Abstract
Objective
To determine the prevalence, clinical significance and the associated risk factors of potential drug-drug interactions (DDIs) at internal medicine ward of University of Gondar (UOG) hospital.
Method
A prospective cross-sectional study was conducted on patients treated in internal medicine ward of UOG hospital from April 29, 2013 to June 2, 2013. Data was collected from medical records and by interviewing the patients face to face. Descriptive analysis was conducted for back ground characteristics and logistic regression was used to determine the associated risk factors.
Result
In our study, we have identified a total number of 413 potential DDIs and 184 types of interacting combinations with 4.13 potential DDIs per patient. Among 413 potential DDIs most were of moderate interactions 61.2% (n=253) followed by 26% (n=107) of minor interactions and 12.8% (n=53) of major interactions. There was significant association of occurrence of potential DDIs only with taking three or more medications.
Conclusion
We have recorded a high rate of prevalence of potential DDI in the internal medicine ward of UOG hospital and a high number of clinically significant DDIs which the most prevalent DDI were of moderate severity. Careful selection of drugs and active pharmaceutical care is encouraged in order to avoid negative consequences of these interactions.
Keywords: Drug-drug interactions, Prevalence, Risk factors, Ethiopia, Prescription
1. Introduction
The term drug-drug interactions (DDIs) refer to alteration in the pharmacokinetics or effects of a drug by the presence of another drug[1]. DDIs are classified as pharmacodynamic or pharmacokinetic, and may result in increased or decreased efficacy, in treatment failure as well as in increased toxicity of medications[2],[3]. DDIs are preventable medication-related problems by avoiding multiple drug treatment, and the potential benefits of drug combinations are weighted against the risk of the occurrence of clinically significant DDIs. The incidence of potential DDIs (pDDIs) is close to 40% in patients taking 5 drugs, and exceeds 80% in patients taking 7 or more medications[4],[5]. Hospitalized patients are more likely to be affected by these DDIs because of severe and multiple illnesses, comorbid conditions, chronic therapeutic regimens, polypharmacy and frequent modification in therapy[6].
A study conducted in Switzerland reported that 56.2% of patients were exposed to one or more major or moderate pDDIs in the internal medicine ward[7]. In a literature review, Becker et al. found that 0.054% of emergency department visits, 0.57% of hospital admissions and 0.12% of re-hospitalizations are caused by DDIs[8].
To the best of our knowledge, a very few number of studies are available regarding the evaluation of potential DDIs in Sub-Saharan region of Africa[9]. In Kenya, about 33.5% of patients receiving anti-retroviral medications were exposed to clinically significant drug interactions with their anti-retroviral medications[10]. In Ethiopia, no previous studies were attempted to document the pharmacoepidemiology of potential DDIs and no attempts has been done to minimize the DDIs in internal medicine wards in hospitals, especially in Gondar University Hospital. Therefore, the aim of the study was to measure the prevalence, clinical significance and associated factors of potential DDIs in internal medicine ward at University of Gondar Teaching Hospital.
2. Materials and methods
A prospective cross sectional pilot study was carried out in internal medicine ward for a period of one month (May-June 2013) in University of Gondar Teaching Hospital, Northwest Ethiopia. University of Gondar Teaching Hospital is a 450-bedded hospital and is the only teaching referral hospital located in Amhara region, Northwest Ethiopia. The internal medicine wards have a capacity of 34 beds for men and 29 beds for women. Ethical approval was obtained from the relevant Institutional Ethics Committee prior to study initiation. Patients admitted consecutively to Internal medicine wards were included in the study. Patients whose medical charts and records were incomplete were excluded from the study.
Demographic information (age and sex), length of hospital stay, main diagnosis, number of drugs and details of comorbidities were obtained from the clinical records. All medications that were prescribed including routine and pro-re-nata (means as required) medications were screened for potential DDIs. We had classified patients in four categories according to their principal diagnosis, as infectious disease, cardiovascular diseases (CVD), endocrine, and others.
Potential DDIs were detected using the Drug Interactions Checker within www.drugs.com database[11]. The detected DDIs were classified as major, moderate and minor, depending on their severity of clinical significance and crossover checked manually for the presence of enough published scientific evidence for the identified interacting agents[12].
