Abstract
Objective
To study the in vitro anthelmintic efficacy of Cassia alata (C. alata), Cassia angustifolia (C. angustifolia) and Cassia occidentalis (C. occidentalis).
Methods
Crude ethanol extract from leaves of the three plants were prepared in rotary evaporator and different concentrations (10, 20 and 40 mg/mL) of leaf extracts were used for treatment on different representatives of helminthes (Heterakis gallinarum, Raillietina tetragona and Catatropis sp.) from domestic fowl (Gallus gallus domesticus). Loss of motility and death were monitored frequently.
Results
C. alata showed early paralysis in all worms treated followed by C. angustifolia. C. occidentalis in combination with C. alata together caused early paralysis in all treated worms than the combination of C. alata with C. angustfolia. While Heterakis gallinarum in control survived for (81.33±2.07) h, treated worms lost their motility at (5.71±0.10) h, (6.60±0.86) h and (13.95±0.43) h with C. angustifolia, C. alata and C. occidentalis respectively at a concentration of 40 mg/mL which showed better efficacy than albendazole. Catatropis sp. survival period was (26.49±1.38) h in control, but with plant treatment, it lost its motility in just (0.57±0.08) h, (1.00±0.12) h and (1.47±0.40) h at 40 mg/mL concentration of C. alata, C. angustifolia and C. occidentalis respectively. Raillietina tetragona on the other hand became paralysed at (1.68±0.27) h, (2.95±0.29) h and (4.13±0.31) h with above concentrations treated with three plants respectively, however in control it survived up to (81.93±4.71) h.
Conclusions
This present study indicated broad spectrum vermifugal activity of all plants tested.
Keywords: Anthelmintic, Cassia, Parasites, Extracts, Paralysis, Vermifugal
1. Introduction
Helminth parasites problem is a major limiting factor in livestock production. Control of helminth infection in domestic animals is widely based on anthelmintic drugs. Its prolonged use has led to the additional problem of emergence of anthelmintic resistant in livestock which has become a constant concern. Dependence of control programs on a limited number of compounds makes drug resistance an even greater concern and careful management of those available is imperative. According to World Health Organization, medicinal plants would be the best source to obtain a variety of drugs. Therefore such plants should be investigated to better understand its safety and efficacy[1]. The origin of many effective drugs is found in traditional medicine practices and has made several researchers to undertake studies for evaluating folklore medicinal plants on their proclaimed anthelmintic efficacy[2]–[4]. Use of medicinal plants and studies on the chemistry and pharmacology of natural products have grown considerably in the second half of 20th century[5],[6]. Medicinal value of plants is attributed to only few active constituents that produce a definite physiological effect. Alkaloids, flavonoids, tannins and phenolic compounds form a major group of such bioactive constituents[7]. Plants and herbs have attained a significant role not only as therapeutic agent but also as health maintaining agent.
Cassia plants are widely distribute in India and West-Bengal in particular and its many species are having medicinal activities. Cassia alata (C. alata) has been recognized for centuries in traditional medicine for its activities such as antimicrobial[8], antifungal[9], purgative[10], anti-inflammatory[9],[11], analgesic[12], antitumor[13], and hypoglycemic[9]. Cassia angustifolia Vahl. (C. angustifolia ) is known for its purgative properties and for regularization of bowel movements[14] while Cassia occidentalis Linn. (C. occidentalis) have been traditionally used for treatment of constipation, skin diseases, as laxative and diuretic[15]. It is also used for treatment of fever, menstrual problems, tuberculosis, diuretic, anemic, liver complaints, and as a tonic for general weakness and illness. Decoction of leaves of C. angustifolia and C. alata is widely practiced in India as compared to other parts of the plant. In addition, their leaves have been listed as a treatment for gastrointestinal worms in medicinal plant resources[14].
Furthermore, these plants have attracted the attention of many scientists because of the presence of flavonoid compounds, anthraquinones and phenols. Kundu and Lyndem[16] reported anthelmintic activity of the above three Cassia plants on Raillietina tetragona (R. tetragona) as well as C. alata on Hymenolepis diminuta[17]. Moreover like commercial drugs themselves, majority of anthelmintic plants are helminth specific, showing activity against a particular species or group of parasites[18]. In order to authenticate their broad anthelmintic spectrum, it is necessary to investigate the effect of all three Cassia species on common helminthes of domestic fowl (Gallus gallus domesticus).
