of necrotizing activity
The minimum necrotizing dose is the least amount of venom which when injected intradermally into rats results in a necrotizing lesion of 5 mm diameter in 3 d later[15]. Neutralization of the necrotizing activity was estimated by mixing a fixed amount of venom with different amounts of CME. The CME–venom mixture was incubated at 37 °C for 1 h and 0.1 mL of the mixture was injected intradermally into mice. The necrotizing lesion was estimated after 3 d.
2.9. Neutralization of coagulant activity
The neutralization of coagulant activity was estimated by mixing different amount of CME, with a fixed amount of venom, incubating for 30 min at 37 °C[4]. Different concentration of incubate were added to the experimental tube in place of 0.1 mL physiological saline, and the clotting time was recorded.
2.10. Minimum defibrinogenating dose (MDD)
The MDD of venom is defined as the minimum amount of venom injected i.v. into mice, causes incoagulable blood 1 h later[15]. Neutralization of this activity was estimated as the amount of CME which prevented defibrinogenation by the MDD. Various amounts of CME were mixed with the MDD of the venom, incubated for 30 min at 37 °C and then injected i.v. into six mice (20-25 g). After 1 h mice were anesthetized with ether and bled by cardiac puncture. The coagulation was observed as described above. The neutralization ability of CME was expressed as the effective dose (ED), defined as the lowest CME/venom ratio in which blood coagulation occurred in the six mice.
2.11. Rat paw edema
The minimum edematic dose of venom/carrageenan is defined as the least amount of venom/carrageenan injected by intraplanter route to rats, produce inflammation in paw[16]. Non fasted male albino rats (150-200 g) were treated with different doses of venom, carrageenan and CME. Venom (1 µg/kg)/carrageenan (1% in 0.01 mL) were injected in right hind paw after 15 min. As a control, equal amount of saline were injected in hind paw.The CME was administered orally. The edematic response was evaluated by the use of a screw gauge at given time intervals.
2.12. Neutralization of cardiotoxic activity
The effect of the CME on Naja venom induced changes in isolated frog heart was determined by the method of Kannappa et al[17]. The isolated frog heart was connected to the perfusion apparatus containing Ringer solution, graded doses of the CME and Naja venom were injected and the changes in heart rate were recorded using kymograph (speed 12 mm/h, INCO instruments, Ambala, India). The effects of Naja venom in the presence of CME were also studied. The experiment was repeated in six isolated preparations[9].
2.13. Neutralization of neurotoxic activity
The effect of CME on Naja venom induced changes on skeletal muscle was determined by the method reported by Kannappa et al[17]. The rectus abdominus muscle of frog was mounted in an organ bath containing Ringer solution. The effect of acetylcholine, CME and Naja venom per se and the effect of acetylcholine and Naja venom in the presence of CME were recorded. The experiment was repeated in six isolated preparations.
2.14. Statistical analysis
Results are expressed as mean±SEM. The data were analyzed by ANOVA complemented by the Tukey–Kramer test. Values of P<0.05 were considered significant.
3. Results
The LD50 of Naja venom was established at 120 µg/rat i.p.. The MHD was 240 µg/rat and the MND at 150 µg/rat respectively. Oral administration of the CME did not produce toxic effects up to 2 g/kg in rats after 72 h.
The data from in vivo studies indicate protection by CME against the Naja venom induced lethality in a dose-dependent manner. Significant protection was observed at 200 and 400 mg/kg. At the dose of 800 mg/kg CME showed higher protection than snake venom antiserum (Table 1). The rate of survived animals was increased to 100%.
Table 1. Effect of the CME on the lethality of Naja venom.
| Group (n=6) | % Survival | % Increase in survival rate |
| Control (Snake Venom) | 50.00 | - |
| Standard (Snake Antivenin) | 83.30a | 66.60 |
| CME (200 mg/kg) | 66.00b | 32.00 |
| CME (400 mg/kg) | 83.30a | 66.60 |
| CME (800 mg/kg) | 100.00a | 100.00 |
aP<0.01 vs. Control, bP<0.05 vs. Control
The MND of venom injected intradermally into rat's shaved dorsal skin caused necrotizing and hemorrhagic lesions. The CME at 200 mg/kg reduced the necrotizing as well as hemorrhagic lesions significantly (Table 2). Blood treated with venom clotted faster than untreated blood. About 61.11 µg of Naja venom clotted human citrated plasma within 60 seconds. In the neutralization assay, the absence of clot formation shows the neutralizing ability of the CME. The CME at (1.81±0.11) mg of dose was able to completely neutralize coagulant activity (Table 3). The Naja venom also induced defibrination in vivo and this activity was neutralized successfully by CME at (2.10±0.21) mg dose (Table 4).