Based on the profile of medications prescribed, the DDIs were identified and classified according to the Drugs.com database. According to severity, pDDIs were classified as: (1) Major interactions are either well documented and have the potential of being harmful to the patient, or have a low incidence of occurrence and have the potential of serious adverse outcome. (2) Moderate interactions are of moderate clinical significance, and are less likely than major interactions to cause harm to the patient, or are less well documented. (3) Minor interactions are of minor clinical significance.
Frequencies expressed as percentages were used to summarize sex, diagnosis, number of drugs dispensed frequency of pDDIs, the drugs involved in the pDDIs and severity of pDDIs. Odds ratio with 95% confidence interval was used to summarize age, length of stay, diagnosis, and number of medications. P-value of 0.05 or less was considered statistically significant. SPSS for Windows version 16 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses.
3. Results
A total of 107 patients admitted in the internal medicine ward during the study period, of which 100 were enrolled in our study. The 7 patients were excluded from the study due to incomplete medical records and did not have sufficient data because the patients were transferred to other departments.
3.1. Demographic information
Of the total 100 patients, 61 were males and 39 were females with median age of 40 and age range of 15-87. The numbers of medications on medical chart were approximately 6 (range 2-15). The most common cause of hospital admission was infectious diseases (50%) followed by CVD (37%). Among all patients 78% (n=78) had at least one or more potential DDI regardless of type of severity (Table 1).
Table 1. General characteristics of patients in internal medicine ward (n=100).
| Variables | Male [n (%)] | Female [n (%)] | |
| Gender | 61 (61%) | 39 (39%) | |
| Age | <20 | 3 (75%) | 1 (25%) |
| 20-34 | 19 (52.7%) | 17 (47.3%) | |
| 35-49 | 23 (67.64%) | 11 (32.36%) | |
| 50-64 | 8 (50%) | 8 (50%) | |
| >64 | 8 (80%) | 2 (20%) | |
| Hospital stay | 1-6 | 12 (48%) | 13 (52%) |
| >6 | 49 (65.3%) | 26 (34.7%) | |
| Diagnosis | Infectious | 31 (62%) | 19 (38%) |
| CVD | 23 (62.1%) | 14 (37.9%) | |
| Endocrine | 3 (50%) | 3 (50%) | |
| Others | 4 (57.1%) | 3 (42.9%) | |
| pDDIs | Present | 47 (60.25%) | 31 (39.75%) |
| Absent | 14 (63.6%) | 8 (34.4%) | |
| No of Medications | 1-3 | 19 (79.2%) | 5 (20.8%) |
| >3 | 42 (55.3%) | 34 (44.7%) | |
3.2. Prevalence of potential DDI
A total of 413 pDDIs and 184 types of interacting combinations were identified in our study. Number of potential DDIs per patient were categorized as 1-3 (n=22, 28.2%), 4-6 (n=35, 44.9%) and more than 6 (n=21, 26.9%). A total of 559 medications were prescribed and the number of medications per patient were found to be 5.59.
3.3. Association with the risk factors
Table 2 shows that there is association of the occurrence of one or more pDDIs only with the number of medications prescribed per patient who took more than three medications [odds ratio (95% CI)=0.084 (0.028,0.251) and P=0.000], but other variables like gender (female) with OR (95% CI)=0.866 (0.325, 2.308) and P=0.774, age<20 (P=0.852), hospital stay (P=0.056) have no association with potential DDIs. Among the 78 patients with pDDIs, most of the DDI present in patients received 5 medications (n=17), followed by patients receiving 4 medications (n=14), 6 medications (n=8), 7 medications (n=7) and 8 medications (n=7).
