Abstract
Objective
To screen the essential oil of Juniperus procera (J. procera) (Cupressaceae) for larvicidal activity against late third instar larvae of Anopheles arabiensis (An. arabiensis) Patton, the principle malaria vector in Ethiopia.
Methods
The essential oil of J. procera was evaluated against the larvae of An. arabiensis under the laboratory and semi-field conditions by adopting the World Health Organization standard protocols. The larval mortality was observed for 24 h of post exposure.
Results
The essential oil of J. procera has demonstrated varying degrees of larvicidal activity against An. arabiensis. The LC50 and LC90 values of J. procera were 14.42 and 24.65 mg/L, respectively under the laboratory conditions, and from this data, a Chi-square value 6.662 was observed to be significant at the P=0.05 level. However, under the semi-field conditions the LC50 and LC90 values of J. procera were 24.51 and 34.21 mg/L, respectively and a Chi-square value 4.615 was significant at the P=0.05 level. The observations clearly showed that larval mortality rate is completely time and dose-dependent as compared with the control.
Conclusions
This investigation indicates that J. procera could serve as a potential larvicidal agent against insect vector of diseases, particularly An. arabiensis. However further studies are strongly recommended for the identification of the chemical constituents and the mode of action towards the rational design of alternative promising insecticidal agents in the near future.
Keywords: Essential oil, Juniperus procera, Anopheles arabiensis, Larvicidal activity, Ethiopia
1. Introduction
Mosquitoes serve as a vector for many dreadful diseases like malaria, filariasis, dengue fever, yellow fever and Japanese encephalitis. Malaria is considered to be a major public health issue in the resource-limited settings of Africa, Asia, and Latin America and beyond[1]. The World Health Organization Malaria Report[2] estimated that 3.3 billion people were at the risk of malaria in 2010, and of all geographical regions, populations living in sub-Saharan Africa have the highest risk of acquiring malaria; among 216 million episodes of malaria in 2010, approximately 81%, or 174 million cases, were observed to be from the African region. It was estimated that 91% of 655 000 of malaria deaths in 2010 were from Africa.
In Africa, malaria remains to be a disease of poverty and a cause of poverty[3]. Although malaria is both preventable and treatable, it has been a cause for severe maternal and childhood morbidity and mortality in Ethiopia[4] and kills a poor African child for every 60 seconds[2]. Besides health impact, it is also one of the major impediments to socio-economic development as the major transmission periods quite coincide with the peak agricultural season[5].
It is well-known that the species within the Anopheles gambiae (An. gambiae) complex and the Anopheles funestus (An. funestus) group play a major role in the transmission of malaria in Africa[6],[7]. The An. gambiae complex contains excellent and efficient vectors of malaria [An. gambiae s.s. and Anopheles arabiensis (An. arabiensis)], as well as minor vectors (Anopheles merus) and non-vectors (Anopheles quadriannulatus species A and B)[7]. In Ethiopia An. arabiensis serves as the major vector, while An. funestus, Anopheles pharoensis and Anopheles nili act as secondary vectors[8].
The emergence of Plasmodium falciparum (P. falciparum) resistance strains, development of insecticide resistance, lack of reliable effective vaccine and unaffordable potential antimalarials[5] have imposed a serious negative impact on malaria control interventions. This causes vector control to be considered as a cornerstone to manage the vector populations by reducing/interrupting the cycle of disease transmission[9]. However, vector control programs are hampered by the continuous evolution of mosquito insecticide resistance and it is a potential threat to the global public health concern. The extensive application of conventional insecticides into the mosquitoes breeding sites may also lead to adverse side effects in the aquatic ecosystem[10].
In this context, new alternative arsenals in terms of plant-based mosquitocidal agents with novel mode of action are urgently needed in the battle against vector-borne diseases particularly malaria. Since prehistoric period over thousand plant species have been employed to repel or kill various insects of public health and agriculture concern[11]. The identification and eventual use of indigenous plants for the mosquito larvae control may be much appreciated especially for developing countries as they are readily available and economical for use[12]. Currently, plant-based essential oils have received renewed attention as potent bioactive compounds against various species of mosquitoes[13]. Indeed, source reduction by targeting the larval stage of mosquitoes with potential larvicides is one of the most appropriate malaria vector controls strategy as the target is exceptionally specific unlike adult control[10],[14].
Essential oils are potentially suitable for mosquito larval control since they constitute a rich source of bio-active molecules that are effective and naturally bio-degradable into non-toxic products[15]–[17]. Malaria control is highly dependent on the use of insecticide-based vector control interventions. However, the evolution of insecticide resistance, the environmental and human health hazard, due to chemical warfare is a matter of grave concern. Therefore, there is an urgent need to screen and devise the resistance-proof, user and eco-friendly plant-based insecticides.
Even today in the resource-limited settings people are using numerous plant-based products as mosquitocidal agents despite their ethnic, cultural and socio-economic diversity. Globally, several researchers are keen to pursue with alternative vector control interventions by exploring our traditional knowledge into practice to devise potential plant-based insecticidal and repellent agents. It could pave the path for the reduction of overreliance on conventional insecticides and subsequently to minimize the environmental and human health hazards. In this context, the objective of the present investigation is to determine the larvicidal potential of Juniperus procera (J. procera) essential oil against Afrotropical malaria vector An. arabiensis under the laboratory and simulated field conditions.