Table 2. Effect of the CME on hemorrhagic and necrotizing lesion.
| Group (n=6) | Hemorrhagic lesion (mm) | Necrotizing lesion (mm) |
| Control (Snake venom) | 10.26±0.20 | 5.12±0.28 |
| Standard (Snake antivenin) | 0.00a | 0.00 a |
| CME (200 mg/kg) | 6.18±0.08b | 3.16±0.21b |
| CME (400 mg/kg) | 4.78±0.20a | 2.92±0.15a |
| CME (800 mg/kg) | 3.25±0.21a | 2.75±0.11a |
aP<0.01 vs. Control, bP<0.05 vs. Control
Table 3. Neutralization of coagulant activity.
| Venom | MCD (µg/mL)a | Effective dose of neutralizationb (mg CME/mg venom) |
| Naja | 61.11±0.21 | 1.81±0.11 |
aDefined as the concentration of venom (µg /ml) which induced coagulation of plasma in 60 seconds (mean±SEM; n=6).
bDefined as the CME/venom ratio at which coagulation time was increased three times when compared to coagulation time of plasma incubated with venom alone (mean±SEM; n=6).
Table 4. Neutralization of defibrinogenating activity.
| Venom | MDD (µg/mL)a | Effective dose of neutralization (mg CME/mg venom) b |
| Naja | 75.32±0.18 | 2.10±0.21 |
aDefined as the minimum amount of venom which, when injected i.v. into mice, causes incoagulable blood 1 h later. Six mice were used per experimental group. bNeutralization ability of CME was expressed as effective dose, defined as the lowest CME /venom ratio in which blood coagulation occurred in the six mice.
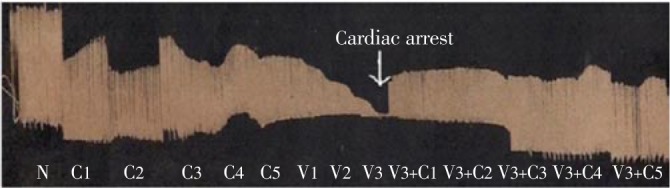
Carrageenan and Naja venom induced significant edema in rat paw. Maximum inflammation was seen at 1 h which gradually decreased over a period of time. Intraplantar Naja venom injection followed by CME produced significant reduction in inflammation at 400 mg/kg dose (Table 5). The venom produced cardiotoxic effect and reduced the heart rate. The venom mixed with different amount of CME did not produce cardiotoxic effects. The effect was significant as compared to constant dose V3 of the venom. The protective action was increased with the increase in dose indicating the dose dependent protection (Table 6 and Figure 1).
Table 5. Carrageenan and Naja venom induced edema and its inhibition by CME.
| Treatment | Edema (mL) |
|||
| 1h | 2h | 3h | 4h | |
| Carrageenan | 7.25±0.21 | 6.84±0.24 | 6.75±0.21 | 5.67±0.21 |
| Naja venom | 9.48±0.11 | 9.25±0.11 | 8.75±0.21 | 8.50±0.25 |
| Snake antivenin | 4.58±0.32a | 4.67±0.27a | 5.05±0.30b | 5.30±0.20b |
| CME (400 mg/kg) | 7.08±0.20b | 8.25±0.11 | 8.25±0.11 | 8.25±0.21 |
| CME (800 mg/kg) | 5.08±0.15a | 5.75±0.30b | 7.25±0.17 | 7.50±0.12 |
aP<0.01 vs. Naja venom, bP<0.05 vs. Naja venom
Table 6. Effect of CME on isolated frog heart.
| Dose | Heart rate/min |
| Normal Saline (N) | 56.33±0.42 |
| C1 (100 µg) | 44.33±0.33 |
| C2 (200 µg) | 40.83±0.30 |
| C3 (400 µg) | 40.83±0.70 |
| C4 (800 µg) | 40.50±0.42 |
| C5 (1600 µg) | 42.60±0.84 |
| V1 (1 µg) | 34.32±0.51 |
| V2 (2 µg) | 28.22±0.49 |
| V3 (4 µg) | 21.13±0.42 |
| V3 + C1 | 24.11±0.21 |
| V3 + C2 | 26.41±0.32 |
| V3 + C3 | 34.50±0.50b |
| V3 + C4 | 40.66±0.42b |
| V3 + C5 | 48.50±0.76a |
aP<0.01 vs. V3, bP<0.05 vs. V3, C1 to C5 -Different doses of CME, V1 to V3: Different doses of Naja venom.