Table 2. Potential DDIs and their associations with the risk factors.
| Variables | Drug interaction |
P value | OR (95% CI) | ||
| Present [n (%)] | Absent [n (%)] | ||||
| Gender | Male | 47 (77%) | 14 (23%) | ||
| Female | 31 (79.5%) | 8 (20.5%) | 0.774 | 0.866 (0.325, 2.308) | |
| Age | <20 | 3 (75%) | 1 (25%) | 0.852 | 0.778 (0.056, 10.861) |
| 20-34 | 29 (80.6%) | 7 (19.4%) | 0.478 | 0.563 (0.115, 2.747) | |
| 35-49 | 26 (76.5%) | 8 (23.5%) | 0.679 | 0.718 (0.150, 3.442) | |
| 50-64 | 13 (81.2%) | 3 (18.8%) | 0.511 | 0.538 (0.085, 3.409) | |
| >64 | 7 (70%) | 3 (30%) | |||
| Hospital stay | 1-6 | 16 (64%) | 9 (36%) | ||
| >6 | 62 (82.7%) | 13 (17.3%) | 0.056 | 0.373 (0.135, 1.026) | |
| Diagnosis | Infectious | 38 (76%) | 12 (24%) | ||
| CVD | 32 (86.5%) | 5 (13.5%) | 0.228 | 0.495 (0.158, 1.554) | |
| Endocrine | 4 (66.7%) | 2 (33.3%) | 0.620 | 1.538 (0.257, 9.745) | |
| Others | 4 (57.1%) | 3 (42.9%) | 0.299 | 2.375 (0.465, 12.141) | |
| No of Medications | 1-3 | 10 (41.7%) | 14 (58.3%) | ||
| >3 | 68 (89.5%) | 8 (10.5%) | 0.000 | 0.084 (0.028, 0.251) | |
3.4. Types of potential DDIs
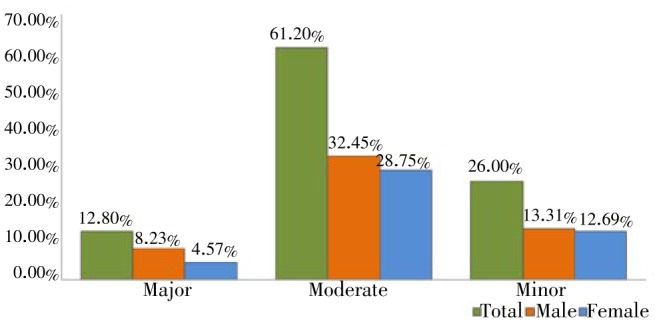
Three types of potential DDIs were classified based on the level of severity, as major, moderate and minor. Among the 413 potential DDIs, most were of moderate interactions 61.2% (n=253), followed by minor interactions 26% (n=107) and major interactions were 12.8% (n=53). Of the moderate interactions, 134 patients were males (52.9%) and females 119 (47.1%). 55 male patients were experiencing minor interactions (51.4%) while 52 patients were females (48.6%). With major interactions, 34 were male patients (64.1%) and 19 were female (35.9%) (Figure 1).
Figure 1. Types and frequency of potential DDIs.
3.5. Common interacting drug combinations
Among most interacting medications, the top 21 commonly occurring pDDIs includes 6 major, 7 moderate and 8 minor pDDIs. Ceftriaxone, furosemide, isoniazid ethambutol, sulfadiazine, pyrimethamine, rifampin, pyrazinamide and phenyton were the drugs most commonly encountered (Table 3).
Table 3. Most frequently identified major or moderate interactions and potential adverse outcomes.
