Abstract
Objective
To investigate the antioxidant potential and anti-acetycholinesterase activity of compounds and extracts from Acacia cyanophylla (A. cyanophylla).
Methods
Three polyphenolic compounds were isolated from ethyl acetate extract of A. cyanophylla flowers. They have been identified as isosalipurposide 1, quercetin 2 and naringenin 3. Their structures were elucidated by extensive spectroscopic methods including 1D and 2D NMR experiments as well as ES-MS. The prepared extracts and the isolated compounds 1-3 were tested for their antioxidant activity using 1′-1′-diphenylpicrylhydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) scavenging assays and reducing power. They have been also investigated for inhibitory effect against acetylcholinesterase using the microplate assay.
Results
In the DPPH test, the EtOAc extract of flowers exhibited the highest antioxidant effect (67.26 µg/mL). Isosalipurposide 1 showed a significant antiradical power against DPPH (81.9 µg/mL). All extracts showed a dose-dependent acetylcholinesterase inhibition. In terms of the IC50 value, the butanolic extract (16.03 µg/mL) was the most potent sample. Isosalipurposide 1 was found to be active against AChE with an IC50 value of 52.04 µg/mL.
Conclusions
The results demonstrated the important antioxidant and anti-acetylcholinesterase activity of pure compounds and extracts from A. cyanophylla.
Keywords: Acacia cyanophylla, Isosalipurposide, Naringenin, Quercetin, Antioxidant activity, Anti-acetylcholinesterase activity
1. Introduction
The use of plants in traditional medicine for treating various ailments remains an integral part of the culture and traditions of a majority of the world's population. In addition, factors such as the availability, affordability and accessibility of medicinal plants have led to their high demand and usage[1]. Secondary metabolites such as alkaloids, flavonoid, tannins, saponins generally produced by plants for their defense mechanisms have been implicated in the therapeutic properties of most medicinal plants[2]. Plants therefore provide an invaluable resourse useful as leads in the development of therapeutic compounds[3]. Indeed, many plant-derived drugs are either in the clinical trial phase or currently used in treatment of ailments such as Alzheimer's disease, and cancer[4]. Oxidative stress arises mainly from the over production of free radicals due to an imbalance in production of antioxidants by the cells[5]. Natural products especially from plants sources have the ability to reduce oxidative stress by acting as antioxidants[6]. Presently, a number of treatments are used against Alzheimer's disease as well as to counter the effect of oxidative stress. These include the use of acetylcholinesterase inhibitors (AChEIs) and high levels of antioxidants. Some adverse effects such as hepatotoxicity, gastrointestinal disturbances, nausea, vomiting, diarrhea and dizziness have been reported with the use of most AChEIs[7]. There is also an increasing concern about the use of some common antioxidants. Butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) for example, have been implicated in incidence of toxicity especially against animal DNA as well as liver damage and carcinogenesis[8]. Consequently, there is need for the discovery and development of novel/alternative natural antioxidants and AChEIs that are safe, affordable and effective at a global level[9].
The genus Acacia is quite large and is widespread in the warm subarid and arid parts of the world, relatively little is known about the chemistry of most species. The most evident substances in many Acacia species are complex phenolic compounds (condensed tannins), polysaccharides (gums) and flavonoid[10]. The difficulty of structure elucidation and the overall lack of toxicity of these substances undoubtedly have contributed to a dearth of chemical study of these plants. The situation is further complicated because identification of Acacia species is difficult and their taxonomic relationships are not clear[10]. Acacia cyanophylla (A. cyanophylla) is a legume shrub species, it was introduced in Tunisia for range land rehabilitation, particularly in the semi arid zones. This shrub represents a potential forage resource particularly during periods of drought.
The most evident substances in A. cyanophylla are flavonoids such as quercetin and kaempferol, while presence of other flavanols has been indicated[11].
Several studies have reported on the plant A. cyanophylla allowed the isolation of some phenolic compounds[10],[12]. Therefore, this present paper describes the isolation, structure elucidation, antioxidant and anti-acetylcholinesterase activities of extracts and isosalipurposide 1, quercetin 2 and naringenin 3 isolated from flowers of A. cyanophylla.
2. Materials and methods
2.1. Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), quercetin, 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), potassium ferricyanide [K3Fe(CN)6], trichloroacetic acid, ferric chloride (FeCl3), acetylcholinesterase (AChE) type VI-S, from electric eel 349 U/mg solid, 411 U/mg protein, 5,5′-dithiobis(2-nitrobenzoic acid), acetylthiocholine iodide, tris(hydroxymethyl) aminomethane, were obtained from Sigma-Aldrich. All other chemicals were of analytical grade purity.