Figure 1. Effect of ethanolic bark extract of Cordia macleodii on isolated frog heart in the presence of Naja venom.
In neurotoxic activity studies on frog rectus abdominus muscle, the addition of Naja venom (6 µg/mL) to the bath produced inhibition of the muscle to the stimulant effect of acetylcholine. This effect was significantly inhibited by the CME at the dose of 400 µg/mL.
4. Discussion
Snake bite remains a public health problem in many countries even though it is difficult to be precise about the actual number of cases[18]. Medicinal plants have been used for many years to treat a great variety of diseases including envenomations by animal bites. Therefore, experimental validation of the traditional use of plants is a necessary step in the development of low-cost phytotherapeutic agents. Many plants are recommended in Indian traditional medicine to treat snakebites some of which have been examined for neutralization of snake venom. The present investigation identified bark of Cordia macleodii, reported in Ayurvedic literature to have significant antivenom activity against Naja venom[6]. Administration of the alcoholic extract of Cordia macleodii bark at 200, 400 and 800 mg/kg body weight markedly reduced the mortality in rats. The Naja venom induced lethality was antagonized by CME in a dose dependent manner. Highly significant protection was observed at the dose of 800 mg/kg which was even more than the snake venom antiserum.
According to the WHO, the anti snake venom compounds should be tested regarding its capacity to neutralize venom effects such as lethality, hemorrhagic-necrotizing effects, neutralization of the coagulant and defibrinogenating activity[15]. Most venom possesses the ability to cause local necrosis and hemorrhage when introduced intradermally. Hence, the minimum necrotizing dose and minimum hemorrhagic dose estimation proves a reasonable test for assessing the antivenom activity[9]. Hemorrhagins of venom causes death because of bleeding from vital organs by damaging vascular endothelium[19]. The Naja venom contains most of the components which are responsible for the destruction of physiological process in the body. Several components are present in Naja venom like enzymes, proteolysins, cardiotoxins, hemorrhagins, cytolysins and neurotoxins. The CME was found to significantly reduce the Naja venom induced necrotic and hemorrhagic lesions. In both the studies the CME showed highly significant resultsindicating the protective effect of CME against Naja venom induced lethality.
As a consequence of Naja bite local necrosis occur, which is often due to clot formation[20]. In patients who suffer from snake bites marked progress of defibrination has been observed[21]. CME effectively antagonized the clotting and defibrination induced by Naja venom indicating that CME possess protective effect against Naja venom poisoning.
The Naja venom containing one of the harmful components, proteolysin, is responsible for the local pain and swelling. In our study Naja venom induced the edema in rat paw indicating the edematogenic activity of the venom. The CME decreased the edema induced by venom indicating the antiedematogenic activity of CME. The Cordia macleodii has been reported to have antiinflammatory activity[10]. The hesperetin-7-rhamnoside Lupa-20, isolated from Cordia species possess anti inflammatory and proteolysin neutralization activity[22]. The CME might be showing antiedematogenic activity due to its anti inflammatory and proteolysin neutralization activity.
Naja venom contains large amount of cardiotoxin and phosphodiesterases. It is harmful for body, decreases heart rate and may cause cardiac arrest. In our study, an attempt was made to determine the possible involvement of the cardiac system in the protective effect against Naja venom. The CME was found to prevent the cardiac arrest produced by venom in dose dependent manner. The Cordia bark has been reported to have cardiotonic activity and the aqueous extract was reported to produce significant positive inotropic and chronotropic effects on rat`s heart[23]. Cordia species has been reported to have cardiac glycoside, cordialin a, cordialin B. The preliminary phytochemical studies on CME indicated the presence of cardiac glycosides[24]. The presence of cardiac glycosides might be responsible for the prevention of cardiac arrest and decrease in the heart rate.
Venom releases an enormous amount of histamine into circulation by mast cell degranulation. The released substances could also add to the various toxic signs and in fact may be responsible for some of the toxicity such as anaphylaxis. Mast cells are a rich source of mediators like histamine and platelet activating factors[25]. The preliminary phytochemical analysis indicated the presence of flavonoids in CME. The flavonoids are reported to have anti-inflammatory, antihistaminic, antibradykinin and antiserotonin properties. The presence of flavonoids might be responsible for the protective effect shown by the CME against snake venom poisoning though it's anti-inflammatory, antihistaminic and antiserotonic activity[26].