| Interaction | Frequency (%) | Potential adverse outcomes | |
| Major | Rifampin+pyrazinamide | 9 (17.0%) | Combination may cause liver damage |
| Rifampin+isoniazid | 9 (17.0%) | Combination may cause liver damage | |
| Cotrimoxazole+leucovorin | 8 (15.0%) | Combination may increase risk of treatment failure | |
| Phenytoin+artemether | 5 (9.4%) | Phenytoin may significantly reduce the blood levels of artemether | |
| Warfarin+heparin | 4 (7.5%) | Combination can cause bleeding more easily | |
| Ceftriaxone+leucovorin | 4 (7.5%) | May cause precipitation of ceftriaxone-calcium salt | |
| Moderate | Ceftriaxone+furosemide | 12 (4.7%) | Furosemide increase the risk of kidney damage by cephalosporin |
| Sulfadiazine+pyrimethamine | 10 (3.9%) | Combination may increase the risk of anemia | |
| Isoniazid+ethambutol | 9 (3.5%) | Combination may increase the risk of nerve damage | |
| Cotrimoxazole+pyrimethamine | 8 (3.2%) | Combination may increase the risk of anemia | |
| Metronidazole+phenytoin | 6 (2.4%) | Combination may increase phenytoin level | |
| Leucovorin+phenytoin | 6 (2.4%) | Leucovorine can decrease the bood level and effects of phenytoin | |
| Sulfadiazine+phenytoin | 6 (2.4%) | Sulfodiazine increase the effect of phenytoin | |
| Minor | Ceftriaxone+phenytoin | 9 (8.4%) | Ceftriaxone may displace phenytoin from serum protein bleeding site |
| Ceftriaxone+heparin | 7 (6.5%) | Combination can increase risk of bleeding | |
| Furosemide+aspirins | 6 (5.6%) | Salicylates in anti-inflammatory dosage may blunt the diuretic and natriuretic response to loop diuretics | |
| Furosemide+doxycycline | 5 (4.7%) | Combination may decrease renal function manifested by increase in clotting factor | |
| Digoxin+spironolactone | 4 (3.7%) | Spironolactone may reduce tubular secretion of digoxin | |
| Warfarin+furosemide | 4 (3.7%) | Furosemide may displace warfarin from plasma protein binding site | |
| Warfarin+spironolactone | 4 (3.7%) | Spironolactone may cause dieresis and hemo concentration of clotting factor | |
| SMX+phenytoin | 4 (3.7%) | SMX may displace phenytoin from serum protein binding site | |
4. Discussion
The present study revealed that the overall prevalence of potential DDIs were 78% which is high compared to other studies which encountered pDDIs of 23%[9], 45%[12], 49.7%[13] and 56.2%[7]. Among them major potential DDIs were 24.25% (n=97), moderate interactions 36% (n=144) and minor interactions 12.25% (n=49) observed in internal medicine ward. These results were contradictory to results obtained in Ismail et al.[12] study, where major interactions 12.8% (n=53), moderate interactions 61.2% (n=253), and minor interactions of 26% (n=107). We recorded average 1.22 and median number of 01 pDDI per patient in internal medicine ward during this study period. Average 1.44 pDDIs per patient and median pDDIs of 2 per patient have been reported by other studies[14],[15].This comparison indicates that pDDIs are most relevant on the general medical ward and need careful attention towards pDDIs. All potential DDIs are not equally harmful; therefore, identification of level of each potential DDIs are integral to assess clinical importance and appropriate management. Studies that have looked at the prevalence of interactions among hospitalized patients have yielded similar results[1]–[15]. The prevalent condition on internal medicine ward were most observed in infectious diseases and CVD. In Gondar hospital particularly in internal medicine ward HIV/AIDS and opportunistic infections lend themselves to use of more complex drug regimen with a high potential for interactions. These patients therefore need closer monitoring to avoid the potential negative outcome of DDIs.
In our study we identified potential DDIs in infectious disease 76% (n=38), in CVD 86.5% (n=32), in endocrine disorder 66.7%(n=4) and other disorders accounts for 57.1%(n=4) but the prevalence of potential DDIs in patient medication which was performed in Uganda was, in infectious 40% (n=12), in CVD 63.6% (n=7), in endocrine disorder 0.00% (n=0) and other disorders account for 13.7% (n=13) also in their study the prevalence of potential DDIs in patients who stayed for less than six days was 21.8% (n=31) and for those who stayed for six days and more, the prevalence was 25.7% (n=23)[9]. But in our study the prevalence for those patients stayed less than six days is 64% (n=16) and for those stayed for more than six days is 82.7% (n=62).
Our findings revealed that the prevalence of potential DDIs was positively associated only with the >3 number of medications (P=0.000). This does not corresponds to other studies reporting that potential DDIs were common in elderly people who were on multi-drug regimen[12],[16],[17]. We have found a potential DDI per patient of 4.13, which is very high compared to Switzerland study which reported a potential DDI per patient of 1.9[18] and the prevalence of major potential DDIs was 2%, moderate potential DDIs was 38% and minor potential DDIs was 60%, which is different from our research that reported a major interaction of 12.83% (n=53), moderate 61.26 (n=253) and minor 25.91% (n=107).