2. Materials and methods
2.1. Preparation of plant extracts
Fresh young leaves of C. angustifolia, C. alata and C. occidentalis were collected, weighed and washed with deionized water, and oven-dried at 50 °C. About 150 g of powdered leaves were extracted with 1 L of ethanol (90%) in a Soxhlet apparatus for 7-8 h. The final crude extracts were recovered using a rotary evaporator and stored at 4 °C until further use.
2.2. Chemicals and drugs
All chemicals used were of standard analytical grade, obtained from Merck (USA). Ethanol was supplied by Bengal Chemicals (Kolkata, India). Two reference drugs praziquantel with trade name Distocide (composed of 600 mg praziquantel) is a product of Chandrabhagat Pharma Pvt. Ltd (Mumbai, India) and albendazole with trade name Zentel (composed of 400 mg albendazole) is a product of Glaxo SmithKline Pharmaceuticals Ltd (Mumbai, India).
2.3. Experimental design
Helminth representatives viz. Heterakis gallinarum (nematoda) (H. gallinarum), Catatropis sp. (trematoda) and R. tetragona (cestoda) were collected from intestine of freshly slaughtered domestic fowl. Live worms were treated in vitro with three leaf extracts at various concentrations (10, 20 and 40 mg/mL, in 0.9% phosphate-buffered saline (pH 7.4). While 5, 10 and 20 mg/mL of albendazole was used as a reference drug for H. gallinarum, praziquantel (0.001, 0.0025 and 0.005 mg/mL) was tested for the other two worms. Control worms were maintained in phosphate-buffered saline with 1% dimethylsulfoxide at (37±1) °C. Combinations of plant extracts in ratio 1:1 (C. alata+C. occidentalis, C. alata+C. angustifolia, C. occidentalis+C. angustifolia) were also tested on fresh worms at (37±1) °C to observe any synergistic or additive effect.
2.4. Statistical analysis
Data were represented as mean±SD for each group (n=6). For determining statistical significance, SD and analysis of variance (ANOVA) at 5% level significance were employed; P<0.005 was considered significant.
3. Results
However, since each tested worms are different, it is therefore convenient to compare different leaf extracts of Cassia plants on a particular parasite.
3.1. Single plant extract treatment
For H. gallinarum, C. angustifolia showed early paralysis at (16.12±3.36) h compared to other two plants at 10 mg/mL, but at 20 mg/mL, both C. alata and C. angustifolia took almost equal time [(11.57±1.43) h and (11.79±3.19) h] to paralyze the worm. At 40 mg/mL paralysis occurred early with C. angustifolia [(5.71±0.1) h] followed by C. alata [(6.6±0.86) h] and C. occidentalis [(13.95±0.43) h] (Table 1). Time of mortality of parasites was reduced to (45.92±1.07) h with C. alata treatment at 10 mg/mL which significantly differed from C. occidentalis [(52.65±3.73) h] and C. angustifolia [(53.75±2.42) h]. Same trend of observation were seen at higher concentration. However parasite survived up to (81.33±2.07) h in control.
Table 1. Effects of crude ethanolic extracts of different Cassia species on three helminths of domestic fowl.
| Worm types | Concentration (mg/mL) |
C. alata |
C. angustifolia |
C. occidentalis |
|||
| PT (h) | TM (h) | PT (h) | TM (h) | PT (h) | TM (h) | ||
| H. gallinarum | 10 | 24.93±1.67 | 45.92±1.07 | 16.12±3.36 | 53.75±2.42 | 29.92±3.35 | 52.65±3.73 |
| 20 | 11.57±1.43 | 27.90±1.20 | 11.79±3.19 | 32.70±1.26 | 22.86±3.61 | 37.69±4.26 | |
| 40 | 6.60±0.86 | 19.42±0.66 | 5.71±0.10 | 17.86±0.18 | 13.95±0.43 | 29.76±4.70 | |
| Catatropis sp. | 10 | 2.83±0.08 | 7.33±0.14 | 3.74±0.17 | 6.70±0.17 | 6.42±0.53 | 12.37±0.88 |
| 20 | 2.32±0.15 | 4.27±0.10 | 1.59±0.10 | 3.67±0.08 | 3.18±0.53 | 9.24±0.17 | |
| 40 | 0.57±0.08 | 2.78±0.31 | 1.00±0.12 | 3.06±0.09 | 1.47±0.40 | 4.71±0.14 | |
| R. tetragona | 10 | 3.10±0.14 | 15.88±0.46 | 14.16±0.03 | 31.14±0.68 | 32.75±0.15 | 48.06±0.11 |
| 20 | 2.89±0.22 | 13.10±0.37 | 5.39±0.46 | 21.15±0.63 | 9.31±0.18 | 26.96±0.24 | |
| 40 | 1.68±0.27 | 8.55±0.32 | 2.95±0.29 | 18.52±0.09 | 4.13±0.31 | 22.40±1.21 | |
Values are expressed as mean±SD. Survivability of parasites in control medium: H. gallinarum=(81.33±2.07) h; R. tetragona=(81.93±4.71) h and Catatropis sp.=(26.49±1.38) h. PT: time of paralysis; TM: time of mortality.