The Cordia macleodii bark is being successfully used for the treatment of snakebites in Chhattisgarh State. Extensive phytochemical studies carried out on this plant and wide class of compounds including flavonol glycosides, pyrollizidine alkaloids, triterpenes, cardiaquinones, sphingolipids, cerebrosides were isolated[19]. Hence it may be postulated that the anti-snake venom activity of CME might be mediated by the cardiotonic, proteolysin neutralization, anti-inflammatory, antiserotonic and antihistaminic activity. It is possible that the protective effect against snake venom may also be due to precipitation of active venom constituents.
However more elaborate work is required to establish the efficacy of CME as potent anti-snake venom drug. Further experimental work is being carried out to isolate and identify the active principles present in the CME that are responsible for anti-snake venom activity.
Acknowledgments
Authors are deeply grateful to the SLT Institute of Pharmaceutical Sciences, G. G. University, Bilaspur (C.G.) and Oriental College of Pharmacy, Raisen Road, Bhopal for providing research facilities and necessary approval of experimental protocol. This research was funded by G. G. University, Bilaspur (C.G.). [Grant No. GGU/pharm/2009-10].
Comments
Background
Most of the snakebites recorded in India are caused by the
Naja naja. Numerous studies are focusing on supplementary alternatives, such as the use of medicinal plants. Cordia macleodii has already demonstrated anti-inflammatory and healing properties. The present researchwork represents antivenom potential of ethanolic extract of Cordia macleodii bark against Naja venom.
Research frontiers
In the current study, the bark of Cordia macleodii neutralized the toxic activities of Naja naja snake venom and the activity was evaluated by using various parameters laid down by WHO.
Related reports
Snake venoms are a complex mixture of toxic enzymes and proteins such as phospholipases A, myotoxins, hemorrhagic metalloproteases, clotting serineproteases, neurotoxins, cytotoxins and others. Snakebite envenomations are frequently treated with parenteral administration of horse- or sheep-derived antivenoms aiming at the neutralization of toxins. Large number of plants have been reported to neutralize the snake vemon toxicities.
Innovations and breakthroughs
Cordia macleodii is a well known medicinal plant used for the treatment of snakebite and cardiac tonic by traditional healers. But no scientific data is reported for its use against snake bite. In present study, authors have scientifically tried to investigate the antivenom properties of Cordia macleodii extract against snake venom.
Applications
Despite the success of serum therapy, it is important to search for different venom inhibitors, either synthetic or natural, which would complement the action of antivenoms, particularly in relation to the neutralization of local tissue damage. Plant extracts constitute an extremely rich source of pharmacologically active compounds, and a number of extracts has been shown to act against snake venom.
Peer review
This is a valuable research work in which authors have demonstrated the antivenom potential of ethanolic extract of Cordia macleodii bark against Naja venom. Cordia macleodii can be promising antidote against Naja venom poisoning.
Footnotes
Foundation Project: Supported by G. G. University, Bilaspur (C.G.). [Grant No. GGU/pharm/2009-10].
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Dhananjaya BL, Zameer F, Girish KS, D'Souza CJ. Anti-venom potential of aqueous extract of stem bark of Mangifera indica L. against Daboia russellii (Russell's viper) venom. Indian J Biochem Biophys. 2011;48:175–183. [PubMed] [Google Scholar]
- 2.Mukharjee AK. Green medicine as a harmonizing tool to antivenom therapy for the clinical management of snakebite: the road ahead. Indian J Med Res. 2012;136:10–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Subramani M, Selvin P, Ramasamy V, Ambikapathi V, Govindarajan P, Antonysamy M. Antitoxin activity of Mimosa pudica root extracts against Naja naja and Bangarus caerulus venoms. Bangladesh J Pharmacol. 2009;4:105–109. [Google Scholar]
- 4.Meenatchisundaram S, Michael A. Antitoxin activity of Mucuna pruriens aqueous extracts against Cobra and Krait venom by in vivo and in vitro methods. Int J Pharm Tech Res. 2010;2:870–874. [Google Scholar]