2. Materials and methods
2.1. Selection of the plant
J. procera (Cupressaceae), was selected from the secondary data i.e. based on some reports in the literature or some bio-ethnological knowledge from the farmers, traditional healers and local residents[18]. In Ethiopia, the local rural residents have been using this indigenous plant for various curative and miscellaneous purposes. The collected voucher specimen has been pressed, numbered, dried, identified and deposited in the Jimma University Regional Herbarium, Ethiopia.
2.2. Taxonomy of J. procera
| Kingdom | Plantae |
| Division | Pinophyta |
| Class | Pinopsida |
| Order | Pinales |
| Family | Cupressaceae |
| Genus | Juniperus |
| Species | procera |
2.3. Description and traditional usage of J. procera
J. procera is commonly known in English as African Juniper or East African Juniper. It is a coniferous tree native to the mountains of eastern Africa from eastern Sudan south to Zimbabwe, and the southwest of the Arabian Peninsula. The stem is used as a tooth brush and leaves are used to treat or cure tonsillitis[19]. It has also been used to treat with intestinal worm problems. The vapour from a leaf decoction has been inhaled several times a day for the treatment of flu like illness.
2.4. Selection of mosquito species
Nearly all members of An. gambiae complex, that are the potent vectors of malaria in tropical Africa, have shown various degrees of resistance to commonly applied insecticides like dichlorodiphenyltrichloroethane (DDT) and pyrethroids[20]. An. arabiensis and An. gambiae s.s. are the most important vectors of human malaria in sub-Saharan Africa[7], and An. arabiensis is the major malarial vector in Ethiopia. Thus, the Adama Malaria Research Centre, Ethiopia laboratory reared An. arabiensis was chosen for the evaluation of larvicidal activity. It was maintained at (27±2) °C, 75%-85% relative humidity, under 14 L:10 D photoperiod cycles. The larvae were fed with dog biscuits and yeast at 3:1 ratio.
2.5. Plant collection and extraction
The leaves of J. procera was collected from the outskirts of Jimma town, Oromiya Region, Ethiopia, and brought to the laboratory. Taxonomic identification of the plants was performed by the botanists and taxonomists in the Department of Biology, College of Natural Science, Jimma University, Ethiopia. The leaves were washed with tap water, shade-dried at room temperature, mechanically ground by an electrical blender, and steam distilled at 100 °C for at least 6 h to obtain the essential oil. The solvent from the extract was removed using a rotary vacuum evaporator to collect the crude extract. Standard stock solutions were prepared at 1% by dissolving the residues in acetone. From this stock solution, different concentrations were prepared and these solutions were used to assess the larvicidal activity.
2.6. Larvicidal bioassay under laboratory conditions
The J. procera essential oil was done at different concentrations ranging from 5 to 30 mg/L by preparing the required stock solutions for the different concentrations. The larvicidal activities of J. procera against An. arabiensis were determined by adopting WHO standard protocols[21]. One percent stock solution of essential oil was made, from which other lower concentrations were prepared in acetone. The desired concentrations of test solution were achieved by adding 1 mL of an appropriate stock solution to 249 mL of tap water taken in a 500 mL beaker. Twenty five An. arabiensis were exposed to various concentrations of J. procera essential oil. A pinch of larval food (125 mg) consisting of yeast powder and dog biscuit (1:1) were provided. The control experiments were also run parallel with each replicate. Every bioassay was carried out in environmentally controlled conditions [temperature ∼ (25±2) °C; relative humidity ∼ (80±10) %; 14 h light and 10 h dark cycle], and replicated four times with mosquitoes from different rearing batches. The percent of mortality was reported from the average of four replicates.
2.7. Larvicidal bioassay under semi-field conditions
The J. procera larvicidal efficacy was also evaluated under the simulated field conditions by following the WHO standard protocols[21] and method of Mwaiko and Savaeli[22]. The artificial containers (plastic bowls) of 18 cm wide (diameter) by 7.5 cm depth of 1.5 L capacity were used for the bioassays in the semi-field and the containers were half-buried in the ground, and 249 mL of water from the natural breeding sites were added into each bowl. Each container was then treated with 1 mL of the stock solution of J. procera essential oil so that final volume was 250 mL each. The concentrations ranging from 15 to 40 mg/L were used for the tests in the semi-field conditions. Batch of 25 wild collected late third instar anopheline larvae were exposed into various test concentrations. Subsequently the containers were covered with nylon mosquito netting to prevent debris and other mosquitoes from egg laying. Four replicates were conducted for the treatments and two for the controls as described above. In both laboratory and field experiments, mortality and survival of larvae were determined after 24 h of exposure and the observations were also made on the behavior of larvae. The moribund and dead larvae in each concentration were combined in quadruplicate and expressed as percentage mortalities.