2.2. Plant material
Flowers of A. cyanophylla were collected in the region of Monastir (Tunisia) in March 2010. The plant was identified by Prof. Fethia Harzallah-Skhiri in the Laboratory of Vegetal Biology and Botanic, High Institute of Biotechnology of Monastir, Tunisia and a voucher specimen (AC-10) was deposited in the same laboratory.
2.3. Isolation and identification of compounds
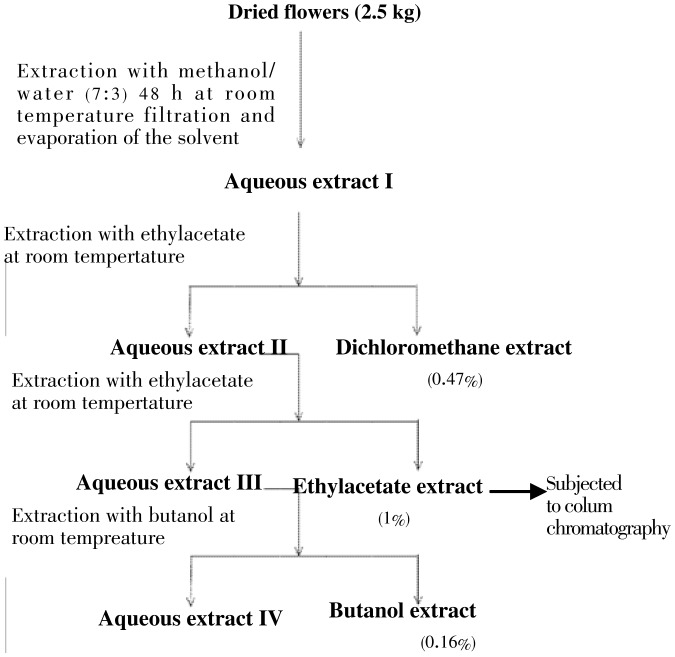
The dried and powdered flowers (2.5 kg) of A. cyanophylla were extracted with the mixture methanol/water (7:3) at room temperature for 48 h. The corresponding aqueous extract was obtained after filtration and evaporation of the organic solvent (MeOH) under reduced pressure. The obtained aqueous extract was extracted successively with dichloromethane, ethyl acetate and n-butanol to yield the corresponding extracts (Figure 1). From all crude extracts, only ethyl acetate (25 g) was further subjected to silica gel column chromatography eluting with CH2Cl2/MeOH gradients. Six main fractions (250 mL × 6) were collected. Fraction 5 (19 g) was purified by column chromatography over silica gel using CH2Cl2/MeOH (9:1) as eluent to afford isosalipurposide 1 (18 g, 0.72%) and quercetin 2 (50 mg).
Figure 1. Extraction of various extracts of A. cyanophylla in solvents of increasing polarity.
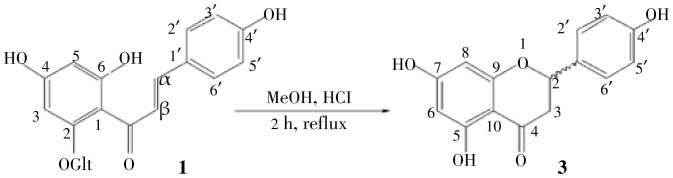
Using the system MeOH/HCl concentrated at reflux for 2 h, we managed the hydrolysis of glucopyranosyl system in C-2. We found from the spectral data of the obtained product explained below that the reaction evolved into a cyclization product 3 (naringenin) involving the phenol group at C-6 and the α, β-unsaturated ketone system (Figure 2).
Figure 2. Synthesis of naringenin 3 from isosalipurposide 1.
The FT-IR spectra were established using a Bio-Rad spectrophotometer with a resolution of 4 cm−1 and scanning a wavelength range from 400 to 4 000 cm−1. 1H-NMR (300 MHz) and 13C-NMR (75 MHz) spectra were recorded in deuterated CD3OD on a Bruker AC-300. All chemical shifts were reported as δ values (mg/L). Double resonance, COSY and HMBC experiments were carried out for complete assignment of proton and carbon signals in the NMR spectra, whenever possible mass spectra were obtained with an automass multi thermo finingam ES-MS spectrometer. Column chromatography was performed on SDS silica gel 60 F254 (70-200 µm) using dichloromethane and methanol mixture as eluents.
2.4. Antioxidant testing assays
The antioxidant activity of extracts and pure compounds was addressed by employing following in vitro assays.
2.4.1. DPPH scavenging assay
The extracts and compounds 1-3 were measured in terms of hydrogen donating or radical scavenging ability using stable radical DPPH following the method given by Sarker et al. (2006) with little modifications[13]. To 1 mL of DPPH, (80 µg/mL in methanolic solution) were added 1 mL of extracts or pure compounds (0.062-1.0 mg/mL). After incubation of 30 min in darkness and at a temperature of 25 °C, absorbance was read at 517 nm wavelength.