With albendazole, both paralysis and time of mortality showed significantly longer time [(45.17±1.84) h and )37.75±1.47) h] compared to all three plant extracts tested, at 10 and 20 mg/mL consecutively (Table 2).
Table 2. Effect of albendazole on H. gallinarum.
| Worm | Concentration (mg/mL) | PT (h) | TM (h) |
| H. gallinarum | 5 | 55.45±1.23 | 72.60±1.85 |
| 10 | 45.17±1.84 | 64.99±1.32 | |
| 20 | 37.75±1.47 | 57.56±1.91 |
Values are expressed as mean±SD. Survivability of parasites in control medium: (81.33±2.07) h. PT: time of paralysis; TM: time of mortality.
For R. tetragona, each plant extract has dose-dependent efficacy (Table 1). In all concentrations, C. alata showed significantly less time to paralyse [(3.1±0.14) h, (2.89±0.22) h and (1.68±0.27) h], followed by C. angustifolia [(14.16±0.03) h, (5.39±0.46) h and (2.95±0.29) h] and C. occidentalis [(32.75±0.15) h, (9.31±0.18) h and (4.13±0.31) h]. Time of mortality also showed a similar pattern with C. alata taking a shorter time and C. occidentalis, a longer time but in control the parasite survived up to (81.93± 4.71) h (Table 1).
Praziquantel treatment on parasites showed both paralytic and mortality time were comparatively short as compared with plant treated parasites even at low concentrations (Table 3).
Table 3. Effect of praziquantel on R. tetragona and Catatropis sp.
| Worm | Concentration (mg/ml) | PT (h) | TM (h) |
| R. tetragona | 0.001 0 | 1.27±0.37 | 21.37±1.87 |
| 0.002 5 | 0.76±0.13 | 19.14±1.18 | |
| 0.005 0 | 0.17±0.01 | 17.07±0.31 | |
| Catatropis sp. | 0.001 0 | 2.50±0.25 | 28.86±0.37 |
| 0.002 5 | 1.09±0.20 | 21.00±0.91 | |
| 0.005 0 | 0.30±0.04 | 13.09±0.37 |
Values are expressed as mean±SD. Survivability of parasites in control medium: R. tetragona=(81.93±4.71) h and Catatropis sp.=(26.49±1.38) h. PT: time of paralysis; TM: time of mortality.
For Catatropis sp. at 10 mg/mL, C. alata caused early paralysis [(2.83±0.08) h] amongst the three plants, followed by C. angustifolia [(3.74±0.17) h] and C. occidentalis [(6.42±0.53) h]. While at 40 mg/mL, there was slight decline in the level of significance in all tested plant extracts when compared between paralysis time and time of mortality. However, control parasite survived up to (26.49±1.38) h (Table 1).
3.2. Treatment with combination of two plant extracts
For H. gallinarum, a combination of C. angustifolia+C. occidentalis was observed to cause early paralysis [(21.95±0.64) h] followed by C. alata+C. angustifolia [(22.53±1.97) h] at 10 mg/mL. However at 40 mg/mL, there was a decline in the level of significance of C. alata+C. occidentalis and C. alata+C. angustifolia. While time of mortality was shown to occur at (23.69±0.53) h by C. angustifolia+C. occidentalis, it took about (27.39±0.51) h in case of C. alata+C. occidentalis (Figure 1).