2.8. Data analysis
It was important to obtain no less than three mortality counts of between 10% and 90%. In cases where the control mortality was between 5%-20%, the observed percent mortality (%M) was corrected by Abbott's formula[23]:
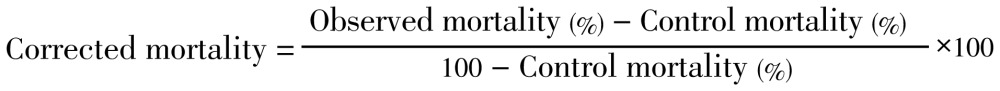 |
The average larval mortality data were subjected to probit analysis for calculating LC50, LC90 and other statistics at 95% confidence limits of upper confidence limit and lower confidence limit, and chi-square values were calculated using the SPSS12.0 (Statistical Package for Social Sciences: Chicago, IL, USA) software. Results with P<0.05 were considered to be statistically significant.
3. Results
The larvicidal activity of J. procera essential oil against late third instar larvae of An. arabiensis is presented in Tables 1, Table 2, Figure 1, Figure 2 and Figure 3.
Table 1. Larvicidal activity of J. procera essential oil against An. arabiensis under laboratory conditions.
| Concentrations ( mg/L) | Mortality (%) (Mean± SD) | Larvicidal activity (95% CI, mg/L) |
|
| LC50 (LCL-UCL) | LC90 (LCL-UCL) | ||
| 5 | 0 | ||
| 10 | 28±4 | ||
| 15 | 49.300±2.309 | ||
| 20 | 64±4 | 14.42 (12.617-16.116) | 24.65 (21.558- 30.253) |
| 25 | 96±4 | ||
| 30 | 100 | ||
| Control | 0 | ||
| Chi-square(χ2) | 6.662 | ||
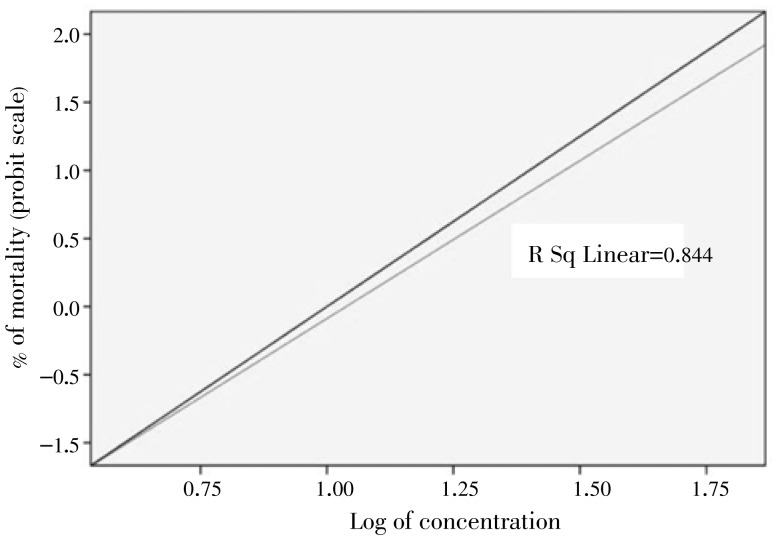
| R2 | 0.844 | ||
Each value (mean±SD) represents mean value of three replicates.
LC50-Lethal concentration that kills 50% of the exposed larvae.
LC90-Lethal concentration that kills 90% of the exposed larvae.
UCL=Upper confidence limit.
LCL=Lower confidence limit.
Table 2. Larvicidal activity of J. procera essential oil against An. arabiensis under field conditions.
| Concentrations (mg/L) | Mortality (%)(Mean±SD) | Larvicidal activity (95% CI, mg/L) |
|
| LC50 (LCL-UCL) | LC90 (LCL-UCL) | ||
| 15 | 0 | ||
| 20 | 33.300±2.309 | ||
| 25 | 50.600±2.309 | ||
| 30 | 70.600±2.309 | 24.51 (22.732-26.232) | 34.21 (31.437- 38.801) |
| 35 | 93.300±2.309 | ||
| 40 | 100 | ||
| Control | 0 | ||
| Chi-square (χ2) | 4.615 | ||
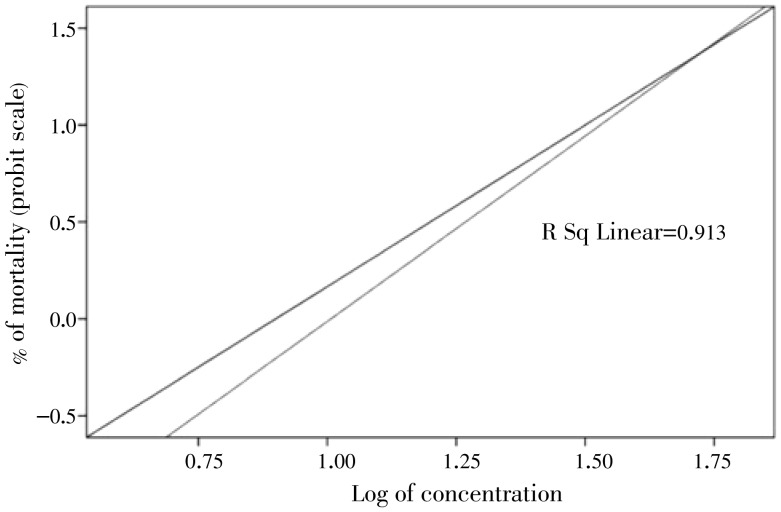
| R2 | 0.913 | ||
Each value (mean±SD) represents mean value of three replicates.