A mixture of 0.5 mL of DPPH• solution and 0.5 mL of ethanol was taken as a blank. Decrease in absorption induced by the tested samples was compared to that of the positive control quercetin. IC50 values calculated denote the concentration required to scavenge 50% of DPPH• radicals. Results were expressed in inhibition percentage at different sample concentrations (mg/mL) after 30 min. Inhibition of free radical DPPH in percent (IP %) was calculated as follow:
%IP= [(A0-A1)/A0] × 100
Where A0 is the absorbance of the control reaction (containing all reagent except the test compound), and A1 is the absorbance of the test compound. Extract concentration providing 50% inhibition (IC50) was calculated from the graph plotted from inhibition percentage against extract concentration. Tests were carried out in triplicate.
2.4.2. Scavenging activity of ABTS radical cation
The ABTS radical cation scavenging activity was measured according to the method described by Chakraborty (2010)[14]. ABTS was dissolved in water to a 7 mmol/L concentration. The ABTS radical cation was produced by adding to the ABTS stock solution 2.45 mmol/L potassium persulphate (final concentration). The completion of radical generation was obtained in the dark at room temperature for 12-16 h. This solution was then diluted with ethanol to adjust its absorbance at 734 nm to 0.70±0.02. To determine the scavenging activity, 50 µL of diluted ABTS solution was added to 950 µL of plant extracts and compound 1 (or water for the control), and the absorbance at 734 nm was measured 6 min after the initial mixing, using ethanol as the blank. The percentage of inhibition was calculated by the equation:
%IP= [(A0-A1)/A0] × 100
Where A0 is the absorbance of control and A1 is the absorbance of reaction mixture, respectively from a plot of concentration against %IP, a linear regression analysis was performed to determine the IC50 value for each plant extract. Tests were carried out in triplicate.
2.4.3. Reducing power assay
This assay has been performed following the method of Oyaizu (1986) with a slight modification[15],[16]. It was used to assess the reducing power of different extracts and compound 1. A volume of 1 mL of different concentrations of extracts and compound 1 (0.062-1.0 mg/mL) and 0.75 mL of distilled water were mixed with 1 mL of 0.2 mol/L sodium phosphate buffer (pH 6.6) and 1 mL (1%) of potassium ferricyanide [K3Fe(CN)6]. The mixture was incubated at 50 °C. After 20 min of incubation, the reaction mixture was acidified with 1 mL of trichloroacetic acid (10%). Finally, 0.25 mL of FeCl3 (0.1%) was added to this solution. Distilled water was used as blank and for control. Absorbance of this mixture was measured at 700 nm using a UV-spectrophotometer. Decreased absorbance indicates ferric reducing power capability of sample. Tests were carried out in triplicate.
2.5. Acetylcholinesterase enzyme inhibitory activity
Inhibition of acetylcholinesterase by the extracts and compound 1 was investigated using the microplate assay. The assay is based on Falé method[17].
Briefly, 90 µL of 50 mmol/L Tris-HCl buffer, pH=8.30 of mixture and 7.5 µL of acetylcholinesterase solution containing 0.26 U/mL were mixed in a microwell plate and left to incubate for 15 min. Subsequently, 22.5 µL of a solution of AHCI (0.023 mg/mL) and 142 µL of 3 mmol/L 5,5′-dithiobis(2-nitrobenzoic acid) were added. The absorbance was read at 405 nm when the reaction reached equilibrium. A control reaction was carried out using water instead of extract or compound 1 and it was considered 100% activity.
%IP=100-(A1/A0) × 100
Where A1 is the absorbance of the extract containing reaction mixture and A0 is the absorbance of the reaction. IP is the percentage of inhibition. Tests were carried out in triplicate and a blank with Tris-HCl buffer instead of enzyme solution was used. In the case of the standards, a blank with methanol was carried out as tested samples were dissolved in this organic solvent.
2.6. Statistical analysis
All experiments were repeated at least three times. Results are reported as mean±SE.