In our study the majority of the major potential DDIs were observed with rifampin with pyrazinamide which may result in severe hepatic injury. Similarly, rifamipin and isoniazid associated to cause higher risk of hepatotoxicity than with either agent alone. These combinations are commonly used and are therapeutically valuable. Caution to be taken in patients with hepatic impairment, malnourished patients, the elderly, and children under 2 years of age. Patients should be monitored for clinical symptoms of liver toxicity including fever, anorexia, vomiting and jaundice[3],[12].Concomitant use of leucovorin and cotrimoxazole for acute treatment of Pneumocystis jiroveci pneumonia in HIV-infected patients has been associated with increased rates of treatment failure and mortality[11],[19]. Co-administration of artemether with phenytoin causes strong induction of CYP3A4 can result in decrease of concentration of artemether and loss of antimalarial efficacy[20]. It is important to monitor all the possible and major pDDIs before prescribing to the patients to minimize the potential consequences and to manage them accordingly. The possible difference between our study and other studies may be due to different factors including, high utilization of drugs having more interaction potential, difference in study design and softwares used.
The potential limitations of the study is using of software for identification of DDIs, which is less sensitive and specific compared to that of other softwares. The study period and sample size was less compared to that of other studies.
The present study has recorded a high prevalence of pDDIs in internal medicine wards and identified 413 potential DDIs, of which the most prevalent DDIs were of moderate severity. We found clinically significant association with increased number of prescribed medications (>3) were more exposed to pDDIs. Identifying and preventing potentially harmful DDIs is a critical component of a pharmacist's mission and the clinical pharmacist must remain vigilant in their monitoring of pDDIs and make appropriate dosage or therapy adjustments. Careful selection of drugs and active pharmaceutical care is encouraged in order to avoid negative consequences of these interactions.
Acknowledgments
The authors are thankful to the patients who gave their informed consent and Department of Internal Medicine, School of Pharmacy for providing facilities and co-operation during this study. This study was supported by University of Gondar, Grant no. UoG/ Re/ Core/ pro/ 0134/ 2011.
Comments
Background
DDIs are a common problem during treatment and give rise to medically important, sometimes serious or even fatal adverse events. DDIs can also cause partial or complete abolishment of treatment efficacy. Hospitalized patients are more likely to be affected by DDIs because of severe and multiple illnesses, comorbid conditions, chronic therapeutic regimens, polypharmacy and frequent modification in therapy. The scarcity of data on DDIs in the study area is the motivation to undertake this stydy.
Research frontiers
The present study emphasizes on prevalence, clinical significance and the associated risk factors of potential DDIs in the study area.
Related reports
Potential DDIs are common. In the study of Kapp et al. (2013), the overall prevalence of pDDIs was 91.5%, while 43.25% of scripts contained at least one pDDI. More than five per cent of prescriptions contained a potentially severe interaction, and one in 200 scripts had a contraindicated combination. Simultaneous prescribing from a regional hospital increased the risk of script containing a potential drug-drug interaction.
Innovations and breakthroughs
The authors in the present study have recorded a high prevalence of pDDIs in internal medicine wards and identified 413 potential DDIs, of which the most prevalent DDIs were of moderate severity. Authors found clinically significant association with increased number of prescribed medications (>3) were more exposed to pDDIs.
Applications
The results of this study can be used to predict treatment recommendations and are developed based on the clinical relevance of the interactions and the possibility to make dose adjustments or treatment monitoring.
Peer review
This is an important work in which authors have demonstrated the prevalence potential risks of DDI from underdeveloped country. The paper provides evidence of the prevalence of DDIs and associated risk factors. The conclusions are valid and the article provides relevant piece of evidence that completes lack of information in study area.