LC50-Lethal concentration that kills 50% of the exposed larvae.
LC90-Lethal concentration that kills 90% of the exposed larvae.
UCL=Upper confidence limit.
LCL=Lower confidence limit.
Figure 1. LC50 and LC90 values of the essential oil of J. procera against An. arabiensis under laboratory and field conditions.
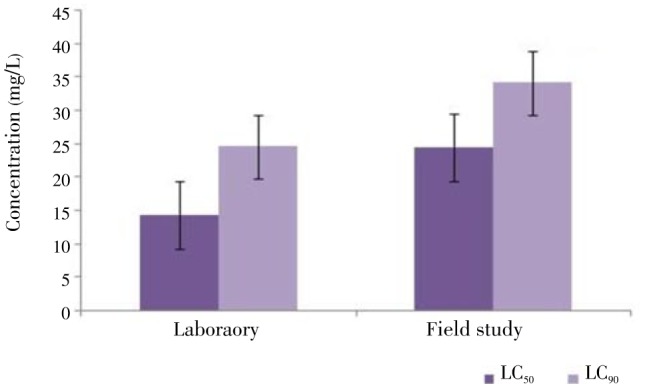
Figure 2. Regression line of log concentration of J. procera essential oil against An. arabiensis under laboratory conditions.
Figure 3. Regression line of log concentration of J. procera essential oil against An. arabiensis under field conditions.
The obtained results established the potentiality of J. procera as a mosquito larvicidal agent. The LC50 and LC90 values were 14.42 and 24.65 mg/L, respectively under the laboratory conditions (Table 1, Figure 1 and Figure 2). In addition, the regression analysis indicates the mortality rate (Y) to be positively correlated with J. procera concentration (X) having a regression value (R) close to one in each case as shown in Figure 2 and Figure 3.
The larvicidal efficacy of J. procera essential oil against late third instar wild-collected anopheline larvae under the semi-field conditions is shown in Table 2, Figure 1 and Figure 3. It shows the highest larvicidal activity with LC50=24.518 and LC90=34.212 mg/L. The results clearly exhibit that the laboratory reared An. arabiensis larvae are more susceptible than the wild-collected anopheline larvae. The bioassay findings evidently suggest that the percent of mortality directly proportional to concentration and the rate of mortality were dose and time dependent (Figure 2 and Figure 3).
4. Discussion
Indeed, malaria is one of the deadliest infectious diseases in the resource-constrained settings. It is a disease of poverty inflicting a serious negative impact on human health and socio-economic development in the poorest countries of the world that cannot afford to succeed[3]. In Ethiopia, at the moment malaria control heavily relies on frontline vector control interventions like regular deployment of insecticide-treated nets and indoor residual spraying. However, their potentialities has been largely undermined due to the wide-spread emergence of insecticide resistance to the most commonly used insecticides like DDT and pyrethroids[9], and due to resistance reported across sub-Saharan Africa and rest of the world too[24].
Besides, there are no new classes of insecticides in the final stage of development[25]. In this perspective, the screening of locally available indigenous ethnomedicinal plants as mosquito larvicidal and adulticidal agents may eventually lead to their usage in plant-based mosquito abatement practices[10]. As per our best of knowledge and understanding so far there are no studies have been reported on the mosquito larvicidal activity of J. procera. Therefore, the present investigation was an attempt to explore on the promising novel mosquito larvicidal agent from an Ethiopian indigenous ethnomedicinal plant J. procera.
The results clearly suggest that the essential oil of J. procera was found to be the most effective one, against the late third instar larvae of An. arabiensis by providing 100% mortality at 30 mg/L with LC50 and LC90 values at 14.42 and 24.65 mg/L, respectively under laboratory conditions. The finding is in accordance with an earlier Ethiopian study reported by Massebo et al. that essential oils of other 11 local plants were evaluated for larvicidal activities against laboratory reared colonies of An. arabiensis and Aedes aegypti larvae[26]. The LC50 values of the oils ranged from 17.5 to 85.9 mg/L against An. arabiensis. However, the LC50 value of Chenopodium ambrosioides and Ocimum lamiifolium essential oil were 17.5 and 20.9 mg/L, respectively against An. arabiensis. Similarly another Ethiopian study have indicated that the crude and column chromatographic fractions of methanol leaf extract of Jatropha curcas (J. curcas) were tested for their larvicidal activities against late third instar larvae of An. arabiensis. The crude methanol leaf extract of J. curcas have had similar larvicidal activity to 0.5 mg/L temephos (positive control) at test concentrations ranging from 125-1000 mg/L while column chromatographic fractions (F1 and F2) of the crude methanol leaf extract of J. curcas showed similar larvicidal activities to 0.5 mg/L temephos at 62.5 and 125 mg/L test concentrations[27].