3. Results
3.1. Characterization of compounds
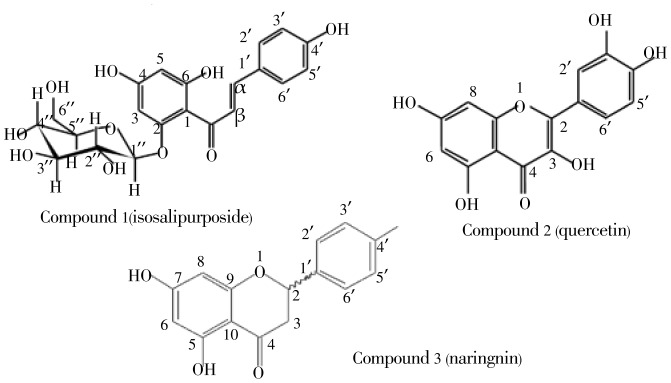
The repeated column chromatograohy of ethyl acetate extract of flowers of A. cyanophylla resulted in the isolation of compound 1 and compound 2 (Figure 3). They are yellow solid, the first, weight 18 g (0.72%) and its molecular formula, C21H22O10, [M+Na]+ at m/z 457 and [M-H]− at m/z 433 was established by ES-MS.
Figure 3. Structure of compounds 1, 2 and 3.
The 13C and 1H-NMR spectra of 1 showed the typical signals for a β-D-glucopyranose with the doublet for the anomeric proton H1″ at δ 5.16 mg/kg and the resonance for the corresponding carbon C1″ at δ 102.2 mg/kg (Table 1). The IR spectrum displayed intense absorption bands for hydroxy (3 482 cm−1), α,β-unsaturated carbonyl (1 624 cm−1) and olefin (1 591 cm−1) functionalities. The 1H-NMR spectrum showed the signals of an olefin at δH 7.69 (H-α) and 8.03 (H-β), a para-substituted aromatic ring at δH 6.86 (H-3′ and H-5′) and 7.65 (H-2′ and H-6′), a 1,2,4,6-tetrasubstitued aromatic ring at δH 6.01 (H-3), 6.23 (H-5). The 13C-NMR and DEPT 135 spectra showed the existence of these structural moieties and one conjugated ketone at δC 194.8 mg/kg. The HMBC spectrum, which displayed correlation between the anomeric proton of the β-D-glucopyranose at δH 5.16 mg/kg (1H, d, J=7.2 Hz, H1″) and C2 at δC 168.2 mg/kg, reinforced the deduction of the glycosilation site as C2. Assignment of the sugar resonances was achieved using the anomeric proton resonance at δH 5.16 mg/kg as a starting point for the interpretation of the 1H-1H COSY spectrum. The above spectral data (Table 1) helped in assigning the structure to the compound 1 as isosalipurposide.
Table 1. 1H- (300 MHz, solvent CD3OD) and 13C- (75 MHz, solvent CD3OD) NMR data for compound 1.
| Atoms | Compound 1 |
|
| 1H (m, J Hz) | 13C | |
| 1 | - | 107.8 |
| 2 | - | 168.2 |
| 3 | 6.01 (d, 2.1 Hz) | 98.8 |
| 4 | - | 162.2 |
| 5 | 6.23 (d, 2.1 Hz) | 96 |
| 6 | - | 166.3 |
| 1′ | - | 128.3 |
| 2′ | 7.65 (d, 8.7 Hz) | 132.2 |
| 3′ | 6.86 (d, 8.7 Hz) | 117.3 |
| 4′ | - | 161.5 |
| 5′ | 6.86 (d, 8.7 Hz) | 117.3 |
| 6′ | 7.65 (d, 8.7 Hz) | 132.2 |
| 1″ | 5.16 (d, 7.2 Hz) | 102.2 |
| 2″ | 3.46-3.58 (m) | 75.4 |
| 3″ | 3.46-3.58 (m) | 78.9 |
| 4″ | 3.46-3.58 (m) | 71.5 |
| 5″ | 3.46-3.58 (m) | 78.8 |
| 6″a, b | 3.76 (m) | 62.8 |
| 3.94 (m) | ||
| α | 7.69 (d, 15.6 Hz) | 128.9 |
| β | 8.03 (d, 15.6 Hz) | 144.6 |
| C=O | - | 194.8 |
Spectral data (Table 2) of compounds 2 and 3 have contributed to the proposal of the two structures of quercetin 2 and naringenin 3 (Figure 3).
Table 2. 1H- (300 MHz, solvent CD3OD) and 13C-(75 MHz, solvent CD3OD) NMR data for compounds 2 and 3.