Figure 1. Effect of combinations of Cassia plant extracts on H. galinarum.
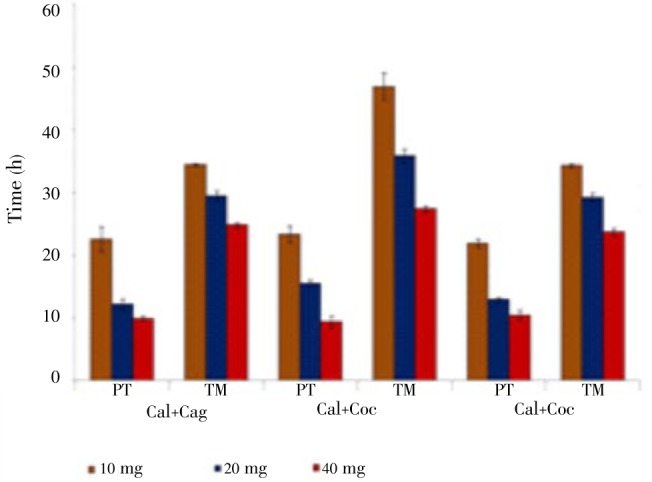
Cal: C. alata; Cag: C. angustifolia; Coc: C. occidentalis.
In R. tetragona at 40 mg/mL, paralysis showed no significantly different in all combinations, but the time of mortality showed more significantly different in C. alata+C. angustifolia with C. angustifolia+C. occidentalis (Figure 2).
Figure 2. Effect of combinations of Cassia plant extracts on R. tetragona.
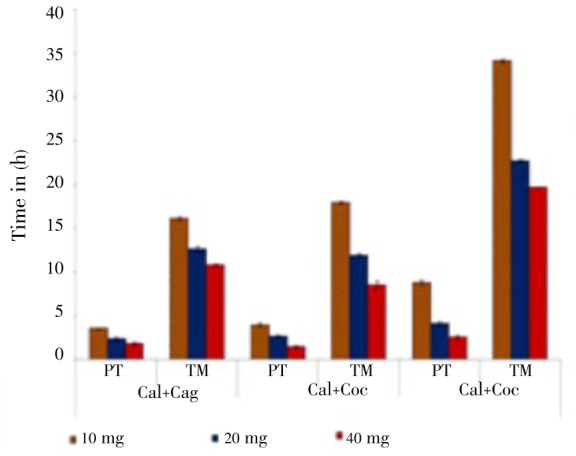
Cal: C. alata; Cag: C. angustifolia; Coc: C. occidentalis.
For Catatropis sp., C. alata + C. angustifolia having more effects in causing early paralysis and mortality in all concentrations followed by C. alata + C. occidentalis and C. angustifolia + C. occidentalis (Figure 3).
Figure 3. Effect of combinations of Cassia plant extracts on Catatropis sp.
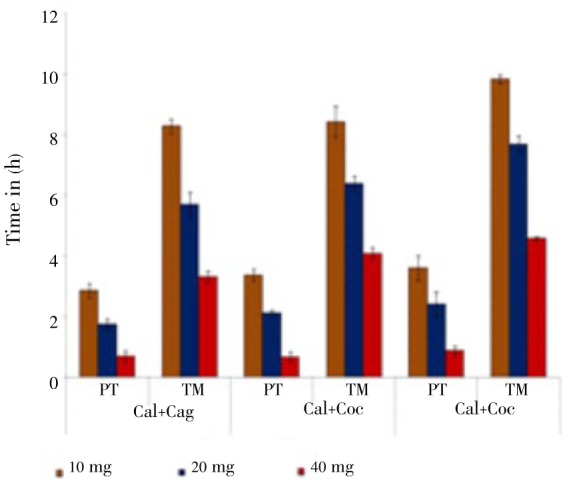
Cal: C. alata; Cag: C. angustifolia; Coc: C. occidentalis.
4. Discussion
The present investigation showed paralysis preceded mortality very significantly and paralysis of worms occurred for a very short interval following exposures of parasites to these plant extracts. Similar in vitro studies were also observed in ethanol extract of Artemesia cina on Moniezia expansa[19]; Artemesia annua, Artemesia absinthium and Fumaria officinalis on Schistosoma mansoni and Fasciola hepatica[20]; Cassia alata on Hymenolepis diminuta[17].