It is interesting to note that the larvicidal effect of J. procera essential oil against anopheline larvae under the semi-field conditions were LC50=24.51 and LC90=34.21 mg/L. Methanol leaf extracts of two Ethiopian traditional medicinal plants Cymbopogon citratus (C. citratus) and Croton macrostachyus (C. macrostachyus) were screened for larvicidal activity against late third instar larvae of An. arabiensis Patton, a potent malaria vector in Ethiopia. C. citratus extract has exhibited potent larvicidal activity than C. macrostachyus at lower concentrations. The LC50 and LC90 values of C. citratus were 74.02 and 158.20 mg/L, respectively and the LC50 and LC90 values of C. macrostachyus were 89.25 and 224.98 mg/L, respectively[10].
The mosquito larvicidal bioassay findings of the present study clearly demonstrated that the percent of mortality rate directly proportional to concentration and the mortality rate was highly dose and exposure time dependent. The results is in accordance with the findings of Elimam et al. which reported that the aqueous extracts from leaves of Ricinus communis against the An. arabiensis and the LC50 values against the 2nd, 3rd and fourth instar larvae of An. arabiensis were 403.65, 445.66 and 498.88 mg/L, respectively[28]. Methanolic extracts of leaves and seeds from Tribulus terrestris was tested against 3rd instar larvae of An. arabiensis under laboratory condition. The seeds extract showed high insecticidal activity at all concentrations compared to the leaves extract and the LC50 were 36.5 and 123.1 mg/L for seeds and leaves extract, respectively[29]. When comparing with the previous studies the present investigation results are quite noteworthy and remarkable even under the semi-field conditions. Therefore, the essential oil of J. procera could serve as a potential larvicidal agent under the field conditions as well.
The essential oils obtained by hydrodistillation of dry leaves from C. citratus (DC.) Stapf, Ocimum canum Sims, Ocimum gratissimum L. and Thymus vulgaris L. were analyzed for their larvicidal activity against the fourth instar larvae of An. gambiae Giles. These essential oils have remarkable larvicidal properties as they could induce 100% mortality in the larvae of An. gambiae at the concentration of 100 mg/L for C. citratus, 200 mg/L for Thymus vulgaris, 350 mg/L for Ocimum gratissimum and 400 mg/L for Ocimum canum. The essential oil of C. citratus was found to be the most efficient, with respective values of LC50=18 mg/L and LC80=25 mg/L[30].
The present investigation results clearly demonstrated that the laboratory reared An. arabiensis larvae were found to be more susceptible to J. procera essential oil than the wild collected larvae of Anopheline population in terms of lethal concentration. The results are quite comparable with the previous studies, which have been conducted in Ethiopia. Three essential oils have already been tested to evaluate their larvicidal activity against third and fourth instars stage of wild-collected anopheline larvae in the simulated field conditions. The essential oil of Ocimum lamiifolium showed highest larvicidal activity (LC50=34 mg/L and LC90=63.5 mg/L) followed by Chenopodium ambrosioides (LC50=47.3 mg/L and LC90=97.9 mg/L); the least activity was exhibited by Piper nigrum (LC50=110.6 mg/L; LC90=162.9 mg/L). In all cases the wild-collected anopheline larvae have had higher LC50 and LC90 values of the essential oils than the laboratory reared An. arabiensis larvae[26]. It could be possibly explained that the wild anopheline larvae exposed to various chemical, biotic and non-biotic factors, might have contributed to the resistance development. In Ethiopia, several anopheline species such as An. arabiensis, Anopheles paharoensis, An. funestus, Anopheles nili, Anopheles coustani, Anopheles marshallii and Anopheles demeilloni had earlier been reported as malaria vectors, the former being the predominant one[31],[32].
The present study results have shown clearly that the Ethiopian ethnomedicinal plant J. procera is highly efficacious for the control of mosquitoes under both laboratory and semi-field conditions. It was also evident that the percent of larval mortality rate was dosage dependent. The present results have been observed to concord with the previous study conducted in Sudan by Zarroug et al., who tested some Sudanese plant extracts against An. arabiensis larvae and obtained significant mortality in the second and fourth instar larvae while using water extracts of Balanites aegyptiaca[33]. Very recently 381 crude plant extracts were investigated by Maharaj et al.[34] for their larvicidal effect on An. arabiensis, and plants were observed to exhibit promising results during screening and were selected for further phytochemical investigations. The results of the testing of the fractions generated identified one fraction of the plant Toddalia asiatica as being potent against the An. arabiensis larvae, among the rest of the tested plants.
Indeed the wide-spread Plasmodium falciparum chloroquine resistant strains have created severe set-back in malaria control. Though currently artemisinin-based combination therapy serves a pivotal role to combat malaria, they are mostly inaccessible and unaffordable in the resource-poor settings[35],[36]. In this context, vector control is a mainstay in the malaria control programme of Ethiopia. It is well-known that the vector control interventions are highly depending upon the chemical insecticides such as DDT and pyrethroids. However, the extensive and injudicious use of synthetic insecticides in the vector control interventions has resulted in environmental and human-health hazards through persistence and accumulation of non-biodegradable toxic components in the ecosystem, development of insecticide resistance among mosquito species, biological magnification in the food chain and toxic effects on human health and non-target organisms[37],[38].