| Atoms | Compound 2 |
Compound 3 |
||
| 1H (m, J Hz) | 13C | 1H (m, J Hz) | 13C | |
| 2 | - | 157.1 | 5.28 (dd, 13 Hz; 3 Hz) | 80.8 |
| 3a, b | - | 136.1 | 2.64 (dd, 17.1 Hz; 3 Hz) | 44.4 |
| 3.06 (dd, 17.1 Hz; 13 Hz) | ||||
| 4 | - | 176.2 | - | 198.1 |
| 5 | - | 161.3 | - | 165.8 |
| 6 | 6.34 (d, 1.8 Hz) | 98.1 | 5.87 (s) | 97.5 |
| 7 | - | 164.4 | - | 168.7 |
| 8 | 6.14 (d, 1.8 Hz) | 93.3 | 5.87 (s) | 96.6 |
| 9 | - | 147.6 | - | 165.2 |
| 10 | - | 103.4 | - | 103.7 |
| 1′ | - | 123 | - | 131.5 |
| 2′ | 7.69 (d, 2.1 Hz) | 114.9 | 7.28 (d, 9.3 Hz) | 129.4 |
| 3′ | - | 146.9 | 6.80 (d, 9.3 Hz) | 116.7 |
| 4′ | - | 145.1 | - | 159.3 |
| 5′ | 6.83 (d, 8.4 Hz) | 115.1 | 6.80 (d, 9.3 Hz) | 116.7 |
| 6′ | 7.59 (dd, 8.7 Hz; 2.1 Hz) | 120.5 | 7.28 (d, 9.3 Hz) | 129.4 |
3.2. Antioxidant acivity
3.2.1. DPPH radical scavenging activity
Compounds 1-3, dichloromethane, EtOAc, n-BuOH and aqueous extracts were screened for DPPH radical scavenging activity. The concentrations of pure compounds 1-3 required to neutralize 50% of DPPH range between 4.58 and 255.5 µg/mL (Table 3).
Table 3. Total antioxidant activity of 1-3 and A. cyanophylla extracts against DPPH, expressed in µg/mL.
| Samples | IC50 (µg/mL) |
| 1 | 81.90±0.81 |
| Quercetin 2 | 4.58±0.19 |
| Naringenin 3 | 255.5±1.31 |
| E1 | 164.64±5.76 |
| E2 | 67.26±2.00 |
| E3 | 122.54±2.86 |
| E4 | 314.66±6.72 |
| Quercetina | 4.77±0.18 |
| Troloxa | 14.56±0.62 |
E1: Dichloromethane extract, E2: Ethyl acetate extract, E3: Butanol extract, E4: Aqueous extract, a: reference compounds.
3.2.2. ABTS radical cation scavenging activity
Compound 1, dichloromethane, EtOAc, n-BuOH and aqueous extracts were screened for ABTS radical scavenging activity (Table 4).
Table 4. Total antioxidant activity of A. cyanophylla extracts against ABTS, expressed in µg/mL.
| Samples | IC50 (µg/mL) |
| 1 | 310.34±2.97 |
| E1 | 78.37±0.18 |
| E2 | 305.68±1.36 |
| E3 | 315.91±1.85 |
| E4 | 399.40±3.57 |
| Troloxa | 200.02±0.74 |
E1: Dichloromethane extract, E2: Ethyl acetate extract, E3: Butanol extract, E4: Aqueous extract, a: reference compound.
3.2.3. Reducing power assay
This method gives a clear idea about the antioxidant power of pure products or extracts. Figure 4 portrays that compound 1, dichloromethane, EtOAc, n-BuOH and aqueous extracts reduced Fe3+ to ferrous ions (Fe2+) at 0.062-1.0 mg/mL concentrations.
Figure 4. Reducing power potential of 1 and extracts in reducing power assay.
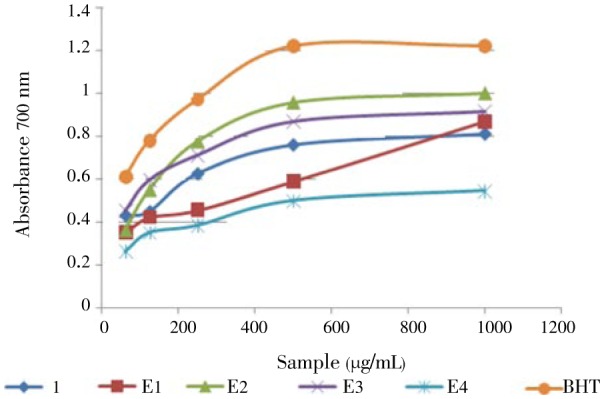
E1: Dichloromethane extract, E2: Ethyl acetate extract, E3: Butanol extract, E4: Aqueous extract, BHT (Butylated hydroxytoluene): reference compound.
3.3. Anti-acetylcholinesterase activity
The acetylcholinesterase inhibitory activity of isosalipurposide 1 and the organic extracts were measured using the microplate assay expressed as IC50 and values are given in Table 5.
Table 5. Anti-acetylcholinesterase activity of isosalipurposide 1 and extracts from A. cyanophylla.
| Samples | IC50 (µg/mL) |
| 1 | 52.04±1.91 |
| E1 | 20.01±0.78 |
| E2 | 27.12±1.35 |
| E3 | 16.03±0.64 |
E1: Dichloromethane extract, E2: Ethyl acetate extract, E3: Butanol extract.