C. angustifolia showed more effect on H. gallinarum compared to other two plants. For R. tetragona and Catatropis sp., it was C. alata that showed more effects followed by C. angustifolia in causing immobility. Though varying rate of efficacy results, these variations in efficacy may be due to different chemical constituents between plant individuals resulting from differences in solubility in ethanol[21]. The effects responsible for anthelmintic activity were similar; all concentrations used have caused moderate level of effects on the motility of tested worms. This inactiveness could lead to expulsion of paralysed worms from the body[22].
Current study inhibited motility at low concentration, increasing the concentration of the plant extracts resulted in early paralysis, thus indicating dose-dependent efficacy of the test materials. Similar observations were also recorded with leaf extract of Stephania glabra and Trichosanthus multiloba on Ancylostoma ceylanicum[23], Alocasia indica on Pheretima posthuma[24], Acacia oxyphylla on Raillietina echinobothrida[25], extracts from coconut, onion, garlic, fig on different cestodes and trematodes[26],[27].
Albendazole is known to cause paralysis of worms so that they are expelled in faeces of man and animals. Experimental plant extracts not only demonstrated this property, but they also caused early death of worms at all concentrations compared to drug. Thus findings from the current study revealed that extracts have shown promising in vitro anthelmintic activity. These plants are known to contain large amount of alkaloids, flavonoids, glycosides, tannins[6]. This anthelmintic activity could be attributed to these bioactive compounds jointly or separately. Classes of secondary metabolites such as alkaloids and flavonoids are considered the source of chemical components responsible for wide therapeutic activities of several medicinal plants[7]. The antimicrobial activities of C. alata, C. angustifolia and C. occidentalis have been ascribed mainly due to the presence of alkaloids, flavonoids, glycosides, tannins[7]. Similarly, Athanasiadou et al. suggested that anthelmintic activity of plant extracts on larvae and adults of gastrointestinal nematodes could be attributed to tannins' capacity to bind to proteins and could operate via several mechanisms[28]. Maqbool et al. suggested that anthelmintic activity of Fumaria parviflora may be due to alkaloids which have the ability to intercalate with DNA synthesis of parasites[29]. Transcuticular diffusion is a common means of entry into helminth parasites for non-nutrient and non-electrolyte substances in nematode, cestode and trematode parasites as opposed to oral ingestion[30],[31]. Possible explanation for better anthelmintic activity of ethanolic extracts could be due to easier transcuticular absorption of ethanolic extracts into body of parasites.
Our current study revealed broad anthelmintic activity of all three plants tested and their broad activities are described for the first time. Delaquis et al. observed naturally occurring combinations of plant compounds showed synergistic and often results in crude extracts having greater antimicrobial activity than the purified individual constituents[32].
Such plant based treatments could be made part of an integrated management plan for control of helminths in developing countries. Further studies are required to isolate and reveal the active compound contained in the crude extracts to establish the mechanism of action.
Acknowledgments
The authors gratefully acknowledge the University Grants Commission (UGC), New Delhi for providing financial assistance through a major research project sanctioned to Larisha M. Lyndem. We also wish to thank the Department of Zoology, Centre for Advanced Studies, Visva-Bharati for providing infrastructural support.
Comments
Background
Helminthiasis has become a worldwide problem for animals and humans. The intensive use of chemical anthelmintics has led to the development of parasite resistance. To try and solve this problem, several alternative methods are studied like phytotherapy, which is used by traditional healers or by indigenous people for humans or animals.
Research frontiers
Studies are being performed in order to scientifically evaluate the traditional anthelmintic use of C. alata, C. angustifolia, C. occidentalis plants used in India and West-Bengal for various ailment. The efficacy of these three leaf extracts was tested against the fowl parasites H. gallinarum, Catatropis sp. and R. tetragona.
Related reports
The lack of effect of some extracts (the water extract here) has to be reconsidered as it was demonstrated in other studies (Raskin et al., 2002) so that it could vary depending on several parameters in the plant (origin, soil, age, season, etc.) and also the type of parasite tested.
Innovations and breakthroughs
An innovation in the paper is that several parasites of different classes were tested.
Applications
The results of this study suggest that the three extracts of the leaves of C. alata, C. angustifolia and C. occidentalis were active against three parasite species of fowl. Thus it is important.