In these perspectives, an ideal insecticide must meet or possess the intrinsic-qualities like low-cost, ready availability, easy-mode of application, pleasurable to use, undisruptive to environment, and safe for human and livestock. Nevertheless, the majority of the existing conventional insecticides are deficient to fulfill these decisive factors. In this prospect, plant-based insecticidal agents are relatively target specific, easily accessible, affordable, bio-degradable, and of user and environmental-friendly nature. Currently, the evolution of multiple resistances among the insect disease vectors is an immense practical problem, challenging the existing vector-borne disease control success. In this context, over the past three decades there has been a renewed interest in designing potential plant-based resistance-proof insecticidal agents as plants possess a number of bio-active molecules and secondary metabolites resulting with complex mode of actions to tackle with the insecticide-resistance issue. Hence, further studies are required to be warranted for intensive screening and evaluation of mosquitocidal agents in terms of larvicidal, adulticidal, ovicidal, repellent and attractant from the traditional ethnomedicinal plant as a natural source of insecticides.
Despite several decades of control efforts still malaria remains to be the leading cause of morbidity and mortality in Ethiopia. At the moment, Plasmodium falciparum resistance strains and insecticide resistance, and unaffordable effective antimalarials have contributed to the resurgence and emergence of malaria incidence and malaria-related illness [36],[39]. In this context, the J. procera plant, well-known by majority of the Ethiopians for its ethnomedicinal values, can be used as a phytotherapeutic agent to treat various ailments. In addition, it has been used as a traditional insect repellent to drive away mosquitoes and other blood-sucking insects by burning its leaves, barks and whole parts of plant in Ethiopia[40]. In the urban areas source reduction is one of the best options to combat mosquito-borne diseases like malaria, filariasis, yellow fever and dengue fever, as other vector control intervention like indoor residual spraying is quite unfeasible or impracticable.
Conclusively, the present investigation exhibited that J. procera could serve as a promising larvicidal agent against An. arabiensis under the laboratory as well as semi-field conditions with lesser LC50 doses. Since the candidate plant enjoys the widespread socio-cultural legitimacy among the majority of the Ethiopians, it enables us to implement as a sustainable alternative intervention in terms of an ideal mosquito larvicidal agent. Therefore, it could serve as a potential supplementary tool in the integrated malaria vector control strategy. However, further studies are strongly recommended for the isolation, identification, analysis, and larvicidal properties of J. procera bioactive ingredients and other secondary metabolites. In addition, the larvicidal mechanism and the toxicity towards the non-target beneficial organisms in the aquatic eco-system must also be scientifically validated. Besides, small-scale pilot studies have to be conducted to investigate its potential residual effects and associated health-risks in the near future.
Acknowledgments
The authors sincerely would like to acknowledge Jimma University Research & Publication division for pursuing this research work by providing financial assistance. We would like to thank Mrs Melita Prakash for her sincere assistance in editing this manuscript. We are also grateful to the staff members of Adama Malaria Research Centre, Ethiopia for their technical assistance in terms of providing mosquito larvae. Without their contribution, this study would have been impossible. Our last but not least heartfelt thanks go to our colleagues from the School of Environmental Health Science, Faculty of Public Health, Jimma University, Jimma, Ethiopia, for their kind support and cooperation.
Comments
Background
Malaria is a leading public health issue in the resource-poor settings. It causes considerable mortality and threatens lives of half of the world population. Malaria is a disease of poverty and a cause of poverty. It is prevalent in the resource-poor settings of Africa, Asia and beyond. Therefore there is need of low-cost indigenous and eco-friendly plant-based insecticidal and repellent agents as vector control tool to address the emerging insecticide resistance issue.
Research frontiers
The present research work depicts larvicidal activity of J. procera essential extract against An. arabiensis under the laboratory and semi-field conditions following WHO standard protocols.
Related reports
Several studies reported the insecticidal activity of the plant-based products against various medically important insects particularly mosquitoes. However, only a limited number of studies carried out by the plants essential oils. This study has been performed with the essential oil of J. procera, an Ethiopian ethnomedicinal plant against a potent malaria vector in the Afro-tropical region, where malaria is more prevalent.
Innovations and breakthroughs
There are studies demonstrated that J. procera can be used as a potent phytotherapuetic agent against various ailments. However, this is the first report on the mosquitocidal potentialities of J. procera.
Applications
From the literature survey it has been found that J. procera is safe to humans. It has ethnomedicinal values against various ailments across Africa. This scientific study support and suggest the use of this plant as a larvicidal agent, which could minimize the environmental pollution and adverse effect on human health.
Peer review
This is a valuable research work in which authors have demonstrated the mosquito-larvicidal activity of J. procera under the laboratory and field conditions. The activity was assessed based on WHO standard procedures. The essential oil of J. procera was found to be a promising larvicidal agent against insect vector of diseases.