4. Discussion
The ethyl acetate extract of A. cyanophylla on thin layer chromatography showed the presence of important number of components. The repeated column chromatography of this mixture caused isolation of an important amount of compound 1 (19 g, 0.072% yield) and compound 2 (50 mg).
The samples were found to be analytically pure by thin layer chromatography. The samples was identified as isosalipurposide and quercetin using spectroscopic techniques viz. 1D, 2D-NMR and mass. Spectral data of compounds 2 and 3 (Figure 3) compared to the literature allowed us to establish their structures as quercetin and naringenin[18],[19], respectively.
The geometry of the olefin of a chalcone moiety in compound 1 was determined to be E on the basis of the 1H-1H coupling constant (JH-α-H-β=15.6 Hz)[20]. Identification of the sugar as β-D-glucopyranose was supported by the 3JH1″-H2″ coupling constant of 7.2 Hz as well as by the trans di-axial couplings between H2″-H3″, H3″-H4″ and H4″-H5″[21].
The assignment of the structure of compound 1 is in consonance with the previous report of Filippo (1978) and Hatem et al. (2012)[11],[12]. For bio-evaluation studies, to the best of our knowledge, no biological studies were performed on this compound.
A review of the literature indicated that chalcones showed anti-infective, anti-inflammatory and cytotoxic properties[22],[23]. Rupashree and Mitali (2011) reported that chalcones also had a leishmanicidal activity and anti-tuberculosis effect as indicated by Hemshekhar et al. (2011)[24],[25].
Proton-radical scavenging action is an important attribute of antioxidants, which is measured by DPPH radical scavenging assay. DPPH is a free radical, a protonated radical, has characteristic absorbance maximum at 517 nm, stable at room temperature, which produces a violet solution in ethanol. In presence of antioxidant compounds, the DPPH is reduced producing a yellow ethanol solution. It is known that blocking one or more phenol groups in the flavonoids and their derivatives such as chalcones inhibited in a manner sometimes significant antioxidant powers[19]. For this reason and in order to improve the possible antioxidant activity of isosalipurposide 1, we thought to regenerate an additional phenol group fixed in C-2 via an acid hydrolysis reaction (Figure 2).
In a preliminary analysis of plant extracts for their antioxidant activity, we found that CH2Cl2, EtOAc, n-BuOH and aqueous extracts of dried flowers of A. cyanophylla exhibited a significant activity. Antiradical properties of flowers of A. cyanophylla are given in Table 1. Quercetin 2 (4.58 µg/mL) has given the most important activity, followed by isosalipurposide 1 (81.9 µg/mL) then naringenin 3 (255.5 µg/mL) (Table 1). We note that the values of IC50 of quercetin used as a reference and that isolated from flowers of A. cyanophylla are almost similar[26]. Isosalipurposide 1 was found to be three times more active than naringenin 3 in which the 2-OH may not contribute to this activity by its implication in a hydrogen bond with the carbonyl function forming a six center pseudo-ring[27]. The transformation of the chalcone 1 (isosalipurposide) to the flavanone 3 (naringenin), led to the disappearance of the 6-OH group, and therefore the creation of the cycle C without the double bond Cα-Cβ. These results are consistent with the relationships between the structure of flavonoids and their ability to trap free radicals. Indeed, numerous studies converge to suggest that the activity depends primarily on the following three criteria[27],[28], the presence of a catechol group in ring B (3′, 4′-OH), the C2-C3 double bond conjugated with the 4-oxo function and the presence of the 3-OH group.
The ethyl acetate extract had the highest antioxidant activity (67.26 µg/mL). This relatively high activity may be due to its richness in phenolic compounds. The CH2Cl2, n-BuOH and aqueous extracts present moderate activities (164.64, 122.54, 314.66 µg/mL, respectively), probably due to the weak amounts of phenolic compounds[29].
The ABTS method gives a measure of the antioxidant activity of extracts or compound 1 by measuring the reduction of the radical cation at 734 nm. Chakraborty (2010) reported that the decolorization of the ABTS+. cation reflects the capacity of an antioxidant to donate electrons or hydrogen atoms to inactive this radical species[14].
Compound 1 (isosalipurposide) showed an important activity through its free OH groups (310.34 µg/mL). With ABTS, the CH2Cl2 extract was found to be the more active one (78.37 µg/mL) compared to that of EtOAc, n-BuOH and aqueous extracts (305.68, 315.91, 399.40 µg/mL, respectively).