Peer review
This is a good study in which the authors evaluated the anthelmintic effet of C. alata, C. angustifolia, and C. occidentalis for the control of gastro-intestinal parasitism. The three different leaf extracts were compared for their efficacy and were found to have different levels of efficacy depending on the parasite species. The results are interesting and suggest that anthelmintic substances are present in leaves of three plant species.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Nascimento GG, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Braz J Microbiol. 2000;31:247–256. [Google Scholar]
- 2.Mehlhorn H, Al-Quaraishy S, Al-Rasheid KA, Jatzlau A, Abdel-Ghaffar F. Addition of a combination of onion (Allium cepa) and coconut (Cocos nucifera) to food of sheep stops gastrointestinal helmintic infections. Parasitol Res. 2010;108:1041–1046. doi: 10.1007/s00436-010-2169-3. [DOI] [PubMed] [Google Scholar]
- 3.Cala AC, Chagas AC, Oliveira MC, Matos AP, Borges LM, Sousa LA, et al. In vitro anthelmintic effect of Melia azedarach L. and Trichilia claussenii C. against sheep gastrointestinal nematodes. Exp Parasitol. 2012;130:98–102. doi: 10.1016/j.exppara.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Ekeanyanwu RC, Etienajirhevwe OF. In vitro anthelmintic potentials of Xylopia aethiopica and Monodora myristica from Nigeria. Afr J Biochem Res. 2012;6:115–120. [Google Scholar]
- 5.Bazh EK, El-Bahy NM. In vitro and in vivo screening of anthelmintic activity of ginger and curcumin on Ascaridia galli. Parasitol Res. 2013;112(11):3679–3686. doi: 10.1007/s00436-013-3541-x. [DOI] [PubMed] [Google Scholar]
- 6.Hossain E, Chandra G, Nandy AP, Mandal SC, Gupta JK. Anthelmintic effect of a methanol extract of Bombax malabaricum leaves on Paramphistomum explanatum. Parasitol Res. 2012;110(3):1097–1102. doi: 10.1007/s00436-011-2594-y. [DOI] [PubMed] [Google Scholar]
- 7.Arunkumar S, Muthuselvam Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci. 2009;5:572–576. [Google Scholar]
- 8.Durapandiyan V, Ayyanar M, Ignacimuthi S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar from Tamil Nadu, India. 2006. BMC Complement Altern Med. 2006;6:35. doi: 10.1186/1472-6882-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makinde AA, Igoli JO, Ama LT, Shaibu SJ, Garba A. Antimicrobial activity of Cassia alata. Afr J Biotechnol. 2007;6:1509–1510. [Google Scholar]
- 10.Rai PP. Anthracene derivatives in leaves and fruits of Cassia alata. Curr Sci. 1978;47:271–272. [Google Scholar]
- 11.Moriyama H, Lizuka T, Nagai M, Miyataka H, Satoh T. Anti-inflammatory activity of heat treated Cassia alata leaf extract and its flavonoid glycoside. Yakugaku Zasshi. 2003;123:607–611. doi: 10.1248/yakushi.123.607. [DOI] [PubMed] [Google Scholar]
- 12.Belkin M, Fitzgerald-Dorothea B, Cogan W. Tumor-damaging capacity of plant materials. I. Plants used as cathartics. J Natl Cancer Inst. 1952;13:139–155. [PubMed] [Google Scholar]
- 13.Paria ND. Medicinal plant resources of South West-Bengal. Kolkata: Directorate of Forests, Government of West Bengal; 2005. pp. 39–40. [Google Scholar]
- 14.Farnsworth NR, Bunyapraphatsara N. Thai medicinal plants: recommended for primary health care system. Bangkok: Medicinal Plants Information Center, Faculty of Pharmacy, Mahidol University; 1992. p. 409. [Google Scholar]
- 15.De-Padua LS, Bunyapraphatsara N, Lemmens RH. Plant resources of South-East Asia: medicinal and poisonous plants. Leiden, Netherlands: Backhuys Publishers; 1999. pp. 167–175. [Google Scholar]
- 16.Kundu S, Lyndem LM. In vitro screening for cestocidal activity of three species of Cassia plants against the tapeworm Raillietina tetragona. J Helminthol. 2012;87:154–159. doi: 10.1017/S0022149X12000156. [DOI] [PubMed] [Google Scholar]