Footnotes
Foundation Project: supported by: Student Research Project of Jimma University, Ethiopia (Grant No.: PG/JU/SRP/1149/2011).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Karunamoorthi K. Global malaria eradication: is it still achievable and practicable? In: Peterson AM, Calamandrei GE, editors. Malaria: etiology, pathogenesis and treatments. New York, USA: Nova Science Publishers; 2012. [Google Scholar]
- 2.World Health Organization . World malaria report 2012. Geneva: World Health Organization; 2012. [Online] Available from: http://www.who.int/malaria/publications/world_malaria_report_2012/en/. [Accessed on 15th December, 2013]. [Google Scholar]
- 3.Karunamoorthi K. Global malaria burden: socialomics implications. J Socialomics. 2012;1:e108. [Google Scholar]
- 4.Karunamoorthi K, Deboch B, Tafere Y. Knowledge and practice concerning malaria, insecticide-treated net (ITN) utilization and antimalarial treatment among pregnant women attending specialist antenatal clinics. J Public Health. 2010;18(6):559–566. [Google Scholar]
- 5.Karunamoorthi K, Bekele M. Prevalence of malaria from peripheral blood smears examination: a 1-year retrospective study from the Serbo Health Center, Kersa Woreda, Ethiopia. J Infect Public Health. 2009;2(4):171–176. doi: 10.1016/j.jiph.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Ketseoglou I, Bouwer G. The susceptibility of five African Anopheles species to Anabaena PCC 7120 expressing Bacillus thuringiensis subsp. israelensis mosquitocidal cry genes. Parasit Vectors. 2012;5:220. doi: 10.1186/1756-3305-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coetzee M, Craig M, Le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000;16:74–77. doi: 10.1016/s0169-4758(99)01563-x. [DOI] [PubMed] [Google Scholar]
- 8.Karunamoorthi K, Yirgalem A. Insecticide risk indicators and occupational insecticidal poisoning in indoor residual spraying. Health Scope. 2013;1(4):163–170. [Google Scholar]
- 9.Karunamoorthi K. Vector control: a cornerstone in the malaria elimination campaign. Clin Microbiol Infect. 2011;17(11):1608–1616. doi: 10.1111/j.1469-0691.2011.03664.x. [DOI] [PubMed] [Google Scholar]
- 10.Karunamoorthi K, Ilango K. Larvicidal activity of Cymbopogon citratus (DC) Stapf. and Croton macrostachyus Del. against Anopheles arabiensis Patton, a potent malaria vector. Eur Rev Med Pharmacol Sci. 2010;14(1):57–62. [PubMed] [Google Scholar]
- 11.Karunamoorthi K, Ramanujam S, Rathinasamy R. Evaluation of leaf extracts of Vitex negundo L. (Family: Verbenaceae) against larvae of Culex tritaeniorhynchus and repellent activity on adult vector mosquitoes. Parasitol Res. 2008;103:545–550. doi: 10.1007/s00436-008-1005-5. [DOI] [PubMed] [Google Scholar]
- 12.Monzon RB, Alvior JP, Luezon LL, Morales AS, Mutuo FE. Larvicidal potential of five Philippine plants against Aedes aegypti (Linnaeus) and Culex quinquefasciatus (Say) Southeast Asian J Trop Med Public Health. 1994;25:755–759. [PubMed] [Google Scholar]
- 13.El-Hag EA, El-Rahman A, El-Nadi H, Zaitoon AA. Effects of methanolic extracts of neem seeds on egg hatchability and larval development of Culex pipiens mosquitoes. Indian Vet J. 2001;78:199–201. [Google Scholar]
- 14.Amer A, Mehlkorn H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res. 2006;99:478–490. doi: 10.1007/s00436-006-0184-1. [DOI] [PubMed] [Google Scholar]
- 15.Lucia A, Gonzalez Audino P, Seccacini E, Licastro S, Zerba E, Masuh H. Larvicidal effect of Eucalyptus grandis essential oil and turpentine and their major components on Aedes aegypti larvae. J Am Mosq Cont Assoc. 2007;3:299–303. doi: 10.2987/8756-971X(2007)23[299:LEOEGE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Cheng SS, Huang CG, Chen WJ, Kuo YH, Chang ST. Larvicidal activity of tectoquinone isolation from red heartwood-type Cryptomeria japonica against two mosquito species. Bioresour Technol. 2008;99:3617–3622. doi: 10.1016/j.biortech.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 17.Cheng SS, Chua MT, Chang EH, Huang CG, Chen WJ, Chang ST. Variations in insecticidal activity and chemical composition of leaf essential oils from Cryptomeria japonica at different ages. Bioresour Technol. 2009;100:465–470. doi: 10.1016/j.biortech.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 18.Karunamoorthi K, Ilango K, Endale A. Ethnobotanical survey of knowledge and usage custom of traditional insect/mosquito repellent plants among the Ethiopian Oromo ethnic group. J Ethnopharmacol. 2009;125(2):224–229. doi: 10.1016/j.jep.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Seshathri K, Thiyagarajan T. Antimicrobial activity of chewing sticks of Jimma-Ethiopia against Streptococcus pyogens. J Phytol. 2011;3(8):34–37. [Google Scholar]