Reducing power was measured by direct electron donation of Fe3+ (CN−)6- Fe2+ (CN−)6[30]. The product was visualized by forming the intense blue color complex and then measured at 700 nm. By increasing the concentration of the extracts and compound 1, we favor the greater the reduction of Fe3+ to Fe2+. This finding justifies the reducing power of these samples[31]–[33]. The ethyl acetate extract from A. cyanophylla might contain a higher amount of reductone, which could react with free radicals to stabilise and terminate radical chain reactions. These results indicate that containing flavonoids and polyphenols may play an important role in reducing power.
Inhibition of AChE, the key enzyme in the breakdown of acetylcholine, is considered one of the treatment strategies against several neurological disorders such as Alzheimer's disease, senile dementia, ataxia and myasthenia gravis[34],[35]. Plants have been traditionally used to enhance cognitive function and to alleviate other symptoms associated nowadays with Alzheimer's disease[36]. The AChE inhibition was determined using an adaptation of the method described in the literature[17].
Isosalipurposide 1 found to be active against acetylcholinesterase with an IC50 value of 52.04 µg/mL by comparison to some results cited in the literature. IC50 of dichloromethane (20.01 µg/mL), ethyl acetate (27.12 µg/mL) and butanol (16.03 µg/mL) extracts of A. cyanophylla expressed in (Table 3) register encouraging results.
Acknowledgments
We are grateful to Dr Fethia Harzallah-Skhiri, High Institute of Biotechnology of Monastir, Tunisia for botanical identification and to Mrs Amna Benzarti, Department of Chemistry, Faculty of Science of Monastir, for NMR analysis. Supported by the Ministry of High Study and Scientific Research (MHSSR) of Tunisia (Grant No. 11/TM06).
Comments
Background
The authors assessed the antioxidant potential and anti-acetylcholinesterase activity of compounds and the crude extract of A. cyanophylla.
Research frontiers
Testing of isolated compounds for activity for identification of compounds will be used in future drug development.
Related reports
Articles on antioxidant and anti-acetylcholienesterase activity of some of the isolated compounds are available.
Innovations and breakthroughs
Determining biological activity of the isolated compounds can be taken further.
Applications
Further tests to assess activity can be carried out in the future study based on this paper.
Peer review
This article provides information on the biological activity of the specific plant which can be taken further. There is a need for the discovery and development of novel/alternative natural antioxidants and AChEIs that are safe, affordable and effective at a global level.
Footnotes
Foundation project: Supported by the Ministry of High Study and Scientific Research (MHSSR) of Tunisia (Grant No. 11/TM06).
Conflict of interest statement: The authors report no conflicts of interest. The authors are alone responsible for the content and writing of the paper.
References
- 1.Petersen LM, Moll EJ, Collins R, Hockings MT. Development of a compendium of local, wild-harvested species used in the informal economy trade, Cape Town, South Africa. Ecol Soc. 2012;17:26–56. [Google Scholar]
- 2.Demkura PV, Ballaré CL. UVR8 Mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol Plant. 2012;5:642–652. doi: 10.1093/mp/sss025. [DOI] [PubMed] [Google Scholar]
- 3.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today. 2008;13:161–171. doi: 10.1016/j.drudis.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 6.Ndhlala AR, Moyo M, Staden JV. Natural antioxidants: fascinating or mythical biomolecules. Molecules. 2010;15:6905–6930. doi: 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syad AN, Shunmugiah KP, Kasi PD. Assessement of anticholinesterase activity of Gelidiella acerosa: implications for its therapeutic potential against Alzheimer's disease. Evid Based Complement Alternat Med. 2012;2012:497242. doi: 10.1155/2012/497242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolatabadi JE, Kashanian S. A review on DNA interaction with synthetic phenolic food additives. Food Res Int. 2010;43:1223–1230. [Google Scholar]
- 9.Senol FS, Orhan I, Yilmaz G, Ciçek M, Sener B. Acetylcholinesterase, butyrycholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem Toxicol. 2010;48:781–788. doi: 10.1016/j.fct.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Nasri N, Elfalleh W, Tlili N, Hannachi H, Triki S, Khaldi A. Minor lipid Components of some Acacia Species: potential dietary health benefits of the unexploited seeds. Lipids Health Dis. 2012;11:49. doi: 10.1186/1476-511X-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghouila H, Meksi N, Haddar W, Mhenni MF, Jannet HB. Extraction, identification and dyeing studies of isosalipurposide a natural chalcone dye from Acacia cyanophylla flowers on wool. Ind Crops Prod. 2012;35:31–36. [Google Scholar]