- 17.Kundu S, Roy S, Lyndem LM. Cassia alata L.: potential role as anthelmintic agent against Hymenolepis diminuta. Parasitol Res. 2012;111:1187–1192. doi: 10.1007/s00436-012-2950-6. [DOI] [PubMed] [Google Scholar]
- 18.Adamu H, Endeshaw T, Teka T, Kife A, Petros B. The prevalence of intestinal parasites in paediatric diarrhoeal and non-diarrhoeal patients in Addis Ababa Hospitals, with special emphasis on opportunistic parasitic infections and with insight into the demographic and socio-economic factors. Ethiop J Health Dev. 2006;20:39–46. [Google Scholar]
- 19.Bashtar AR, Hassanein M, Abdel-Ghaffar F, Al-Rasheid K, Hassan S, Mehlhorn H, et al. Studies on monieziasis of sheep I. Prevalence and antihelminthic effects of some plant extracts, a light and electron microscopic study. Parasitol Res. 2011;108:177–186. doi: 10.1007/s00436-010-2060-2. [DOI] [PubMed] [Google Scholar]
- 20.Ferriera JF, Peaden P, Keiser J. In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. absinthium, Asimina triloba, and Fumaria officinalis: trematocidal plant alcoholic extracts. Parasitol Res. 2011;109:1585–1592. doi: 10.1007/s00436-011-2418-0. [DOI] [PubMed] [Google Scholar]
- 21.Hördegen P, Hertzberg H, Heilmann J, Langhans W, Maurer V. The anthelmintic efficacy of five plant products against gastrointestinal trichostrongyloids in artificially infected lambs. Vet Parasitol. 2003;117:51–60. doi: 10.1016/j.vetpar.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Al-Shaibani MS, Phulan IR, Shiekh M. Anthelmintic activity of Fumaria parviflora (Fumariaceae) against gastrointestinal nematodes of sheep. Int J Agric Biol. 2009;11:431–436. [Google Scholar]
- 23.Martin RJ. Gama aminobutyric acid and piperazine activated single channel currents from Ascaris suum body muscle. Br J Pharmacol. 1985;84:445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyndem LM, Tandon V, Das B. Anthelmintic efficacy of medicinal plants from Northeast India against hookworms: an in vitro study on Ancylostoma ceylanicum. Pharmacologyonline. 2008;3:697–707. [Google Scholar]
- 25.Mulla WA, Thorat VS, Patil RV, Burade KB. Anthelmintic activity of leaves of Alocasia indica Linn. Int J Pharm Tech Res. 2010;2:26–30. [Google Scholar]
- 26.Dasgupta S, Roy B, Tandon V. Ultrastructural alterations for the tegument of Raillietina echinobothrida treated with stem bark of Acacia oxyphylla (Leguminosae) J Ethnopharmacol. 2010;127:568–571. doi: 10.1016/j.jep.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Ghaffar F, Semmler M, Al-Rasheid SB, Strassen B, Fischer K, Aksu G, et al. The effects of different plant extracts on intestinal nematodes and trematodes. Parasitol Res. 2011;108:979–984. doi: 10.1007/s00436-010-2167-5. [DOI] [PubMed] [Google Scholar]
- 28.Athanasiadou S, Kyriazakis F, Jackson F, Coop RI. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet Parasitol. 2001;99:205–219. doi: 10.1016/s0304-4017(01)00467-8. [DOI] [PubMed] [Google Scholar]
- 29.Maqbool A, Hayat CS, Tanveer A. Comparative efficacy of various indigenous and allopathic drugs against fascioliasis in buffaloes. Vet Arch. 2004;74:107–114. [Google Scholar]
- 30.Alvarez LI, Mottier ML, Lanusse CE. Drug transfer into target helminth parasites. Trends Parasitol. 2007;23:97–104. doi: 10.1016/j.pt.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Eguale T, Tilahun G, Debella A, Fleke A, Makonnen E. Haemonchus contortus: in vitro and in vivo anthelmintic activity of aqueous and hydro-alcoholic extracts of Hedera helix. Exp Parasitol. 2007;116:340–345. doi: 10.1016/j.exppara.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Delaquis PJ, Stanich K, Girard B, Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol. 2002;74:101–109. doi: 10.1016/s0168-1605(01)00734-6. [DOI] [PubMed] [Google Scholar]