Footnotes
Foundation Project: Supported by University of Gondar (Grant no. UoG/ Re/ Core/ pro/ 0134/ 2011).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Segura-Bedmar I, Martínez P, de Pablo-Sánchez C. Extracting drug-drug interactions from biomedical texts. BMC Bioinformatics. 2010;1(Suppl 5):9. [Google Scholar]
- 2.Hansten PD, Horn JR. Drug interactions analysis and management. St. Louis, MO, USA: Facts & Comparisons; 2009. [Google Scholar]
- 3.Baxter K, editor. Stockley's drug interactions. London, UK: Pharmaceutical Press; 2010. [Google Scholar]
- 4.Kapp PA, Klop AC, Jenkins LS. Drug interactions in primary health care in the George subdistrict, South Africa: a cross-sectional study. South Afr Fam Pract. 2013;55(1):78–84. [Google Scholar]
- 5.Grattagliano I, Portincasa P, D'Ambrosio G, Palmieri VO, Palasciano G. Avoiding drug interactions: here's help. J Fam Pract. 2010;59:322–329. [PubMed] [Google Scholar]
- 6.Zwart-van-Rijkom JEF, Uijtendaal EV, Ten Berg MJ, Van Solinge WW, Egberts ACG. Frequency and nature of drug-drug interactions in a Dutch university hospital. Br J Clin Pharmacol. 2009;68:187–193. doi: 10.1111/j.1365-2125.2009.03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonbach P, Dubied A, Krähenbühl S, Beer JH. Prevalence of drug-drug interactions at hospital entry and during hospital stay of patients in internal medicine. Eur J Intern Med. 2008;19:413–420. doi: 10.1016/j.ejim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Becker ML, Kallewaard M, Caspers P, Visser LE, Leufkens H, Stricker B. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641–651. doi: 10.1002/pds.1351. [DOI] [PubMed] [Google Scholar]
- 9.Lubinga SJ, Uwiduhaye E. Potential drug-drug interactions on in-patient medications prescriptions at Mbarara Regional Referral Hospital (MRRH) in western Uganda: Prevalence, clinical importance and associated factors. Afr Health Sci. 2011;11(3):499–507. [PMC free article] [PubMed] [Google Scholar]
- 10.Moura CS, Acurcio FA, Belo NO. Drug-drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12(3):266–272. doi: 10.18433/j35c7z. [DOI] [PubMed] [Google Scholar]
- 11.Cerner Multum, Inc . Drug Interactions Checker. USA: Cerner Multum, Inc.; 2013. [Online] Available from: http://www.drugs.com/drug_interactions.html. [Accessed on July 23 2013] [Google Scholar]
- 12.Ismail M, Iqbal Z, Khattak MB, Javaid A, Khan TM. Prevalence, type and predictors of potential drug-drug interactions in pulmonary ward of a tertiary care hospital. Afr J Pharm Pharmacol. 2011;5(10):1303–1309. [Google Scholar]
- 13.Cruciol-Souza JM, Thomson JC. Prevalence of potential drug-drug interactions and its associated factors in a Brazilian teaching hospital. J Pharm Pharmaceut Sci. 2006;9(3):427–433. [PubMed] [Google Scholar]
- 14.Egger SS, Drewe J, Schlienger RG. Potential drug-drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol. 2003;58:773–778. doi: 10.1007/s00228-002-0557-z. [DOI] [PubMed] [Google Scholar]
- 15.Fokter N, Mozina M, Brvar M. Potential drug-drug interactions and admissions due to drug-drug interactions in patients treated in medical departments. Wien Klin Wochenschr. 2010;122:81–88. doi: 10.1007/s00508-009-1251-2. [DOI] [PubMed] [Google Scholar]
- 16.Riechelmann RP, Moreira F, Smaletz O, Saad ED. Potential for drug interactions in hospitalized cancer patients. Cancer Chemother Pharmacol. 2005;56:286–290. doi: 10.1007/s00280-004-0998-4. [DOI] [PubMed] [Google Scholar]
- 17.Doubova SV, Reyes-Morales H, Torres-Arreola LDP, Suárez-Ortega M. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv Res. 2007;7:147. doi: 10.1186/1472-6963-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertoli R, Bissig M, Caronozolo D, Odorico M, Pons M, Bernasconi E. Assessment of potential drug-drug interactions at hospital discharge. Swiss Med Wkly. 2010 doi: 10.4414/smw.2010.13043. [DOI] [PubMed] [Google Scholar]
- 19.McEvoy GK, Snow ED, editors. AHFS: drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2012. pp. 41–46. [Google Scholar]
- 20.Coartem (Artemether/Lumefantrine) tablet: safety labeling changes approved by FDA center for Drug evaluation and research (CDER) U.S Food and Drug Administration. Silver spring, MD, USA. Aug, 2012.