- 12.Imperato F. A new chalcone glucoside and isosalipurposide from acacia cyanophylla. Phytochemistry. 1978;17:822–823. [Google Scholar]
- 13.Sarker SD, Latif Z, Gray AI. Natural products isolation. New Jersey, USA: Humana Press Inc; 2006. p. 529. [Google Scholar]
- 14.Chakraborty K, Paulraj R. Sesquiterpenoids with free radicalscavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010;122:31–41. [Google Scholar]
- 15.Oyaizu M. Studies on products of the browning reaction. Antioxidative activities of products of browning reaction prepared from glucose amine. Jpn J Nutr Diet. 1986;44:307–315. [Google Scholar]
- 16.Gülçin İ, Bursal E, Şehitoğlu MH, Bilsel M, Gören AC. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol. 2010;48:2227–2238. doi: 10.1016/j.fct.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 17.Falé PL, Borges C, Madeira PJ, Ascensão L, Araujo ME, Florêncio MH, et al. Rosmarinic acid, scutellarein 4′-methyl ether 7-O-glucoronide and (16S)-coleon E are the main compounds responsible for the antiacetylcolinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“falso boldo”) Food Chem. 2009;114:798–805. [Google Scholar]
- 18.Liu LL, Yang JL, Shi YP. Phytochemicals and biological activities of Pulicaria species. Chem Biodivers. 2010;7:327–349. doi: 10.1002/cbdv.200900014. [DOI] [PubMed] [Google Scholar]
- 19.Deodhar M, Black DS, Kumar M. Acid catalyzed stereoselective rearrangement and dimerization of flavenes: synthesis of dependensin. Tetrahedron. 2007;63:5227–5235. [Google Scholar]
- 20.Ninomiya M, Efdi M, Inuzuka T, Koketsu M. Chalcone glycosides from aerial parts of Brassica rapa L.‘hidabeni’, turnip. Phytochem Lett. 2010;3:96–99. [Google Scholar]
- 21.Chaari A, Ben Jannet H, Salmona G, Mighri Z. Nauplathizine, a new unusual O-heteroside from Nauplius aquaticus (L) Nat Prod Res. 2005;19:523–528. doi: 10.1080/14786410412331302127. [DOI] [PubMed] [Google Scholar]
- 22.Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42:125–137. doi: 10.1016/j.ejmech.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Orlikova B, Tasdemir D, Golais F, Dicato M, Diederich M. Dietary chalcones with chemopeventive and chemoterapeutic potential. Genes Nutr. 2011;6:125–147. doi: 10.1007/s12263-011-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen R, Chatterjee M. Plant derived therapeutics for the treatment of leishmaniasis. Phytomedicine. 2011;18:1056–1069. doi: 10.1016/j.phymed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Hemshekhar M, Sunitha K, Sebastin MS, Devaraja S, Kemparaju K, Vishwanath BS, et al. An overview on genus garcinia: Phytochemical and therapeutical aspects. Phytochem Rev. 2011;10:325–351. [Google Scholar]
- 26.Mohamad H, Abas F, Permana D, Lajis NH, Ali AM, Sukari MA, et al. DPPH free radical scavenger components from the Fruits of Alpinia rafflesiana Wall. ex. Bak. (Zingiberaceae) Z Naturforsch C. 2004;59:811–815. doi: 10.1515/znc-2004-11-1208. [DOI] [PubMed] [Google Scholar]
- 27.Erkan K, Giuseppe M. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem. 2010;119:343–348. [Google Scholar]
- 28.Lizcano LJ, Bakkali F, Ruiz-Larrea MB, Ruiz-Sanz JI. Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use. Food Chem. 2010;119:1566–1570. [Google Scholar]
- 29.Meot-Duros L, Magné C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol Biochem. 2009;47:37–41. doi: 10.1016/j.plaphy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Yen G, Chen H. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. [Google Scholar]
- 31.Hajlaoui H, Mighri H, Noumi E, Snoussi M, Trabelsi N, Ksouri R, et al. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: A high effectiveness against Vibrio spp. strains. Food Chem Toxicol. 2010;48:2186–2192. doi: 10.1016/j.fct.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 32.Singh R, Singh B, Singh S, Kumar N, Kumar S, Arora S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol In Vitro. 2008;22:1965–1970. doi: 10.1016/j.tiv.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Sundararajan R, Haja NA, Venkatesan K, Mukherjee K, Saha B P, Bandyopadhyay A, et al. Cytisus scoparius link - A natural antioxidant. BMC Comp Altern Med. 2006;6:8. doi: 10.1186/1472-6882-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Orhan G, Orhan I, Sener B. Recent developments in natural and synthetic drug research for Alzheimer's disease. Lett Drug Design Disco. 2006;3:268–274. [Google Scholar]
- 36.Howes MJ, Houghton PJ. Plant used in Chinese and Indian traditional medecine for improvement of memory and coqnitive function. Pharmacol Biochem Behav. 2003;75:513–527. doi: 10.1016/s0091-3057(03)00128-x. [DOI] [PubMed] [Google Scholar]