- 20.Warrel DA, Gilles HM. Bruce-Chwatt's essential malariology. 3rd edition. Oxford: Taylor & Francis; 1993. p. 340. [Google Scholar]
- 21.World Health Organization . Guidelines for laboratory and field testing of mosquito larvicides. Geneva: World Health Organization; 2005. [Online] Available from: http://whqlibdoc.who.int/hq/2005/who_cds_whopes_gcdpp_2005.13.pdf. [Accessed on 22th December, 2013]. [Google Scholar]
- 22.Mwaiko GL, Savaeli ZX. Lemon peel oil extract as mosquito larvicide. East Afr Med J. 1994;71:797–799. [PubMed] [Google Scholar]
- 23.Abbott WS. A method for computing the effectiveness of the insecticide. 1925. J Am Mosq Control Assoc. 1987;3:302–303. [PubMed] [Google Scholar]
- 24.Corbel V, N'Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101:207–216. doi: 10.1016/j.actatropica.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Kilama W, Ntoumi F. Malaria: a research agenda for the eradication era. Lancet. 2009;374:1480–1482. doi: 10.1016/S0140-6736(09)61884-5. [DOI] [PubMed] [Google Scholar]
- 26.Massebo F, Tadesse M, Bekele T, Balkew M, Gebre-Michael T. Evaluation on larvicidal effects of essential oils of some local plants against Anopheles arabiensis Patton and Aedes aegypti Linnaeus (Diptera, Culicidae) in Ethiopia. Afr J Biotechnol. 2009;8(17):4183–4188. [Google Scholar]
- 27.Zewdneh T, Mamuye H, Asegid T, Yalemtsehay M, Beyene P. Larvicidal effects of Jatropha curcas L. against Anopheles arabiensis (Diptera: Culicidea) Momona Ethiop J Sci. 2011;3(1):52–64. [Google Scholar]
- 28.Elimam AM, Elmalik KH, Ali FS. Larvicidal, adult emergence inhibition and oviposition deterrent effects of foliage extract from Ricinus communis L. against Anopheles arabiensis and Culex quinquefasciatus in Sudan. Trop Biomed. 2009;26(2):130–139. [PubMed] [Google Scholar]
- 29.El-Sheikh TM, Hanan AM, Shalaby NM. Insecticidal and repellent activities of methanolic extract of Tribulus terrestris L. against the malarial vector Anopheles arabiensis (Diptera: Culicidae) Egypt Acad J Biol Sci. 2012;5(2):13–22. [Google Scholar]
- 30.Tchoumbougnang F, Dongmo PM, Sameza ML, Mbanjo EG, Fotso GB, Zollo PH, et al. [Larvicidal activity against Anopheles gambiae Giles and chemical composition of essential oils from four plants cultivated in Cameroon] Biotechnol Agron Soc Environ. 2009;13:77–84. French. [Google Scholar]
- 31.Adugna N, Petros B, Woldegiorgis M, Tilahun D, Lulu M. A study on the status of Anopheles tenebrosus (Donitz, 1902) in the transmission of malaria in Sille, Southern Ethiopia. Ethiop J Health Dev. 1998;12:75–80. [Google Scholar]
- 32.Taye A, Hadis M, Adugna N, Tilahun D, Wirtz RA. Biting behavior and Plasmodium infection rates of Anopheles arabiensis from Sille, Ethiopia. Acta Trop. 2006;97:50–54. doi: 10.1016/j.actatropica.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Zarroug IM, Nuggud AD, Bashir AK, Mageed AA. Evaluation of Sudanese plants. Int J Crude Drug Res. 1988;26:77–80. [Google Scholar]
- 34.Maharaj R, Maharaj V, Crouch NR, Bhagwandin N, Folb PI, Pillay P, Gayaram R. Screening of selected ethnomedicinal plants from South Africa for larvicidal activity against the mosquito Anopheles arabiensis. Malar J. 2012;11:320. doi: 10.1186/1475-2875-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karunamoorthi K, Tsehaye E. Ethnomedicinal knowledge, belief and self-reported practice of local inhabitants on traditional antimalarial plants and phytotherapy. J Ethnopharmacol. 2012;141(1):143–150. doi: 10.1016/j.jep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Karunamoorthi K, Sabesan S, Jegajeevanram K, Vijayalakshmi J. The role of traditional anti-malarial plants in the battle against global malaria burden. Vector Borne Zoonotic Dis. 2013;13:521–544. doi: 10.1089/vbz.2011.0946. [DOI] [PubMed] [Google Scholar]
- 37.Bansal SK, Singh KV, Sharma S, Sherwani MR. Comparative larvicidal potential of different plant parts of Withania somnifera against vector mosquitoes in the semi-arid region of Rajasthan. J Environ Biol. 2011;32(1):71–75. [PubMed] [Google Scholar]
- 38.Devine GJ, Furlong MJ. Insecticide use: contexts and ecological successions. Agr Human Values. 2007;24:281–306. [Google Scholar]
- 39.Karunamoorthi K, Bekele M. Changes in malaria indices in an Ethiopian health centre: a five year retrospective analysis. Health Scope. 2012;1(3):118–126. [Google Scholar]
- 40.Karunamoorthi K, Mulelam A, Wassie F. Assessment of knowledge and usage custom of traditional insect/mosquito repellent plants in Addis Zemen Town, South Gonder, North Western Ethiopia. J Ethnopharmacol. 2009;121(1):49–53. doi: 10.1016/j.jep.2008.09.027. [DOI] [PubMed] [Google Scholar]