- 5.Tan NH, Fung SY, Sim SM, Marinello E, Guerranti R, Aguiyi JC. The protective effect of Mucuna pruriens seeds against snake venom poisoning. J Ethnopharmacol. 2009;123:356–358. doi: 10.1016/j.jep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. New Delhi: CSIR Publication; 1956. [Google Scholar]
- 7.Meenatchisundaram S, Parameshwari G, Michael A. Studies on antivenom activity of Andrographis paniculata and Aristolochia indica plant extracts against Daboia russelli venom by in vivo and in vitro methods. Indian J Sci Tech. 2009;2:76–79. [Google Scholar]
- 8.Lakhmale SP, Acharya R, Yewatkar N. Ethnomedicinal claims on antivenom activity of certain fruit and seed drugs–a review. Ayurpharm Int J Ayur Alli Sci. 2012;1:21–29. [Google Scholar]
- 9.Shirwaikar A, Rajendran K, Bodla R, Kumar CD. Neutralization potential of Viper russelli venom by ethanol extract of Acalypha indica. J Ethnopharmacol. 2004;94:267–273. doi: 10.1016/j.jep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Kumarapppan C, Jaswanth A, Kumarasunderi K. Antihaemolytic and snake venom neutralizing effect of some Indian medicinal plants. Asian Pac J Trop Med. 2011;4:743–747. doi: 10.1016/S1995-7645(11)60185-5. [DOI] [PubMed] [Google Scholar]
- 11.Gomes A, Das R, Sarkhel S, Mishra R, Mukharjee S, Bhattacharya S, et al. Herbs and herbal constituents active against snake bites. Indian J Exp Biol. 2010;48:865–878. [PubMed] [Google Scholar]
- 12.Amui SF, Puga RD, Soares AM, Giuliatti S. Plant-antivenom: database of anti-venom medicinal plants. Electorn J Biotechnol. 2011;14 doi: 10.2225/vol14-issue1-fulltext-1. [DOI] [Google Scholar]
- 13.Qureshi NN, Kuchekar BS, Logade NA, Haleem MA. Analgesic, anti-inflammatory and acute toxicity studies on Cordia macleodii and Leucas ciliata leaves. Int J Pharm Tech Res. 2010;2:1311–1315. [Google Scholar]
- 14.OECD . OECD guidelines for testing of chemicals, acute oral toxicity-acute toxic class method No. 423. Paris: OECD; 2001. pp. 1–17. [Google Scholar]
- 15.Theakston RD, Reid HA. Development of simple standard assay procedures for the characterization of snake venoms. Bull World Health Organ. 1983;61:949–956. [PMC free article] [PubMed] [Google Scholar]
- 16.Alam MI, Gomes A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol. 2003;86:75–80. doi: 10.1016/s0378-8741(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 17.Kannappa RM, Viswanathan S, Thirugnanasambantham P, Kameshwaran L. Effect of Leucus aspera on snake venom poisoning in mice and its possible mechanism of action. Fitoterpia. 1993;64:442–446. [Google Scholar]
- 18.Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1998;76:515–524. [PMC free article] [PubMed] [Google Scholar]
- 19.Warrell DA. Russell's viper biology, venom and treatment of bites. Trans R Soc Trop Med Hyg. 1989;83:732–740. doi: 10.1016/0035-9203(89)90311-8. [DOI] [PubMed] [Google Scholar]
- 20.Theakston RD, Phillips RE, Warrell DA, Galagedera Y, Abeysekera DT, Dissanayaka P, et al. Envenoming by the common krite (Bungarus caeruleus) and Sri Lankan cobra (Naja naja naja): efficacy and complications of therapy with Haffkine antivenom. Trans R Soc Trop Med Hyg. 1990;84:301–308. doi: 10.1016/0035-9203(90)90297-r. [DOI] [PubMed] [Google Scholar]
- 21.Li QB, Yu QS, Huang GW, Tokeshi Y, Nakamurac M, Kinjohc K, et al. Hemostatic disturbances observed in patients with snakebite in south China. Toxicon. 2000;38:1355–1366. doi: 10.1016/s0041-0101(99)00092-6. [DOI] [PubMed] [Google Scholar]
- 22.Thirupathi K, Sathesh Kumar S, Raju SV, Ravikumar B, Krishna DR, Krishna Mohan G. A review of medicinal plants of the genus Cordia: their chemistry and phamacological uses. J Nat Remedies. 2008;8:1–10. [Google Scholar]
- 23.Chauhan MG, Chavan SS. Pharmacognosy and biological activity of Cordia rothii Roem. & Schult. Bark. Indian J Tradit Knowl. 2009;8:598–601. [Google Scholar]
- 24.Khandelwal KR. Practical pharmacognosy techniques and experiment. New Delhi: Nirali Prakashan; 2008. [Google Scholar]
- 25.Slater NT, Freedman JE, Larson-Prior LJ. Russell's viper venom proteins: molecular probes for neurotransmitter receptors: a review. Comp Biochem Physiol C. 1988;91:51–60. doi: 10.1016/0742-8413(88)90168-5. [DOI] [PubMed] [Google Scholar]
- 26.Prabhakar MC, Bano H, Kumar I, Shamsi MA, Khan MS. Pharmacological investigation on vitexin. Planta Med. 1981;43:396–403. doi: 10.1055/s-2007-971532. [DOI] [PubMed] [Google Scholar]


